Gas Phase Toluene Adsorption Using Date Palm-Tree Branches Based Activated Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Procedure for Activated Carbon
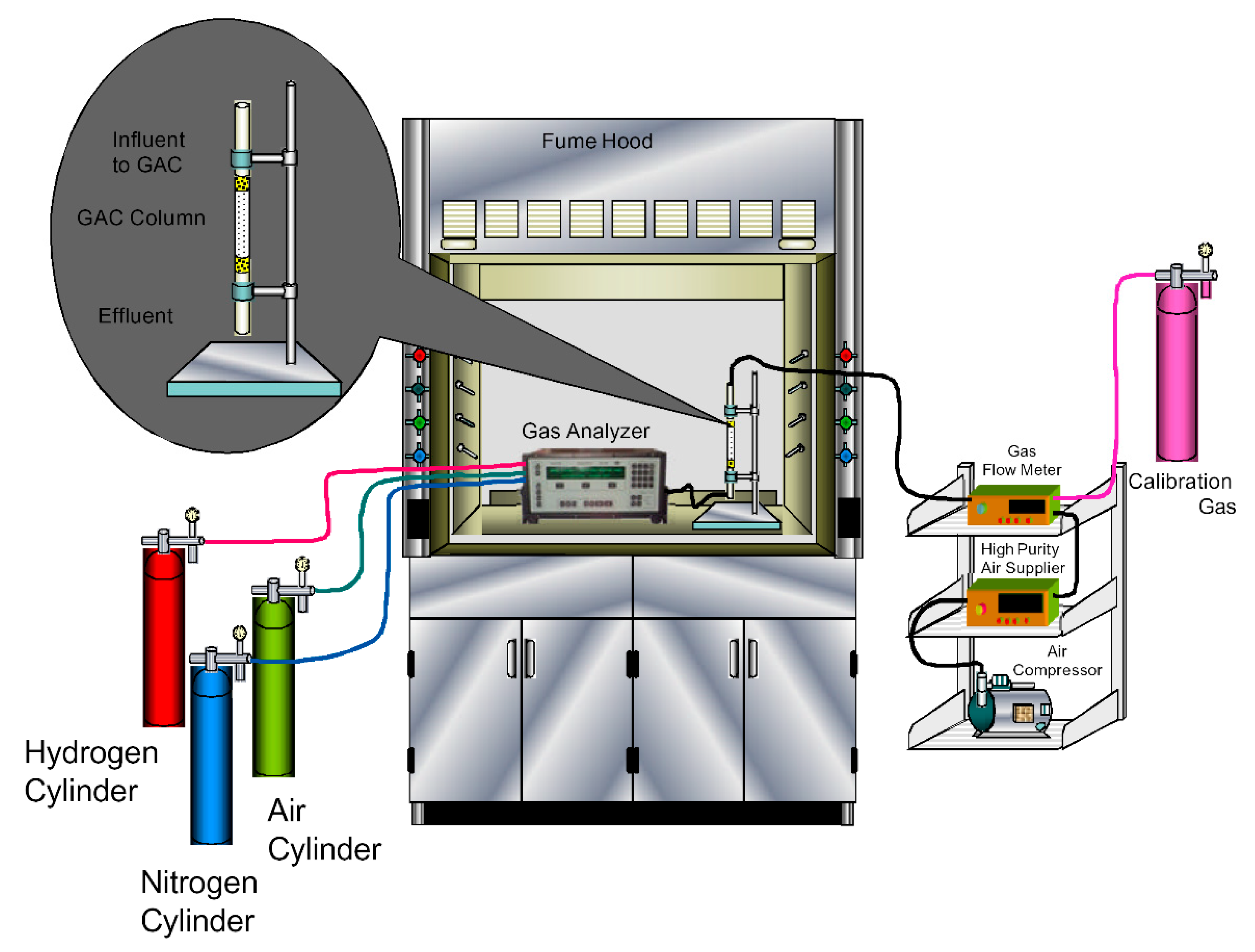
2.3. Toluene Gas Treatment
2.4. Analytical Methods
2.5. Response Surface Methodology (RSM) Modeling
3. Results and Discussion
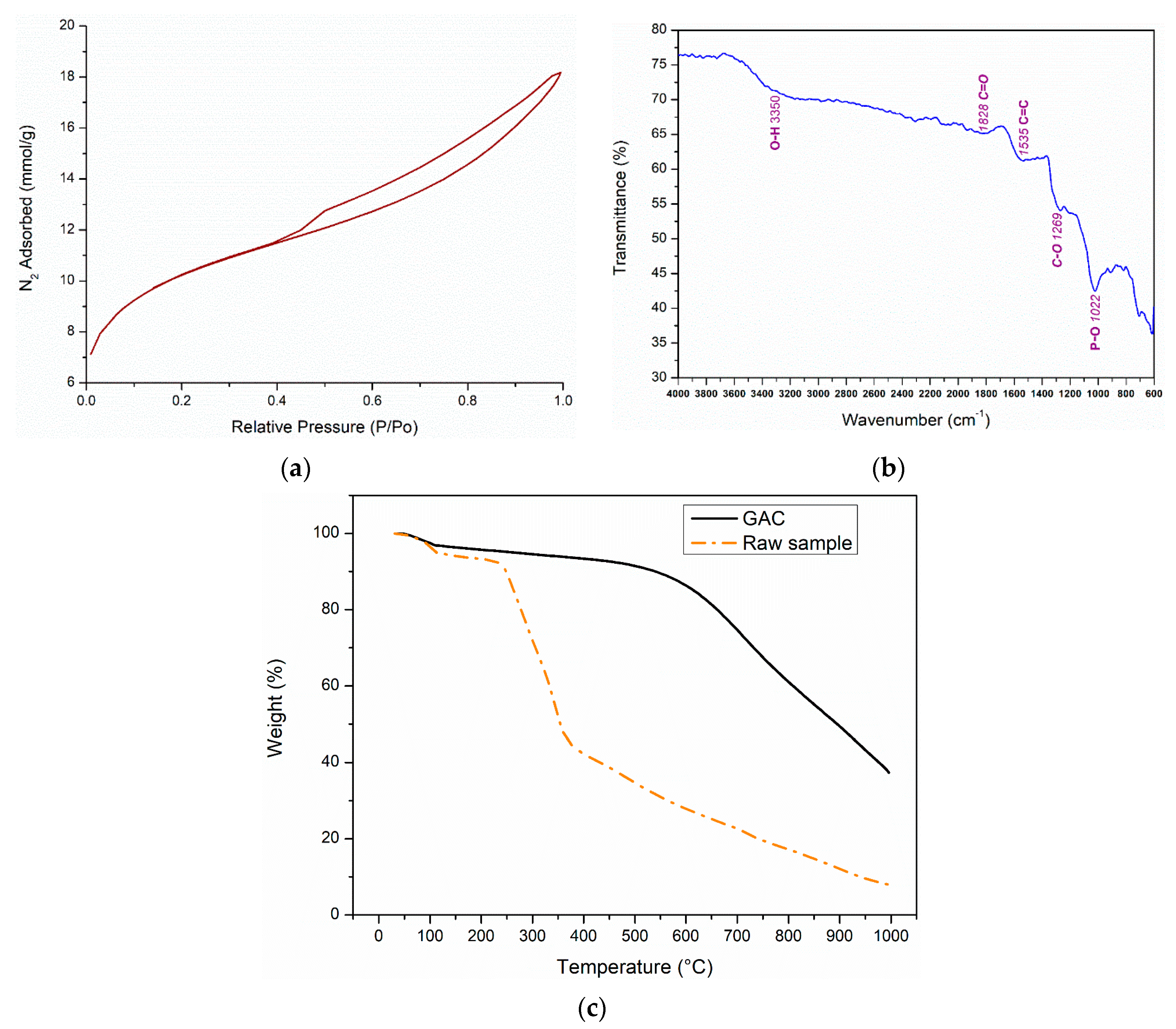
3.1. Activated Carbon (AC) Characterization
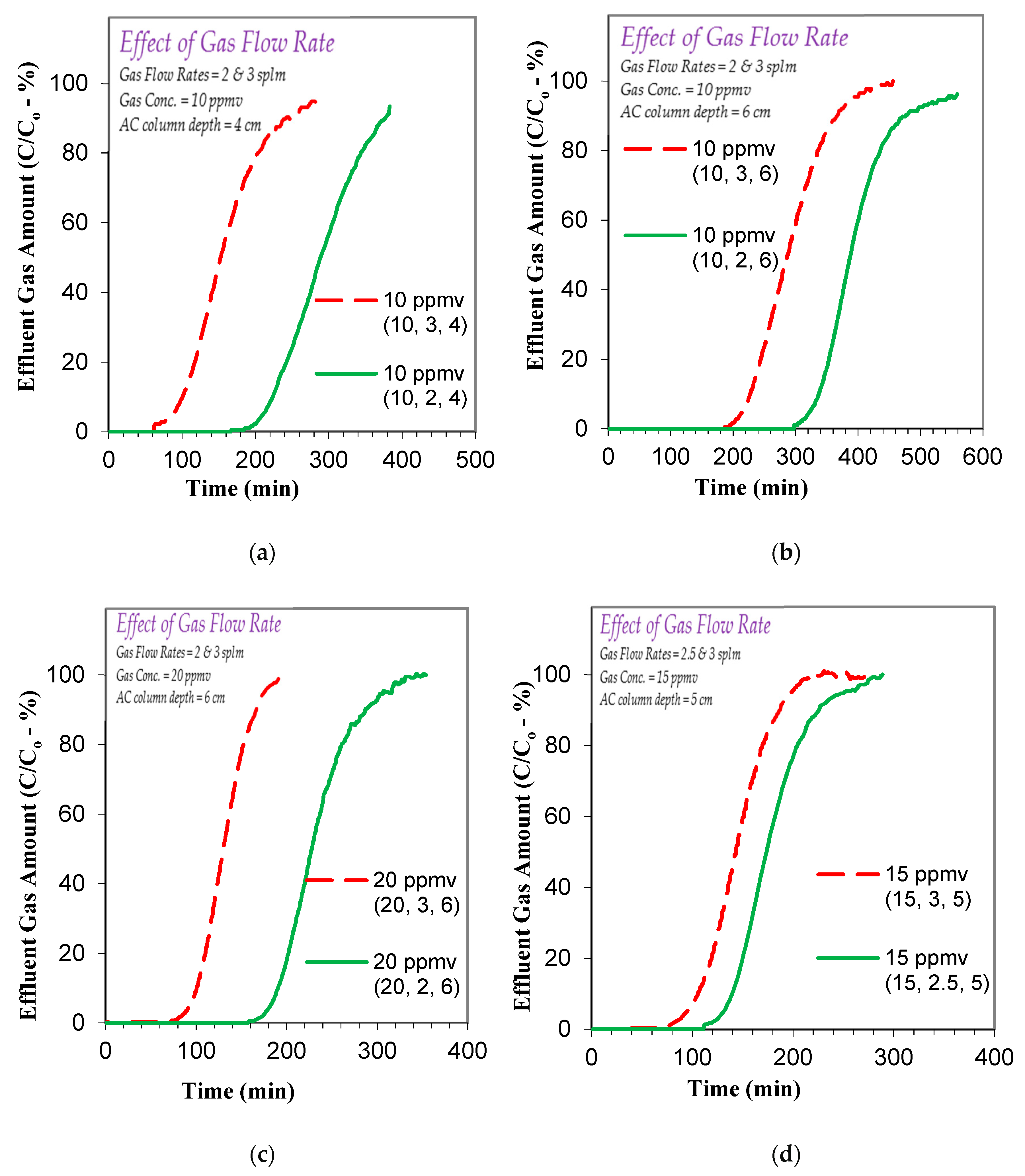
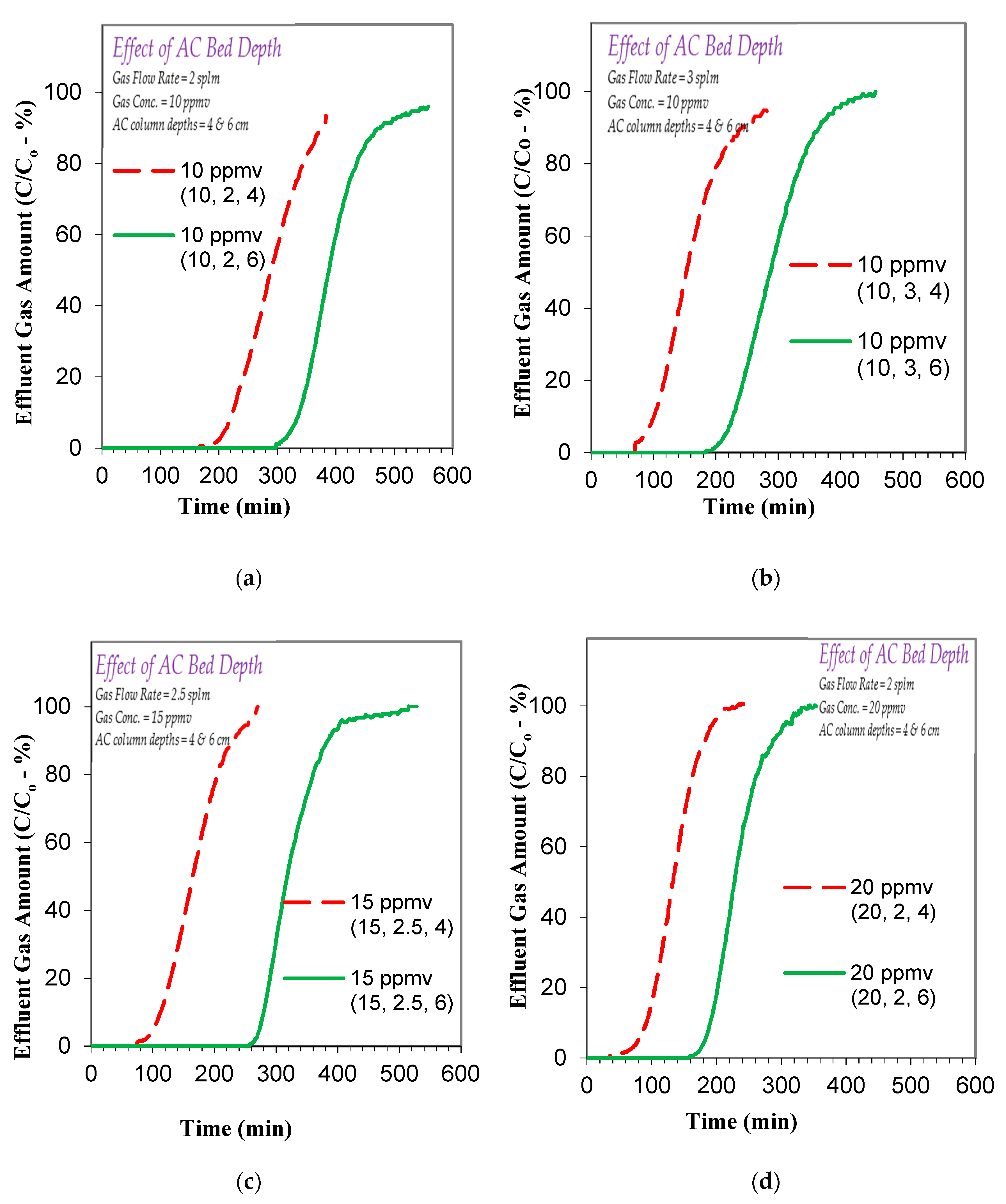
3.2. Effect of Operational Parameters on Toluene Gas Adsorption
 C6H5CH3 (interface)
C6H5CH3 (interface)
 C6H5CH3 (bulk-solid)
C6H5CH3 (bulk-solid)
 GAC-O-C6H5CH3
GAC-O-C6H5CH3
3.3. RSM Modeling
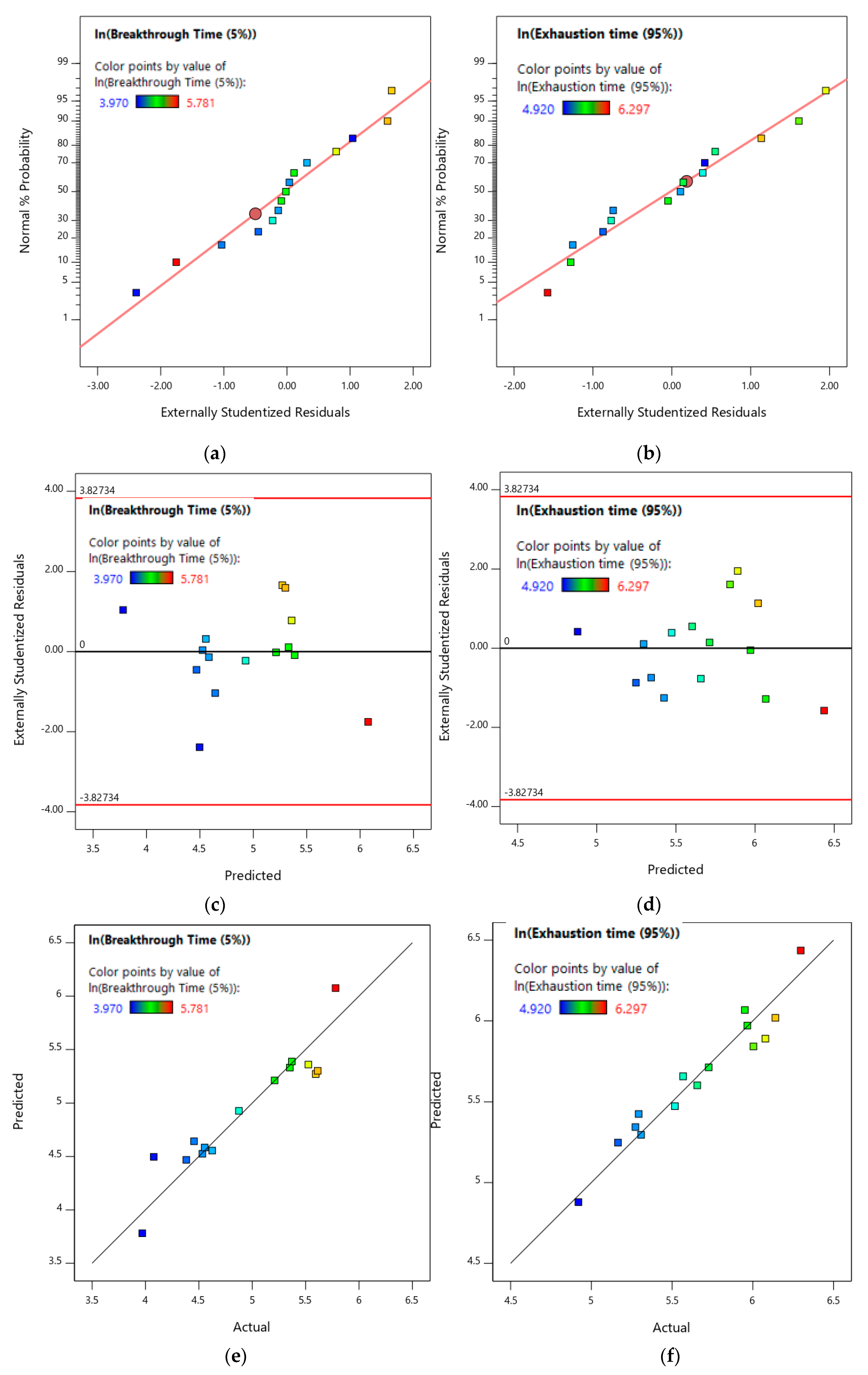
3.3.1. Toluene Gas Adsorption Breakthrough Time and Exhaustion Time Models
3.3.2. Effect of Factors on Toluene Gas Adsorption Breakthrough and Exhaustion Times
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Fadel, M.; Massoud, M. Methane emissions from wastewater management. Environ. Pollut. 2001, 114, 177–185. [Google Scholar] [CrossRef]
- Loibl, W.; Orthofer, R.; Winiwarter, W. Spatially disaggregated emission inventory for anthropogenic NMVOC in Austria. Atmos. Environ. Part A Gen. Top. 1993, 27, 2575–2590. [Google Scholar] [CrossRef]
- Shin, H.C.; Park, J.W.; Park, K.; Song, H.C. Removal characteristics of trace compounds of landfill gas by activated carbon adsorption. Environ. Pollut. 2002, 119, 227–236. [Google Scholar] [CrossRef]
- Easter, C.; Quigley, C.; Burrowes, P.; Witherspoon, J.; Apgar, D. Odor and air emissions control using biotechnology for both collection and wastewater treatment systems. Chem. Eng. J. 2005, 113, 93–104. [Google Scholar] [CrossRef]
- Saggar, S.; Singh, J.; Giltrap, D.L.; Zaman, M.; Luo, J.; Rollo, M.; Kim, D.G.; Rys, G.; Van der Weerden, T.J. Quantification of reductions in ammonia emissions from fertiliser urea and animal urine in grazed pastures with urease inhibitors for agriculture inventory: New Zealand as a case study. Sci. Total Environ. 2013, 465, 136–146. [Google Scholar] [CrossRef]
- Sutton, M.A.; Dragosits, U.; Tang, Y.S.; Fowler, D. Ammonia emissions from non-agricultural sources in the UK. Atmos. Environ. 2000, 34, 855–869. [Google Scholar] [CrossRef]
- Rumburg, B.; Neger, M.; Mount, G.H.; Yonge, D.; Filipy, J.; Swain, J.; Kincaid, R.; Johnson, K. Liquid and atmospheric ammonia concentrations from a dairy lagoon during an aeration experiment. Atmos. Environ. 2004, 38, 1523–1533. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Bai, Z.H.; Winiwarter, W.; Kiesewetter, G.; Heyes, C.; Ma, L. Mitigating ammonia emission from agriculture reduces PM2.5 pollution in the Hai River Basin in China. Sci. Total Environ. 2017, 609, 1152–1160. [Google Scholar] [CrossRef]
- Burgess, J.E.; Parsons, S.A.; Stuetz, R.M. Developments in odour control and waste gas treatment biotechnology: A review. Biotechnol. Adv. 2001, 19, 35–63. [Google Scholar] [CrossRef]
- Bianchi, A.P.; Varney, M.S. Volatilisation processes in wastewater treatment plants as a source of potential exposure to VOCs. Ann. Occup. Hyg. 1997, 41, 437–454. [Google Scholar] [CrossRef]
- Wu, B.Z.; Feng, T.Z.; Sree, U.; Chiu, K.H.; Lo, J.G. Sampling and analysis of volatile organics emitted from wastewater treatment plant and drain system of an industrial science park. Anal. Chim. Acta 2006, 576, 100–111. [Google Scholar] [CrossRef]
- Muezzinoglu, A. A study of volatile organic sulfur emissions causing urban odors. Chemosphere 2003, 51, 245–252. [Google Scholar] [CrossRef]
- Atasoy, E.; Dögeroglu, T.; Kara, S. The estimation of NMVOC emissions from an urban-scale wastewater treatment plant. Water Res. 2004, 38, 3265–3274. [Google Scholar] [CrossRef] [PubMed]
- Escalas, A.; Guadayol, J.M.; Cortina, M.; Rivera, J.; Caixach, J. Time and space patterns of volatile organic compounds in a sewage treatment plant. Water Res. 2003, 37, 3913–3920. [Google Scholar] [CrossRef]
- Nikolaou, A.D.; Golfinopoulos, S.K.; Kostopoulou, M.N.; Kolokythas, G.A.; Lekkas, T.D. Determination of volatile organic compounds in surface waters and treated wastewater in Greece. Water Res. 2002, 36, 2883–2890. [Google Scholar] [CrossRef]
- Sree, U.; Bauer, H.; Fuerhacker, M.; Ellinger, R.; Schmidt, H.; Puxbaum, H. Hydrocarbons emissions from a municipal wastewater treatment pilot plant in Vienna. Water. Air. Soil Pollut. 2000, 124, 177–186. [Google Scholar] [CrossRef]
- Tansel, B.; Eyma, R.R. Volatile organic contaminant emissions from wastewater treatment plants during secondary treatment. Water. Air. Soil Pollut. 1999, 112, 315–325. [Google Scholar] [CrossRef]
- Simonich, S.L.; Begley, W.M.; Debaere, G.; Eckhoff, W.S. Trace analysis of fragrance materials in wastewater and treated wastewater. Environ. Sci. Technol. 2000, 34, 959–965. [Google Scholar] [CrossRef]
- Kimochi, Y.; Inamori, Y.; Mizuochi, M.; Xu, K.Q.; Matsumura, M. Nitrogen removal and N2O emission in a full-scale domestic wastewater treatment plant with intermittent aeration. J. Ferment. Bioeng. 1998, 86, 202–206. [Google Scholar] [CrossRef]
- Lin, T.Y.; Sree, U.; Tseng, S.H.; Chiu, K.H.; Wu, C.H.; Lo, J.G. Volatile organic compound concentrations in ambient air of Kaohsiung petroleum refinery in Taiwan. Atmos. Environ. 2004, 38, 4111–4122. [Google Scholar] [CrossRef]
- Pearson, J.; Stewart, G.R. The deposition of atmospheric ammonia and its effects on plants. New Phytol. 1993, 125, 283–305. [Google Scholar] [CrossRef]
- Cheng, W.H.; Hsu, S.K.; Chou, M.S. Volatile organic compound emissions from wastewater treatment plants in Taiwan: Legal regulations and costs of control. J. Environ. Manag. 2008, 88, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Vohra, M.S. Adsorption-Based Removal of Gas-Phase Benzene Using Granular Activated Carbon (GAC) Produced from Date Palm Pits. Arab. J. Sci. Eng. 2015, 11, 3007–3017. [Google Scholar] [CrossRef]
- Seo, J.; Kato, S.; Ataka, Y.; Chino, S. Performance test for evaluating the reduction of VOCs in rooms and evaluating the lifetime of sorptive building materials. Build. Environ. 2009, 44, 207–215. [Google Scholar] [CrossRef]
- Rodrigues, C.C.; de Moraes, D.; da Nóbrega, S.W.; Barboza, M.G. Ammonia adsorption in a fixed bed of activated carbon. Bioresour. Technol. 2007, 98, 886–891. [Google Scholar] [CrossRef]
- Fortier, H.; Westreich, P.; Selig, S.; Zelenietz, C.; Dahn, J.R. Ammonia, cyclohexane, nitrogen and water adsorption capacities of an activated carbon impregnated with increasing amounts of ZnCl2, and designed to chemisorb gaseous NH3 from an air stream. J. Colloid Interface Sci. 2008, 320, 423–435. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Petit, C. On the reactive adsorption of ammonia on activated carbons modified by impregnation with inorganic compounds. J. Colloid Interface Sci. 2009, 338, 329–345. [Google Scholar] [CrossRef]
- Vohra, M. Treatment of gaseous ammonia emissions using date palm pits based granular activated carbon. Int. J. Environ. Res. Public Health 2020, 17, 1519. [Google Scholar] [CrossRef]
- Guo, J.; Xu, W.S.; Chen, Y.L.; Lua, A.C. Adsorption of NH 3 onto activated carbon prepared from palm shells impregnated with H 2SO 4. J. Colloid Interface Sci. 2005, 281, 285–290. [Google Scholar] [CrossRef]
- Iyobe, T.; Asada, T.; Kawata, K.; Oikawa, K. Comparison of removal efficiencies for ammonia and amine gases between woody charcoal and activated carbon. J. Heal. Sci. 2004, 50, 148–153. [Google Scholar] [CrossRef]
- Labaran, B.A.; Vohra, M.S. Application of activated carbon produced from phosphoric acid-based chemical activation of oil fly ash for the removal of some charged aqueous phase dyes: Role of surface charge, adsorption kinetics, and modeling. Desalin. Water Treat. 2016, 57, 16034–16052. [Google Scholar] [CrossRef]
- Guo, J.; Lua, A.C. Adsorption of sulfur dioxide onto activated carbons prepared from oil-palm shells impregnated with potassium hydroxide. J. Chem. Technol. Biotechnol. 2000, 75, 971–976. [Google Scholar] [CrossRef]
- Guo, J.; Lua, A.C. Adsorption of sulphur dioxide onto activated carbon prepared from oil-palm shells with and without pre-impregnation. Sep. Purif. Technol. 2003, 30, 265–273. [Google Scholar] [CrossRef]
- Guo, J.; Luo, Y.; Lua, A.C.; Chi, R.A.; Chen, Y.L.; Bao, X.T.; Xiang, S.X. Adsorption of hydrogen sulphide (H2S) by activated carbons derived from oil-palm shell. Carbon 2007, 45, 330–336. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; Gonalves, M.; Rodríguez-Reinoso, F. Oxidation of activated carbon with aqueous solution of sodium dichloroisocyanurate: Effect on ammonia adsorption. Microporous Mesoporous Mater. 2011, 142, 577–584. [Google Scholar] [CrossRef]
- de la Cruz-Lovera, C.; Manzano-Agugliaro, F.; Salmerón-Manzano, E.; de la Cruz-Fernández, J.L.; Perea-Moreno, A.J. Date seeds (Phoenix dactylifera L.) valorization for boilers in the mediterranean climate. Sustainability 2019, 11, 711. [Google Scholar] [CrossRef]
- Houache, O.; Al-Maamari, R.; Al-Rashidi, B.; Jibril, B. Study of date palm stem as raw material in preparation of activated carbon. J. Eng. Res. 2009, 6, 47–51. [Google Scholar] [CrossRef]
- Daraghmeh, A.; Hussain, S.; Saadeddin, I.; Servera, L.; Xuriguera, E.; Cornet, A.; Cirera, A. A Study of Carbon Nanofibers and Active Carbon as Symmetric Supercapacitor in Aqueous Electrolyte: A Comparative Study. NanoScale Res. Let. 2017, 12, 639. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Vunain, E.; Kenneth, D.; Biswick, T. Synthesis and characterization of low-cost activated carbon prepared from Malawian baobab fruit shells by H3PO4 activation for removal of Cu(II) ions: Equilibrium and kinetics studies. Appl. Water Sci. 2017, 7, 4301–4319. [Google Scholar] [CrossRef]
- Cui, Y.; Atkinson, J.D. Tailored activated carbon from glycerol: Role of acid dehydrator on physiochemical characteristics and adsorption performance. J. Mater. Chem. A 2017, 5, 16812–16821. [Google Scholar] [CrossRef]
- Pei, J.; Zhang, J.S. Determination of adsorption isotherm and diffusion coefficient of toluene on activated carbon at low concentrations. Build. Environ. 2012, 48, 66–76. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 2005, 43, 1758–1767. [Google Scholar] [CrossRef]
- Gil, R.R.; Ruiz, B.; Lozano, M.S.; Martín, M.J.; Fuente, E. VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem. Eng. J. 2014, 245, 80–88. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Fletcher, A.J.; Thomas, K.M.; Cazorla-Amorós, D.; Linares-Solano, A. Competitive adsorption of a benzene-toluene mixture on activated carbons at low concentration. Carbon 2006, 44, 1455–1463. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Benzene and toluene adsorption at low concentration on activated carbon fibres. Adsorption 2011, 17, 473–481. [Google Scholar] [CrossRef]
- Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical activated carbons for low concentration toluene adsorption. Carbon 2010, 48, 2625–2633. [Google Scholar] [CrossRef]
- Long, C.; Li, Y.; Yu, W.; Li, A. Removal of benzene and methyl ethyl ketone vapor: Comparison of hypercrosslinked polymeric adsorbent with activated carbon. J. Hazard. Mater. 2012, 203–204, 251–256. [Google Scholar] [CrossRef]
- Ahmed, S.A.A.; Vohra, M.S. Treatment of aqueous selenocyanate (SeCN−) using combined TiO2 photocatalysis and 2-Line ferrihydrite Adsorption. Desalin. Water Treat. 2020. accepted. [Google Scholar] [CrossRef]


| Factors | Level −1 | Level 0 | Level 1 |
|---|---|---|---|
| A = Toluene Gas Flow Rate (slpm) | 2 | 2.5 | 3 |
| B = AC Bed Depth (cm) | 4 | 5 | 6 |
| C = Toluene Gas Concentration (ppmv) | 10 | 15 | 20 |
| Exp No. | Factor A: Influent Toluene Gas Flow Rate (slpm) | Factor B: GAC Bed Depth (cm) | Factor C: Influent Toluene Gas Concentration (ppmv) |
|---|---|---|---|
| 1 | 2 | 5 | 15 |
| 2 | 3 | 6 | 20 |
| 3 | 2 | 4 | 10 |
| 4 | 2.5 | 4 | 15 |
| 5 | 2.5 | 6 | 15 |
| 6 | 2 | 4 | 20 |
| 7 | 3 | 4 | 10 |
| 8 | 2 | 6 | 10 |
| 9 | 2.5 | 5 | 20 |
| 10 | 2 | 6 | 20 |
| 11 | 3 | 4 | 20 |
| 12 | 2.5 | 5 | 10 |
| 13 | 2.5 | 5 | 15 |
| 14 | 3 | 6 | 10 |
| 15 | 3 | 5 | 15 |
| Property | Value |
|---|---|
| SSABET | 800.87 m2/g |
| t-Plot Micropore Area | 335.25 m2/g |
| t-Plot External Surface Area | 465.62 m2/g |
| t-Plot Micropore Volume | 0.150 cm3/g |
| Total Pore Volume | 0.437 cm3/g |
| Average Pore Width (4 V/A by BET) | 30.32 Å |
| Parameter Changed | Change in Parameter | Change in Breakthrough Time Observed | Change in Exhaustion Time Observed | Reason |
|---|---|---|---|---|
| Influent Gas Concentration | Increased 10 to 20 ppmv | Decreased | Decreased | Fixed adsorption sites on the GAC surface |
| Flow Rate | Increased 2 to 3 slpm | Decreased | Decreased | Faster consumption of adsorption sites at higher flow rates |
| Column Depth | Increased 4 to 6 cm | Increased | Increased | Availability of more adsorbent based surface complexation sites |
| Source | p-Value | Significance |
|---|---|---|
| Model | <0.0001 | Significant |
| A: Toluene Gas Flow Rate (slpm) | 0.0006 | Significant |
| B: Activated Carbon Column Depth (cm) | 0.0003 | Significant |
| C: Toluene Gas Concentration (ppmv) | <0.0001 | Significant |
| Source | p-Value | Significance |
|---|---|---|
| Model | <0.0001 | significant |
| A: Toluene Gas Flow Rate (slpm) | <0.0001 | significant |
| B: Activated Carbon Column Depth (cm) | 0.0004 | significant |
| C: Toluene Gas Concentration (ppmv) | <0.0001 | significant |
| Statistical Parameter | Response | |
|---|---|---|
| Breakthrough Time | Exhaustion Time | |
| R2 | 0.8840 | 0.9346 |
| Adjusted R2 | 0.8524 | 0.9168 |
| Predicted R2 | 0.7982 | 0.8845 |
| Adequate Precision | 19.3374 | 25.5566 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vohra, M.; Al-Suwaiyan, M.; Hussaini, M. Gas Phase Toluene Adsorption Using Date Palm-Tree Branches Based Activated Carbon. Int. J. Environ. Res. Public Health 2020, 17, 9287. https://doi.org/10.3390/ijerph17249287
Vohra M, Al-Suwaiyan M, Hussaini M. Gas Phase Toluene Adsorption Using Date Palm-Tree Branches Based Activated Carbon. International Journal of Environmental Research and Public Health. 2020; 17(24):9287. https://doi.org/10.3390/ijerph17249287
Chicago/Turabian StyleVohra, Muhammad, Mohammad Al-Suwaiyan, and Minaam Hussaini. 2020. "Gas Phase Toluene Adsorption Using Date Palm-Tree Branches Based Activated Carbon" International Journal of Environmental Research and Public Health 17, no. 24: 9287. https://doi.org/10.3390/ijerph17249287
APA StyleVohra, M., Al-Suwaiyan, M., & Hussaini, M. (2020). Gas Phase Toluene Adsorption Using Date Palm-Tree Branches Based Activated Carbon. International Journal of Environmental Research and Public Health, 17(24), 9287. https://doi.org/10.3390/ijerph17249287





