Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis
Abstract
1. Introduction
2. Potentially Malignant Lesions of the Oral Mucosa
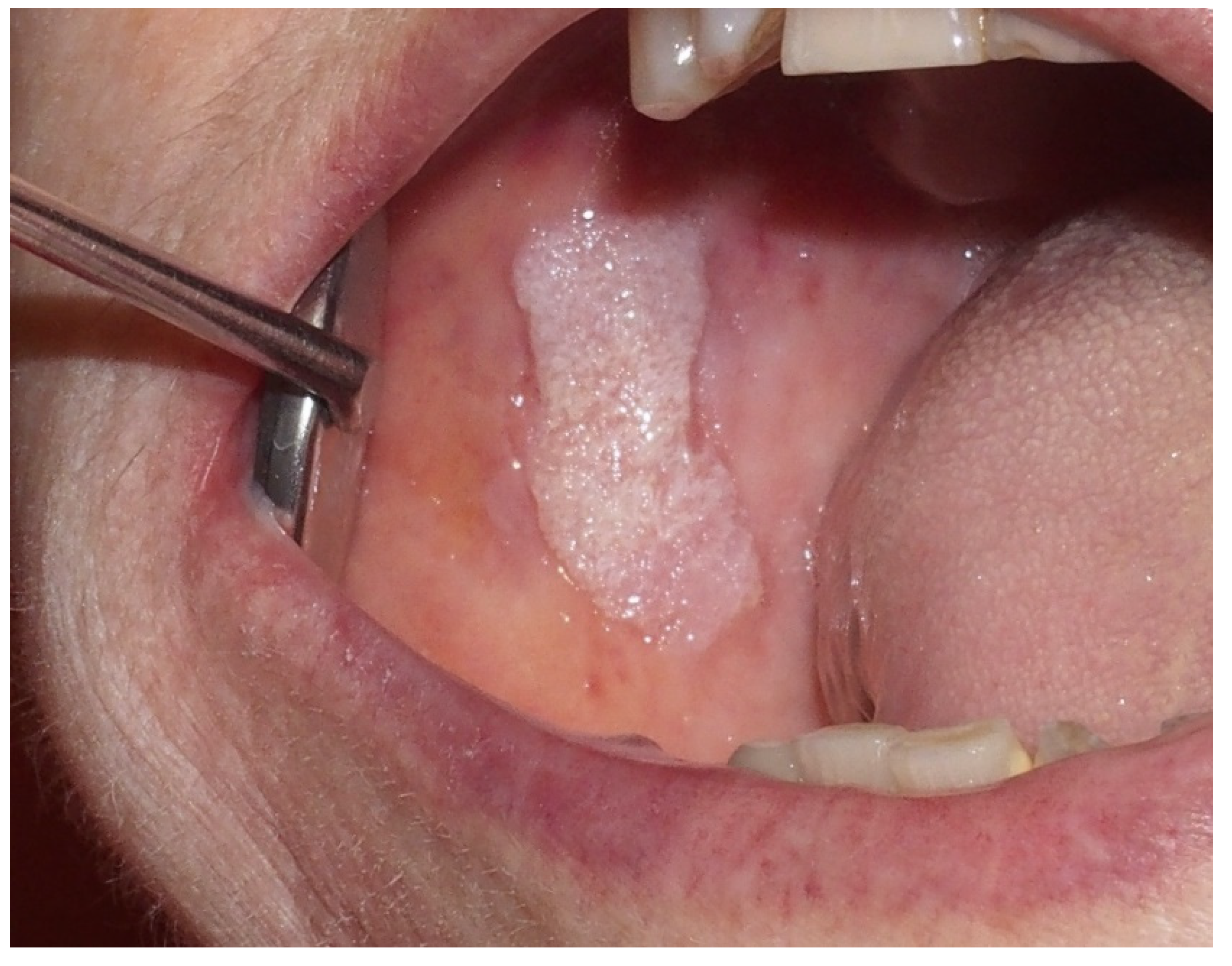
2.1. Leukoplakia
- Leukoplasic lesion present in more than 2 oral sites, most frequently the gingiva, alveolar processes, and palate;
- Presence of a verrucous area;
- Lesions that have spread or enlarged during development of the disease;
- Recurrence in a previously treated area;
- Exclusion of invasive OSCC with biopsy.
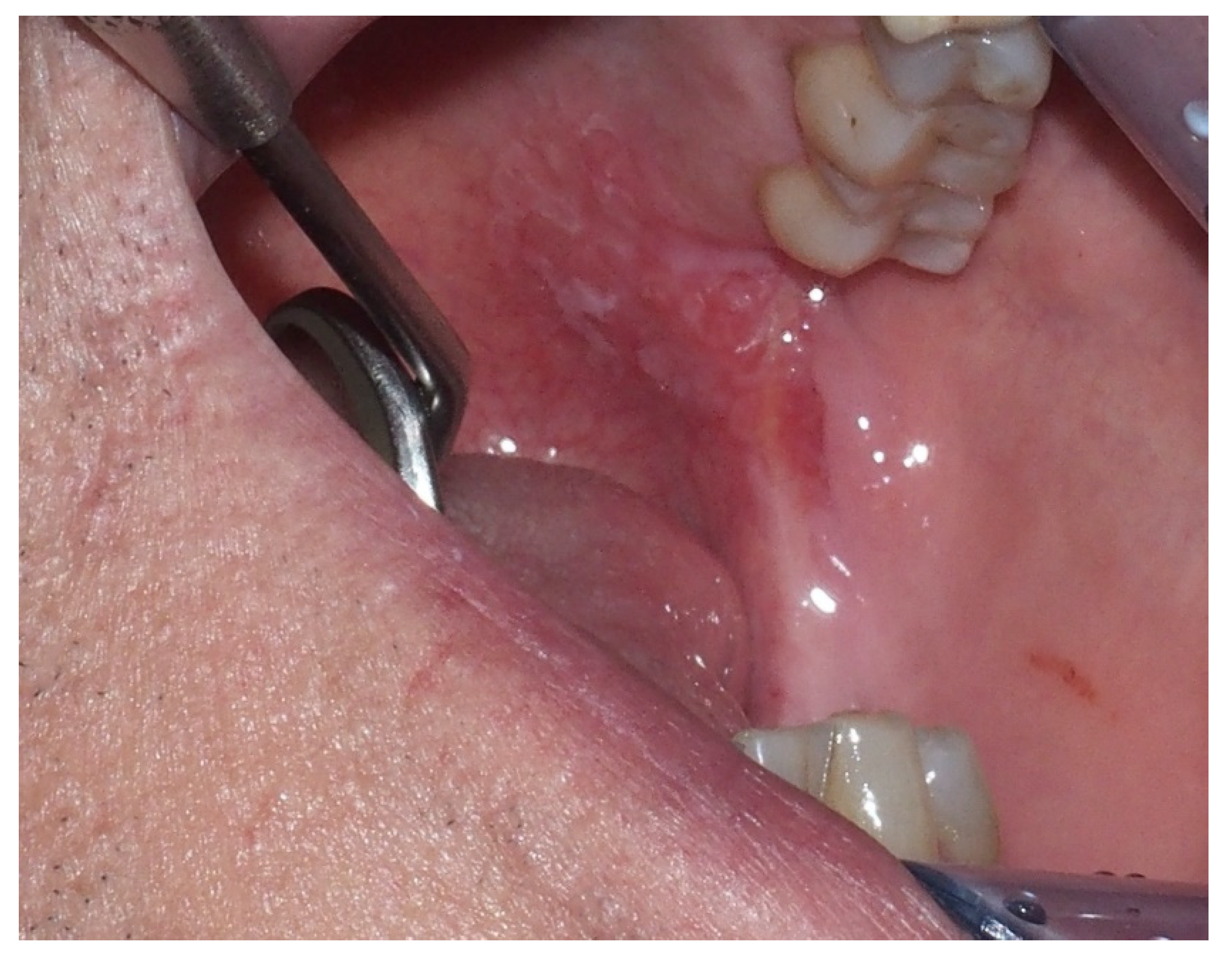
2.2. Erythroplakia
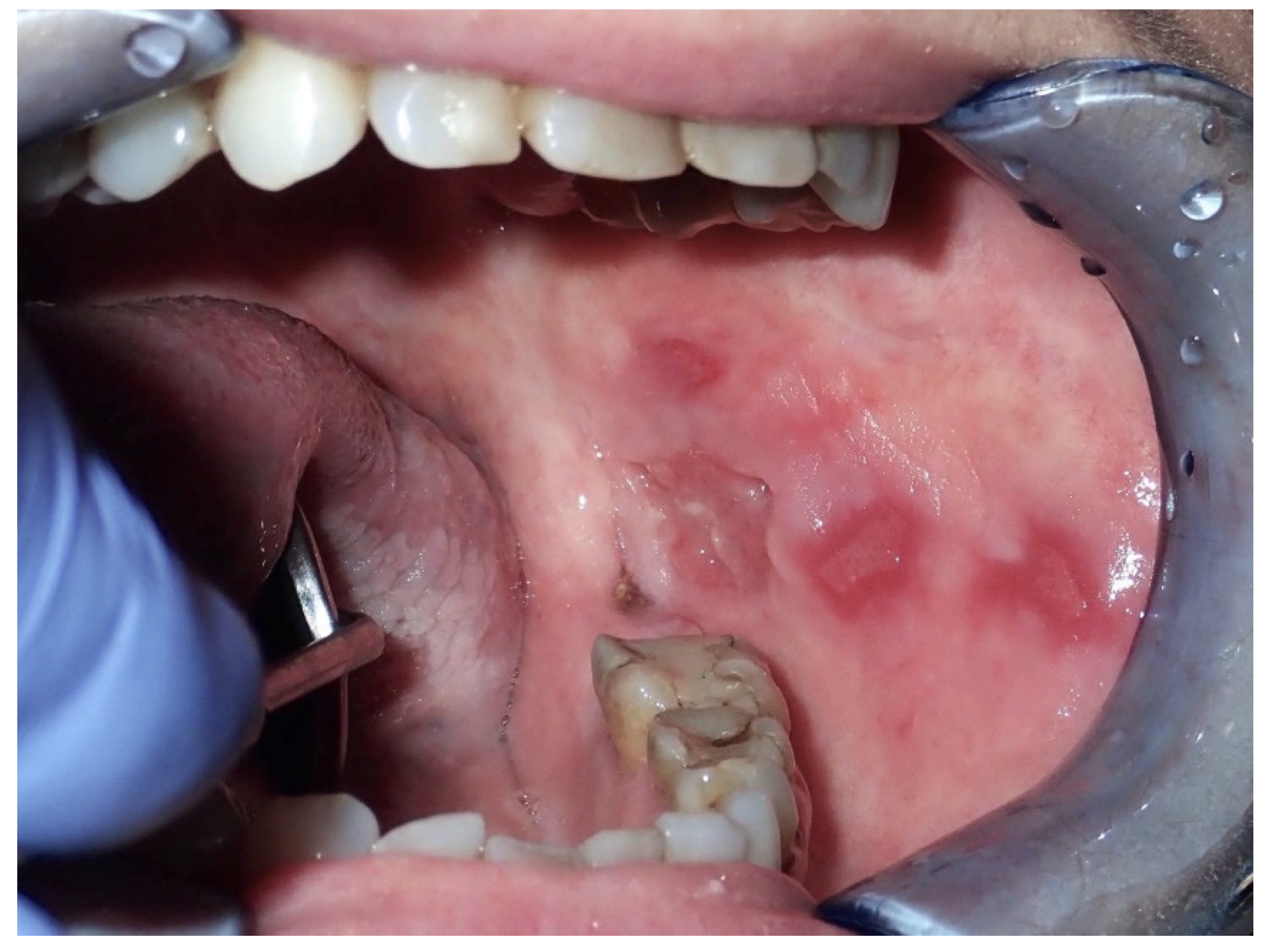
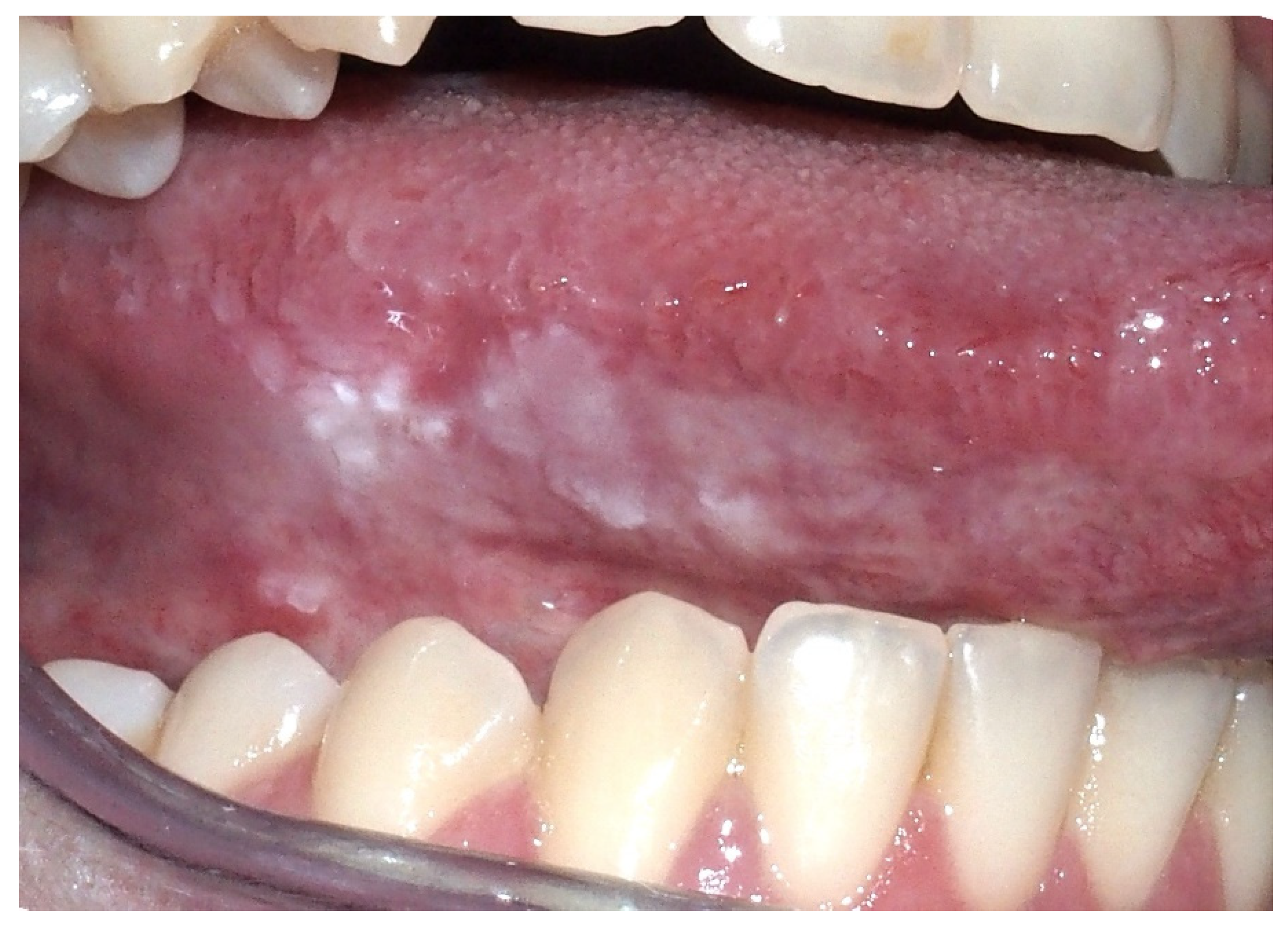
2.3. Oral Lichen Planus
2.4. Submucosal Fibrosis
2.5. Chronic Inflammation
2.6. Oral Bacteria and Cancer
2.7. Other Potentially Premalignant Lesions
2.8. Risk of Transformation
3. Early Diagnosis
3.1. Toluidine Blue Staining
3.2. Autofluorescence Imaging
3.3. Salivary Biomarkers for Point-of-Care Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Approval
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.-P.; Shin, H.-I.; Choi, S.-Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal 2017, 23, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C. Essentials of oral cancer. In International Journal of Clinical and Experimental Pathology; E-Century Publishing Corporation: Madison, WI, USA, 2015; Volume 8, pp. 11884–11894. [Google Scholar] [CrossRef]
- Kane, S.; Gupta, M.; Kakade, A.; Cruz, A.D. Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur. J. Surg. Oncol. 2006, 32, 795–803. [Google Scholar] [CrossRef]
- Montero, P.H.; Patel, S.G. Cancer of the Oral Cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.; Kerr, A.R.; Epstein, J.B. Oral and Pharyngeal Cancer Control and Early Detection. J. Cancer Educ. 2010, 25, 279–281. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.; Prasad, G.; Farah, C. Oral mucosal malignancy and potentially malignant lesions: An update on the epidemiology, risk factors, diagnosis and management. Aust. Dent. J. 2010, 55 (Suppl. 1), 61–65. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Seoane, J.; Varela-Centelles, P.; Diz, P.; Takkouche, B. Is diagnostic delay related to advanced-stage oral cancer? A meta-analysis. Eur. J. Oral Sci. 2009, 117, 541–546. [Google Scholar] [CrossRef] [PubMed]
- McGurk, M.; Chan, C.; Jones, J.; O’Regan, E.; Sherriff, M. Delay in diagnosis and its effect on outcome in head and neck cancer. Br. J. Oral Maxillofac. Surg. 2005, 43, 281–284. [Google Scholar] [CrossRef]
- Groome, P.; Rohland, S.L.; Hall, S.F.; Irish, J.C.; MacKillop, W.; O’Sullivan, B. A population-based study of factors associated with early versus late stage oral cavity cancer diagnoses. Oral Oncol. 2011, 47, 642–647. [Google Scholar] [CrossRef]
- Laura, Q.M.; Chow, M.D. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Ford, P.; Farah, C. Early detection and diagnosis of oral cancer: Strategies for improvement. J. Cancer Policy 2013, 1, e2–e7. [Google Scholar] [CrossRef]
- Trimarchi, M.; Bertazzoni, G.; Bussi, M. Cocaine induced midline destructive lesions. Rhinol. J. 2014, 52, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, M.; Bellini, C.; Fabiano, B.; Gerevini, S.; Bussi, M. Multiple mucosal involvement in cicatricial pemphigoid. Acta Otorhinolaryngol. Ital. 2009, 29, 222–225. [Google Scholar] [PubMed]
- Biafora, M.; Bertazzoni, G.; Trimarchi, M. Maxillary Sinusitis Caused by Dental Implants Extending into the Maxillary Sinus and the Nasal Cavities. J. Prosthodont. 2014, 23, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, M.; Bondi, S.; Della Torre, E.; Terreni, M.; Bussi, M. Palate perforation differentiates cocaine-induced midline destructive lesions from granulomatosis with polyangiitis. Acta Otorhinolaryngol. Ital. 2017, 37, 281–285. [Google Scholar] [CrossRef]
- Lanzillotta, M.; Campochiaro, C.; Trimarchi, M.; Arrigoni, G.; Gerevini, S.; Milani, R.; Bozzolo, E.; Biafora, M.; Venturini, E.; Cicalese, M.P.; et al. Deconstructing IgG4-related disease involvement of midline structures: Comparison to common mimickers. Mod. Rheumatol. 2017, 27, 638–645. [Google Scholar] [CrossRef]
- Wong, T.; Wiesenfeld, D. Oral Cancer. Aust. Dent. J. 2018, 63, S91–S99. [Google Scholar] [CrossRef]
- Joseph, B. Oral Cancer: Prevention and Detection. Med. Princ. Pract. 2002, 11 (Suppl. 1), 32–35. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Singhvi, H.R.; Malik, A. The role of chronic mucosal trauma in oral cancer: A review of literature. Indian J. Med. Paediatr. Oncol. 2017, 38, 44–50. [Google Scholar] [CrossRef]
- Van Der Waal, I. Oral potentially malignant disorders: Is malignant transformation predictable and preventable? Med. Oral Patol. Oral Cir. Bucal 2014, 19, e386–e390. [Google Scholar] [CrossRef]
- Lingen, M.W.; Kalmar, J.R.; Karrison, T.; Speight, P.M. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008, 44, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Van Der Waal, I.; De Bree, R.; Brakenhoff, R.; Coebergh, J.-W. Early diagnosis in primary oral cancer: Is it possible? Med. Oral Patol. Oral Cir. Bucal 2011, 16, e300–e305. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Ramadas, K.; Thomas, G.; Muwonge, R.; Thara, S.; Mathew, B.; Rajan, B. Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet 2005, 365, 1927–1933. [Google Scholar] [CrossRef]
- Lim, K.; Moles, D.R.; Downer, M.C.; Speight, P.M. Opportunistic screening for oral cancer and precancer in general dental practice: Results of a demonstration study. Br. Dent. J. 2003, 194, 497–502. [Google Scholar] [CrossRef]
- Villa, A.; Villa, C.; Abati, S. Oral cancer and oral erythroplakia: An update and implication for clinicians. Aust. Dent. J. 2011, 56, 253–256. [Google Scholar] [CrossRef]
- Rethman, M.P.; Carpenter, W.; Cohen, E.E.; Epstein, J.; Evans, C.A.; Flaitz, C.M.; Graham, F.J.; Hujoel, P.P.; Kalmar, J.R.; Koch, W.M.; et al. Evidence-Based Clinical Recommendations Regarding Screening for Oral Squamous Cell Carcinomas. J. Am. Dent. Assoc. 2010, 141, 509–520. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; Van Der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Neville, B.W.; Day, T.A. Oral Cancer and Precancerous Lesions. CA Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef]
- Axell, T.; Pindborg, J.J.; Smith, C.J.; Van Der Waal, I. An International Collaborative Group on Oral White Lesions* Oral white lesions with special reference to precancerous and tobacco-related lesions: Conclusions of an international symposium held in Uppsala, Sweden, May 18–21 1994. J. Oral Pathol. Med. 1996, 25, 49–54. [Google Scholar] [CrossRef]
- Carrard, V.C.; Van Der Waal, I. A clinical diagnosis of oral leukoplakia; A guide for dentists. Med. Oral Patol. Oral Cir. Bucal 2017, 23, e59–e64. [Google Scholar] [CrossRef]
- Bewley, A.F.; Farwell, D.G. Oral leukoplakia and oral cavity squamous cell carcinoma. Clin. Dermatol. 2017, 35, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Haim, S. Oral leukoplakia. Harefuah 1981, 101, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Bouquot, J.E.; Whitaker, S.B. Oral leukoplakia--rationale for diagnosis and prognosis of its clinical subtypes or “phases”. Quintessence Int. 1994, 25, 133–140. [Google Scholar] [PubMed]
- Wetzel, S.L.; Wollenberg, J. Oral Potentially Malignant Disorders. Dent. Clin. N. Am. 2020, 64, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Maymone, M.B.; Greer, R.O.; Kesecker, J.; Sahitya, P.C.; Burdine, L.K.; Cheng, A.-D.; Maymone, A.C.; Vashi, N.A. Premalignant and Malignant Mucosal lesions: Clinical and Pathological Findings Part II. Premalignant and malignant mucosal lesions. J. Am. Acad. Dermatol. 2018, 81, 59–71. [Google Scholar] [CrossRef]
- Cabay, R.J.; Morton, T.H.; Epstein, J.B. Proliferative verrucous leukoplakia and its progression to oral carcinoma: A review of the literature. J. Oral Pathol. Med. 2007, 36, 255–261. [Google Scholar] [CrossRef]
- Lapiedra, R.C.; Martínez, D.B.; López, L.A.M.; Esparza-Gómez, G.; Bagán, J.V. Proliferative verrucous leukoplakia: A proposal for diagnostic criteria. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e839–e845. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Clinical features and presentation of oral potentially malignant disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 582–590. [Google Scholar] [CrossRef]
- Lapthanasupkul, P.; Poomsawat, S.; Punyasingh, J. A clinicopathologic study of oral leukoplakia and erythroplakia in a Thai population. Quintessence Int. 2007, 38, e448–e455. [Google Scholar]
- Reichart, P.A.; Philipsen, H.P. Oral erythroplakia—A review. Oral Oncol. 2005, 41, 551–561. [Google Scholar] [CrossRef]
- Olson, M.A.; Rogers, R.S.; Bruce, A.J. Oral lichen planus. Clin. Dermatol. 2016, 34, 495–504. [Google Scholar] [CrossRef]
- Sharma, M.; Shetty, S.S.; Radhakrishnan, R. Oral Submucous Fibrosis as an Overhealing Wound: Implications in Malignant Transformation. Recent Pat. Anticancer Drug Discov. 2018, 13, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Arakeri, G.; Brennan, P.A. Oral submucous fibrosis: An overview of the aetiology, pathogenesis, classification, and principles of management. Br. J. Oral Maxillofac. Surg. 2013, 51, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Morassi, M.L.; Trimarchi, M.; Nicolai, P.; Gregorini, G.; Maroldi, R.; Specks, U.; Facchetti, F. Cocaina, ANCA e granulomatosi di Wegener [Cocaine, ANCA, and Wegener’s granulomatosis]. Pathologica 2001, 93, 581–583. (In Italian) [Google Scholar] [PubMed]
- Piemonte, E.D.; Lazos, J.P.; Brunotto, M. Relationship between chronic trauma of the oral mucosa, oral potentially malignant disorders and oral cancer. J. Oral Pathol. Med. 2010, 39, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, S.E.; Lamont, R.J. Oral Bacteria and Cancer. PLOS Pathog. 2014, 10, e1003933. [Google Scholar] [CrossRef]
- Robledo-Sierra, J.; Ben-Amy, D.P.; Varoni, E.; Bavarian, R.; Simonsen, J.L.; Paster, B.J.; Wade, W.G.; Kerr, R.; Peterson, D.E.; Lau, E.F.; et al. World Workshop on Oral Medicine VII: Targeting the oral microbiome Part 2: Current knowledge on malignant and potentially malignant oral disorders. Oral Dis. 2019, 25, 28–48. [Google Scholar] [CrossRef]
- Trimarchi, M.; Bellini, C.; Toma, S.; Bussi, M. Back-and-forth endoscopic septoplasty: Analysis of the technique and outcomes. Int. Forum Allergy Rhinol. 2012, 2, 40–44. [Google Scholar] [CrossRef]
- Brennan, M.; Migliorati, C.A.; Lockhart, P.B.; Wray, D.; Al-Hashimi, I.; Axéll, T.; Bruce, A.J.; Carpenter, W.; Eisenberg, E.; Epstein, J.B.; et al. Management of oral epithelial dysplasia: A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, S19.e1–S19.e12. [Google Scholar] [CrossRef]
- Reibel, J. Prognosis of Oral Pre-malignant Lesions: Significance of Clinical, Histopathological, and Molecular Biological Characteristics. Crit. Rev. Oral Biol. Med. 2003, 14, 47–62. [Google Scholar] [CrossRef]
- Scully, C.; Bagan, J. Oral squamous cell carcinoma: Overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009, 15, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.; Eveson, J.W.; Reichart, P.; Sidransky, D.E. World Health Organization classification of tumours. In Pathology & Genetics. Head and Neck Tumours; Int Agency Res Cancer [IARC] Press: Lyon, France, 2005. [Google Scholar]
- Walsh, T.; Liu, J.L.; Brocklehurst, P.; Glenny, A.M.; Lingen, M.; Kerr, A.R.; Ogden, G.R.; Warnakulasuriya, S.; Scully, C. Clinical assessment to screen for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Practice, C. Guideline for the early detection of oral cancer in British Columbia 2008. J. Can. Dent. Assoc. 2008, 74, 245. [Google Scholar]
- Pentenero, M.; Val, M.; Rosso, S.; Gandolfo, S. Microbiopsy a first-level diagnostic test to rule out oral dysplasia or carcinoma in general dental practice. Oral Dis. 2018, 24, 109–111. [Google Scholar] [CrossRef]
- Omar, E. Current concepts and future of noninvasive procedures for diagnosing oral squamous cell carcinoma—A systematic review. Head Face Med. 2015, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-N.; Lu, R.; Zhang, J.; Zhou, G. Inter-and intra-observer agreement on the judgment of toluidine blue staining for screening of oral potentially malignant disorders and oral cancer. Clin. Oral Investig. 2018, 23, 1709–1714. [Google Scholar] [CrossRef]
- Chainani-Wu, N.; Madden, E.; Cox, D.; Sroussi, H.; Epstein, J.; Silverman, S. Toluidine blue aids in detection of dysplasia and carcinoma in suspicious oral lesions. Oral Dis. 2015, 21, 879–885. [Google Scholar] [CrossRef]
- Liu, J.L.; Walsh, T.; Kerr, A.R.; Lingen, M.; Brocklehurst, P.; Ogden, G.R.; Warnakulasuriya, S.; Scully, C. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst. Rev. 2012, 29. [Google Scholar] [CrossRef]
- Zhang, L.; Williams, M.; Poh, C.F.; Laronde, D.; Epstein, J.B.; Durham, S.; Nakamura, H.; Berean, K.; Hovan, A.; Le, N.D.; et al. Toluidine Blue Staining Identifies High-Risk Primary Oral Premalignant Lesions with Poor Outcome. Cancer Res. 2005, 65, 8017–8021. [Google Scholar] [CrossRef]
- De Veld, D.C.; Skurichina, M.; Witjes, M.J.; Duin, R.P.; Sterenborg, H.J.C.M.; Roodenburg, J.L. Autofluorescence and diffuse reflectance spectroscopy for oral oncology. Lasers Surg. Med. 2005, 36, 356–364. [Google Scholar] [CrossRef]
- Tiwari, L.; Kujan, O.; Farah, C.S. Optical fluorescence imaging in oral cancer and potentially malignant disorders: A systematic review. Oral Dis. 2019, 26, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Awan, K. Efficacy of Autofluorescence Imaging as an Adjunctive Technique for Examination and Detection of Oral Potentially Malignant Disorders: A Systematic Review. J. Contemp. Dent. Pract. 2015, 16, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Awan, K.H.; Morgan, P.R.; Warnakulasuriya, S. Evaluation of an autofluorescence based imaging system (VELscope™) in the detection of oral potentially malignant disorders and benign keratoses. Oral Oncol. 2011, 47, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Tecco, S.; Parisi, M.R.; Gastaldi, G.; Polizzi, E.; D’Amicantonio, T.; Zilocchi, I.; Gardini, I.; Gherlone, E.F.; Lazzarin, A.; Capparè, P. Point-of-care testing for hepatitis C virus infection at an Italian dental clinic: Portrait of the pilot study population. New Microbiol. 2019, 42, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Romanos, G.E.; Mariani, E.; Benasciutti, E.; Gherlone, E. Corticocancellous porcine bone in the healing of human extraction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral Maxillofac. Implant. 2011, 26, 866–872. [Google Scholar]
- Kaur, J.; Jacobs, R.; Huang, Y.; Salvo, N.; Politis, C. Salivary biomarkers for oral cancer and pre-cancer screening: A review. Clin Oral Investig. 2018, 22, 633–640. [Google Scholar] [CrossRef]
- Capparé, P.; Teté, G.; Romanos, G.E.; Nagni, M.; Sannino, G.; Gherlone, E.F. The ‘All-on-four’ protocol in HIV-positive patients: A prospective, longitudinal 7-year clinical study. Int. J. Oral Implantol. 2019, 12, 501–510. [Google Scholar]
- Gherlone, E.F.; Capparè, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. A Prospective Longitudinal Study on Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients with 1-Year Follow-up: The Role of CD4+ Level, Smoking Habits, and Oral Hygiene. Clin. Implant. Dent. Relat. Res. 2015, 18, 955–964. [Google Scholar] [CrossRef]
- Moretti, M.; Lissoni, A.; Gastaldi, G.; Arrigoni, G.; Doglioni, C.; Abati, S. Expression of hexokinase ii in oral keratotic lesions with or without inflammation. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Franzmann, E.J.; Donovan, M.J. Effective early detection of oral cancer using a simple and inexpensive point of care device in oral rinses. Expert Rev. Mol. Diagn. 2018, 18, 837–844. [Google Scholar] [CrossRef]




|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. https://doi.org/10.3390/ijerph17249160
Abati S, Bramati C, Bondi S, Lissoni A, Trimarchi M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. International Journal of Environmental Research and Public Health. 2020; 17(24):9160. https://doi.org/10.3390/ijerph17249160
Chicago/Turabian StyleAbati, Silvio, Chiara Bramati, Stefano Bondi, Alessandra Lissoni, and Matteo Trimarchi. 2020. "Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis" International Journal of Environmental Research and Public Health 17, no. 24: 9160. https://doi.org/10.3390/ijerph17249160
APA StyleAbati, S., Bramati, C., Bondi, S., Lissoni, A., & Trimarchi, M. (2020). Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. International Journal of Environmental Research and Public Health, 17(24), 9160. https://doi.org/10.3390/ijerph17249160







