Decompressive Craniectomy Improves QTc Interval in Traumatic Brain Injury Patients
Abstract
:1. Introduction
2. Methods
2.1. Patient Selection
2.2. Patients’ Monitoring and Management
2.3. Anaesthesia for Decompressive Craniectomy
2.4. Electrocardiography and Vectorcardiography Measurement, and Studied Time Points
2.5. Statistical Analysis
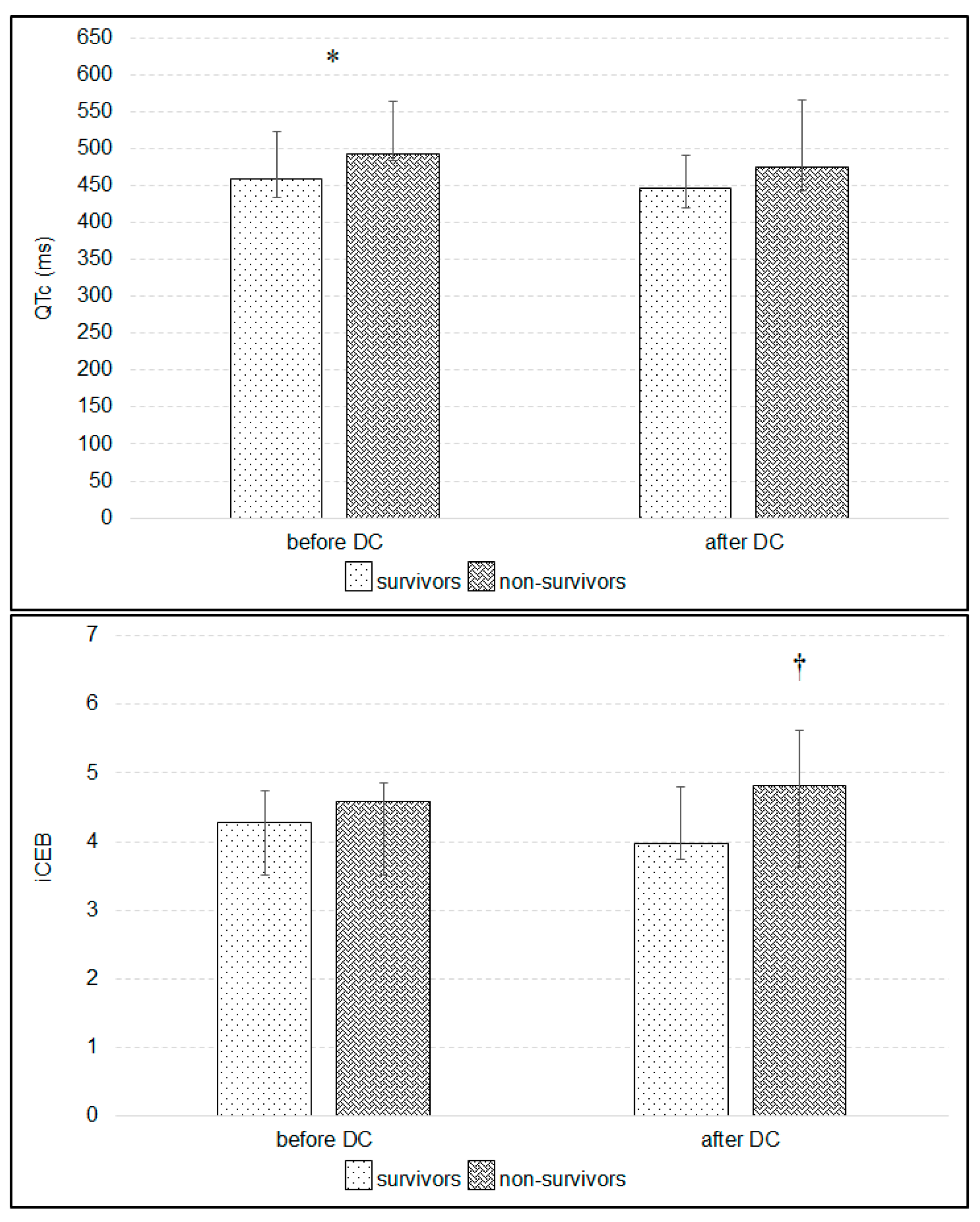
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hasanin, A.; Kamal, A.; Amin, S.; Zakaria, D.; El Sayed, R.; Mahmoud, K.; Mukahtar, A. Incidence and outcome of cardiac injury in patients with severe head trauma. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglini, D.; Robba, C.; Lopes da Silva, A.; Dos Santos Samary, C.; Leme Silva, P.; Dal Pizzol, F.; Pelosi, P.; Rocco, P.R.M. Brain-heart interaction after acute ischemic stroke. Crit. Care 2020, 24, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Menynar, A.; Goyal, A.; Latifi, R.; Al-Thani, H.; Frishman, W. Brain-heart interactions in traumatic brain injury. Cardiol. Rev. 2017, 25, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Sijercic, S.; Krdzalic, A.; Avdagic, H.; Krdzalic, G. Incidence of cardiac dysfunction after brain injury. Med. Arch. 2018, 72, 316–318. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Korantzopoulos, P.; Tsivgoulis, G.; Kyritsis, A.P.; Kosmidou, M.; Giannopoulos, S. Electrocardiographic abnormalities and cardiac arrhythmias in structural brain lesions. Int. J. Cardiol. 2013, 167, 328–334. [Google Scholar] [CrossRef]
- Dabrowski, W.; Schlegel, T.T.; Wosko, J.; Rola, R.; Rzecki, Z.; Malbrain, M.L.N.G.; Jaroszynski, A. Changes in spatial QRS-T angle and QTc interval in patients with traumatic brain injury with or without intra-abdominal hypertension. J. Electrocardiol. 2018, 51, 499–507. [Google Scholar] [CrossRef]
- Jachuck, S.J.; Ramani, P.S.; Clark, F.; Kalbag, R.M. Electrocardiographic abnormalities associated with raised intracranial pressure. Br. Med. J. 1975, 1, 242–244. [Google Scholar] [CrossRef] [Green Version]
- Keller, C.; Williams, A. Cardiac dysrhythmias associated with central nervous system dysfunction. J. Neurosci. Nurs. 1993, 25, 349–355. [Google Scholar] [CrossRef]
- Grosse-Wortmann, L.; Bindl, L.; Seghaye, M.C. Multiple types of cardiac arrhythmias in child with head injury and raised intracranial pressure. Pediatr. Cardiol. 2006, 27, 286–288. [Google Scholar] [CrossRef]
- Frangiskakis, J.M.; Hravnak, M.; Crago, E.A.; Tanabe, M.; Kip, K.E.; Gorcsan, J.; Horowitz, M.B.; Kassam, A.B.; London, B. Ventricular arrhythmia risk after subarachnoid haemorrhage. Neurocrit. Care 2009, 10, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, C.; Thalamus, J.; Kristoffersen, D.T.; Svendsen, M.V.; Holla, Ø.L.; Heldal, K.; Haugaa, K.H.; Hysing, J. QT prolongation predicts short-term mortality independent of comorbidity. Europace 2019, 21, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Whang, W.; Shimbo, D.; Levitan, E.B.; Newman, J.D.; Rautaharju, P.M.; Davidson, K.W.; Muntner, P. Relations between QRS|T angle, cardiac risk factors, and mortality in the third national health and nutrition examination survey (NHANES III). Am. J. Cardio 2012, 109, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elming, H.; Brendorp, B.; Køber, L.; Sahebzadah, N.; Torp-Petersen, C. QTc interval in the assessment of cardiac risk. Card. Electrophysiol. Rev. 2002, 6, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.R.; Yan, G.X.; Gallacher, D.J. A new biomarker—Index of Cardiac Electrophysiological Balance (iCEB)—Plays an important role in drug-induced cardiac arrhythmias: Beyond QT-prolongation and Torsades de Pointes (TdPs). J. Pharmacol. Toxicol. Methods 2013, 68, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Sivri, S.; Celik, M. Evaluation of index of cardiac-electrophysiological balance before and after hemodialysis in patients with end-stage renal disease. J. Electrocardiol. 2019, 54, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Robyns, T.; Lu, H.R.; Gallacher, D.J.; Garweg, C.; Ector, J.; Willems, R.; Janssens, S.; Nuyens, D. Evaluation of index of cardio-electrophysiological balance (iCEB) as a new biomarker for the identification of patients at increased arrhythmic risk. Ann. Noninvasive Electrocardiol. 2016, 21, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Taggart, P.; Critchey, H.; van Duijvendoden, S.; Lambiase, P.D. Significance of neuro-cardial control mechanisms governed by higher regions of the brain. Auton. Neurosci. 2016, 199, 54–65. [Google Scholar] [CrossRef]
- Machhada, A.; Ang, R.; Ackland, G.L.; Ninkina, N.; Buchman, V.L.; Lythgoe, M.F.; Trapp, S.; Tinker, A.; Marina, N.; Gourine, A.V. Control of ventricular excitability by neurons of the dorsal motor nucleus of the vagus nerve. Heart Rhythm 2015, 12, 2285–2293. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.A.; Wijdicks, E.F.M. Decompressive craniectomy in acute brain injury. Handb. Clin. Neurol. 2017, 140, 299–318. [Google Scholar]
- Hutchinson, P.J.; Kolias, A.G.; Timofeev, I.S.; Corteen, E.A.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; Eynon, C.A.; et al. RESCUEicp Trial Collaborators. Trial of decompressive craniectomy for traumatic intracranial hypertension. N. Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.H.; Deng, Y.H.; Lee, T.C.; Chen, W.F. Rotterdam computed tomography score as a prognosticator in head-injured patients undergoing decompressive craniectomy. Neurosurgery 2012, 71, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, W.; Siwicka-Gieroba, D.; Robba, C.; Badenes, R.; Bialy, M.; Iwaniuk, P.; Schlegel, T.T.; Jaroszynski, A. Plasma hyperosmolality prolongs QTc interval and increases risk for atrial fibrillation in traumatic brain injury patients. J. Clin. Med. 2020, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the management of severe traumatic brain injury, Fourth edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Cortez, D.L.; Schlegel, T.T. When deriving the spatial QRS-T angle from 12-lead electrocardiogram, which transform in more Frank: Regression or inverse Dower? J. Electrocardiol. 2010, 43, 302–309. [Google Scholar] [CrossRef]
- Edenbrandt, L.; Pahlm, O. Vectorcardiogram synthesis from 12-lead ECG: Superiority of the inverse Dover matrix. J. Electrocardiol. 1988, 21, 361–367. [Google Scholar] [CrossRef]
- Xue, J.Q. QT interval measurement what can we really expect? Comput. Cardiol. 2006, 33, 385–388. [Google Scholar]
- Oppenheimer, S.M. Neurogenic cardiac effects of cerebrovascular disease. Curr. Opin. Neurol. 1994, 7, 20–24. [Google Scholar] [CrossRef]
- Chen, W.; Sheng, J.; Peng, G.; Yang, J.; Wang, S.; Li, K. Early stage alterations of catecholamine and adrenocorticotropic hormone levels in posttraumatic acute diffuse brain swelling. Brain Res. Bull. 2017, 130, 47–52. [Google Scholar] [CrossRef]
- Nguyen, H.; Zaroff, J.G. Neurogenic stunned myocardium. Curr. Neurol. Neurosci. Rep. 2009, 9, 486–491. [Google Scholar] [CrossRef]
- Kôiv, L.; Merisalu, E.; Zilmer, K.; Tomberg, T.; Kaasik, A.-E. Changes of sympatho-adrenal and hypothalamo-pituitary-adrenocortical system in patients with head injury. Acta Neurol. Scand. 1997, 96, 52–58. [Google Scholar] [CrossRef]
- Desgranges, F.P.; Javouhey, E.; Mottolese, C.; Migeon, A.; Szathmari, A.; Baudin, F.; de Queiroz, M.; Cogniat, B.; Chassaed, D. Intraoperative blood loss during decompressive craniectomy for intractable intracranial hypertension after severe traumatic brain injury in children. Childs Nerv. Syst. 2014, 30, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Daney, P. QT interval lengthening in cardiac disease relates more to left ventricular systolic dysfunction than to autonomic function. Eur. J. Heart Fail. 2000, 2, 265–271. [Google Scholar]
- Tian, R.; Liu, W.; Dong, J.; Zhang, J.; Xu, L.; Zhang, B.; Tao, X.; Li, J.; Liu, B. Prognostic predictors of early outcomes and discharge status of patients undergoing decompressive craniectomy after severe traumatic brain injury. World Neurosurg. 2019, 126, e101–e108. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, E.J.; Melis, M.; Gordon, E.; Ullman, J.S.; Germano, I.M. Decompressive craniectomy for severe traumatic brain injury: A systematic review. World Neurosurg. 2016, 88, 411–420. [Google Scholar] [CrossRef]
- Vandael, E.; Vandenberk, B.; Vandenberghe, J.; Willems, R.; Foulon, V. Risk factors for QTc- prolongation: Systematic review of the evidence. Int. J. Clin. Pharm. 2017, 39, 16–25. [Google Scholar] [CrossRef]
- Yamazaki, T.; Froelicher, V.F.; Myers, J.; Chun, S.; Wang, P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm 2005, 2, 73–78. [Google Scholar] [CrossRef]
- Kück, K.; Isaksen, J.L.; Graff, C.; Skaaby, T.; Linneberg, A.; Hansen, T.; Kanters, J.K. Spatial QRS-T angle variants for prediction of all-cause mortality. J. Electrocardiol. 2018, 51, 768–775. [Google Scholar] [CrossRef] [Green Version]
- Ӧzdemir, L.; Sӧkmen, E. Effect of habitual cigarette smoking on the index of cardiac electrophysiological balance in apparently healthy individuals. J. Electrocardiol. 2020, 59, 41–44. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, X.; Gao, H.; Ren, Y.; Li, H.; He, Y.; Wang, G. Effect of different concentrations of desflurane on the index of cardiac electrophysiological balance in gynecologic surgery patients. Can. J. Physiol. Pharmacol. 2020, 98, 332–335. [Google Scholar] [CrossRef]
- Tse, G.; Yan, B.P. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace 2017, 19, 712–721. [Google Scholar] [CrossRef]
- Oji, M.; Terao, Y.; Toyoda, T.; Kuriyama, T.; Miura, K.; Fukusaki, M.; Sumikawa, K. Differential effects of propofol and sevoflurane on QT interval during anesthetic induction. J. Clin. Monit. Comput. 2013, 27, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Hnatkova, K.; Seegers, J.; Barthel, P.; Novotny, T.; Smetana, P.; Zabel, M.; Schmidt, G.; Malik, M. Clinical value of different QRS-T angle expressions. Europace 2018, 20, 1352–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| n = 48 | R = 0.61, R2 = 0.37 Corrected R2 = 0.37 F(2.45) = 13.5, p < 0.001 | |||||
|---|---|---|---|---|---|---|
| b * | SD of b * | b | SD of b | t | p | |
| 5.99 | 0.366 | 16.343 | 0.0000 | |||
| Heart rate | −0.633 | 0.123 | −0.02 | 0.004 | −5.131 | 0.0000 |
| Dose of norepinephrine | 0.276 | 0.123 | 0.647 | 0.288 | 2.241 | 0.0300 |
| Before DC | After DC | |
|---|---|---|
| I | 0.01 | 0 |
| [−0.01; 0.02] | [−0.01; 0.02] | |
| II | 0 | 0.02 * |
| [−0.03; 0.02] | [−0.01; 0.04] | |
| III | 0 | 0.01 ** |
| [−0.02; 0.01] | [−0.01; 0.03] | |
| aVR | 0 | −0.01 |
| [−0.03; 0.02] | [−0.03; 0.01] | |
| aVL | 0 | 0 |
| [−0.01; 0.01] | [−0.01; 0.01] | |
| aVF | 0 | 0.01 ** |
| [−0.02; 0.02] | [−0.01; 0.04] | |
| V1 | 0 | 0 |
| [−0.01; 0.01] | [−0.01; 0.01] | |
| V2 | 0 | 0 |
| [−0.01; 0.02] | [−0.02; 0.02] | |
| V3 | 0.01 | 0.01 |
| [−0.01; 0.04] | [−0.02; 0.03] | |
| V4 | −0.01 | −0.01 |
| [−0.05; 0.03] | [−0.03; 0.03] | |
| V5 | −0.01 | 0 |
| [−0.03; 0.03] | [−0.02; 0.04] | |
| V6 | 0 | 0.01 * |
| [−0.02; 0.03] | [−0.01; 0.05] |
| Lead | Time Point | Survivors | Non-Survivors | p Value |
|---|---|---|---|---|
| I | before DC | 0.01 | −0.01 | 0.005 |
| [0; 0.02] | [−0.01; 0] | |||
| after DC | 0.01 | −0.01 | 0.020 | |
| [0; 0.02] | [−0.01; 0] | |||
| II | before DC | 0.01 | −0.03 | 0.001 |
| [−0.01; 0.04] | [−0.05; 0.01] | |||
| after DC | 0.02 | −0.01 | 0.007 | |
| 0.01; 0.05] | [−0.04; 0.01] | |||
| III | before DC | 0 | −0.01 | 0.016 |
| [−0.01; 0.02] | [−0.04; 0] | |||
| after DC | 0.01 | −0.01 | 0.025 | |
| [0; 0.03] | [−0.03; 0.01] | |||
| aVR | before DC | 0 | 0.03 | 0.006 |
| [−0.04; 0.01] | [0; 0.05] | |||
| after DC | −0.02 | 0 | 0.023 | |
| [−0.03; 0] | [−0.01; 0.02] | |||
| aVL | before DC | 0 | 0 | 0.533 |
| [−0.01; 0.01] | [0; 0.01] | |||
| after DC | 0 | 0.01 | 0.339 | |
| [−0.01; 0.01] | [0; 0.01] | |||
| aVF | before DC | 0.01 | −0.02 | 0.004 |
| [−0.01; 0.03] | [−0.04; 0] | |||
| after DC | 0.02 | −0.01 | 0.036 | |
| [0.01; 0.04] | [−0.03; 0.02] | |||
| V1 | before DC | −0.01 | 0.01 | 0.032 |
| [−0.02; 0.02] | [0; 0.03] | |||
| after DC | 0 | 0.01 | 0.115 | |
| [−0.01; 0.02] | [0; 0.03] | |||
| V2 | before DC | 0 | 0 | 0.787 |
| [−0.01; 0.02] | [−0.02; 0.03] | |||
| after DC | 0 | 0.01 | 0.444 | |
| [−0.02; 0.02] | [−0.01; 0.02] | |||
| V3 | before DC | 0.01 | 0 | 0.166 |
| [0; 0.04 | [−0.03; 0.02] | |||
| after DC | 0.01 * | 0 | 0.390 | |
| [−0.02; 0.03 | [−0.03; 0.03] | |||
| V4 | before DC | 0 | −0.05 | 0.012 |
| [−0.03; 0.04] | −0.09; 0.02] | |||
| after DC | 0 | −0.03 | 0.139 | |
| [−0.03; 0.03] | [−0.06; 0.01] | |||
| V5 | before DC | 0.01 | −0.02 | 0.008 |
| [−0.02; 0.04] | [−0.06; −0.01] | |||
| after DC | 0.01 | −0.03 | 0.015 | |
| [−0.01; 0.05] | [−0.05; 0] | |||
| V6 | before DC | 0.01 | −0.01 | 0.008 |
| [−0.01; 0.04] | [−0.04; 0.01] | |||
| after DC | 0.02 | −0.02 | 0.003 | |
| [0.01; 0.05] | [−0.04; 0] |
| R = 0.64, R2 = 0.41 Corrected R2 = 0.38 F(2.3) = 11.2 p < 0.001 | |||||||
| b * | SD of b * | b | SD of b | t | p | ||
| survivors | 6.136 | 0.435 | 14.078 | 0.0000 | |||
| Heart rate | −0.666 | 0.142 | −0.023 | 0.004 | −4.682 | 0.0000 | |
| Dose of norepinephrine | 0.296 | 0.142 | 0.615 | 0.295 | 2.085 | 0.0451 | |
| non-survivors | R = 0.63, R2 = 0.4 Corrected R2 = 0.28 F(2.1) = 3.4 p < 0.075 | ||||||
| 6.152 | 0.785 | 7.836 | 0.0000 | ||||
| Heart rate | −0.655 | 0.253 | −0.019 | 0.008 | −2.588 | 0.027 | |
| Dose of norepinephrine | 0.2323 | 0.2533 | 0.7631 | 0.8323 | 0.9168 | 0.3807 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabrowski, W.; Siwicka-Gieroba, D.; Robba, C.; Badenes, R.; Kotfis, K.; Schlegel, T.T.; Jaroszynski, A. Decompressive Craniectomy Improves QTc Interval in Traumatic Brain Injury Patients. Int. J. Environ. Res. Public Health 2020, 17, 8653. https://doi.org/10.3390/ijerph17228653
Dabrowski W, Siwicka-Gieroba D, Robba C, Badenes R, Kotfis K, Schlegel TT, Jaroszynski A. Decompressive Craniectomy Improves QTc Interval in Traumatic Brain Injury Patients. International Journal of Environmental Research and Public Health. 2020; 17(22):8653. https://doi.org/10.3390/ijerph17228653
Chicago/Turabian StyleDabrowski, Wojciech, Dorota Siwicka-Gieroba, Chiara Robba, Rafael Badenes, Katarzyna Kotfis, Todd T. Schlegel, and Andrzej Jaroszynski. 2020. "Decompressive Craniectomy Improves QTc Interval in Traumatic Brain Injury Patients" International Journal of Environmental Research and Public Health 17, no. 22: 8653. https://doi.org/10.3390/ijerph17228653
APA StyleDabrowski, W., Siwicka-Gieroba, D., Robba, C., Badenes, R., Kotfis, K., Schlegel, T. T., & Jaroszynski, A. (2020). Decompressive Craniectomy Improves QTc Interval in Traumatic Brain Injury Patients. International Journal of Environmental Research and Public Health, 17(22), 8653. https://doi.org/10.3390/ijerph17228653






