How Frequently Is Asthma Objectively Demonstrated before Starting a Biologic? Quality Assessment of a Group Practice of Allergists and Immunologists
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Asthma Confirmation
3.2. Asthma Comorbidities
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
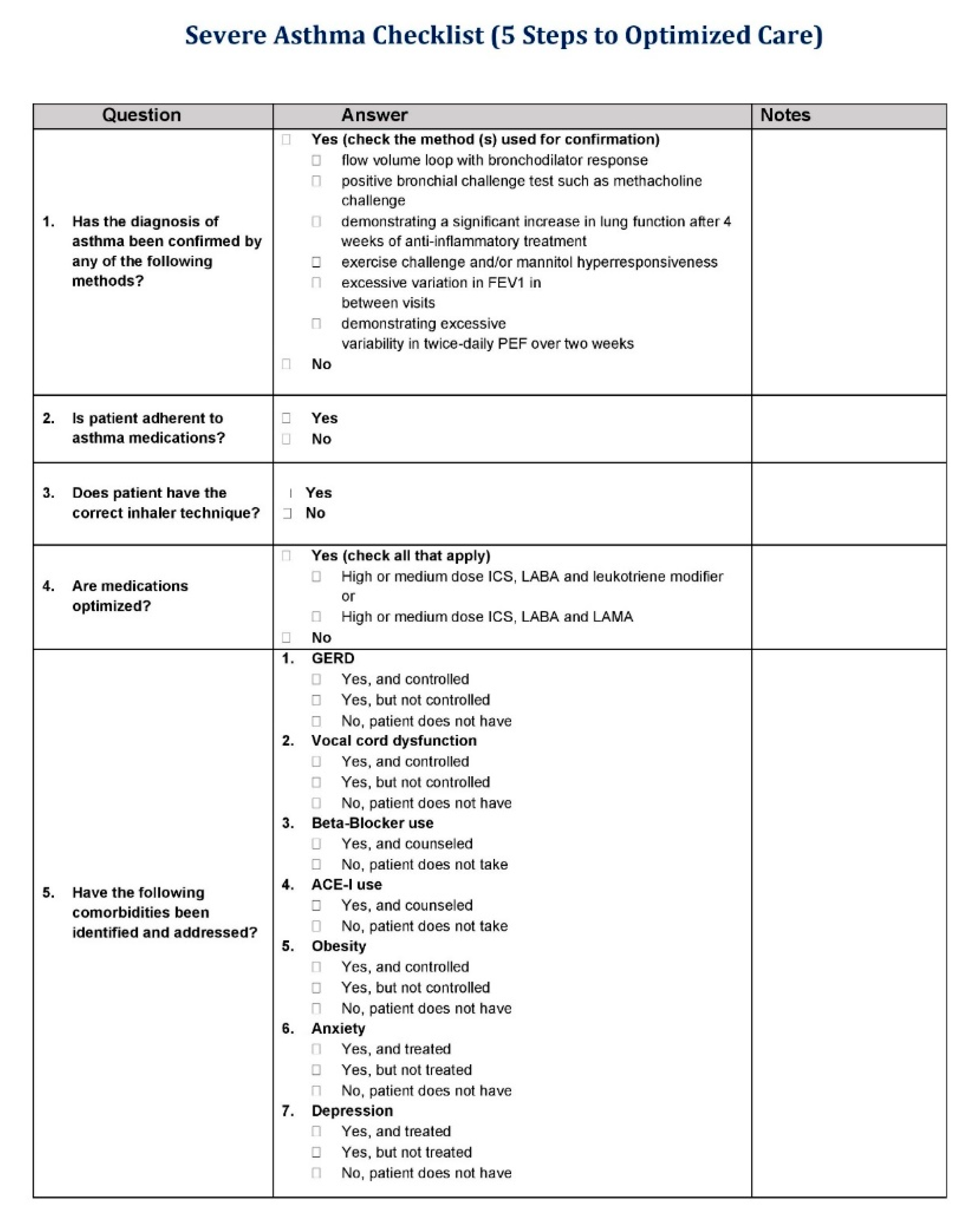
Appendix A
References
- Carr, T.F.; Bleecker, E.R. Asthma heterogeneity and severity. World Allergy Organ. J. 2016, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- To, T.M.; Wang, C.; Guan, J.; McLimont, S.; Gershon, A.S. What Is the Lifetime Risk of Physician-diagnosed Asthma in Ontario, Canada? Am. J. Respir. Crit. Care Med. 2010, 181, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2020. Available online: www.ginasthma.org (accessed on 12 October 2020).
- Subbarao, P.; Mandhane, P.J.; Sears, M.R. Asthma: Epidemiology, etiology and risk factors. Can. Med. Assoc. J. 2009, 181, E181–E190. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/asthma/data-visualizations/prevalence.htm#anchor_1569598317284 (accessed on 12 October 2020).
- Akinbami, L.J.; Moorman, J.E.; Bailey, C.; Zahran, H.S.; King, M.; Johnson, C.A.; Liu, X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. In NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2012; pp. 1–8. [Google Scholar]
- To, T.M.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz Álvaro, A.; Boulet, L.-P. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health 2012, 12, 204. [Google Scholar] [CrossRef]
- Nurmagambetov, T.; Kuwahara, R.; Garbe, P. The economic burden of asthma in the United States, 2008–2013. Ann. Am. Thorac. Soc. 2018, 15, 348–356. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Adibi, A.; Safari, A.; Fitzgerald, J.M.; Sadatsafavi, M. The Projected Economic and Health Burden of Uncontrolled Asthma in the United States. Am. J. Respir. Crit. Care Med. 2019, 200, 1102–1112. [Google Scholar] [CrossRef]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of Asthma Phenotypes Using Cluster Analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef]
- Moore, W.C.; Bleecker, E.R.; Curran-Everett, D.; Erzurum, S.C.; Ameredes, B.T.; Bacharier, L.; Calhoun, W.J.; Castro, M.; Chung, K.F.; Clark, M.P.; et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J. Allergy Clin. Immunol. 2007, 119, 405–413. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Robinson, D.; Humbert, M.; Buhl, R.; Cruz, A.A.; Inoue, H.; Korom, S.; Hanania, N.A.; Nair, P. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: Current knowledge and therapeutic implications. Clin. Exp. Allergy 2017, 47, 161–175. [Google Scholar] [CrossRef]
- Barlow, J.L.; McKenzie, A.N. Type-2 innate lymphoid cells in human allergic disease. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 397–403. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; Fitzgerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Solèr, M.; Matz, J.; Townley, R.; Buhlz, R.; O’Brien, J.; Fox, H.; Thirlwell, J.; Gupta, N.; Della Cioppa, G. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur. Respir. J. 2001, 18, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.; Corren, J.; Lanier, B.Q.; McAlary, M.; Fowler-Taylor, A.; Della Cioppa, G.; Van As, A.; Gupta, N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001, 108, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, E.R.; Fitzgerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- Pavord, I.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.G.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Doroudchi, A.; Pathria, M.; Modena, B.D. Asthma biologics. Ann. Allergy Asthma Immunol. 2019, 124, 44–56. [Google Scholar] [CrossRef]
- Settipane, R.A.; Kreindler, J.L.; Chung, Y.; Tkacz, J. Evaluating direct costs and productivity losses of patients with asthma receiving GINA 4/5 therapy in the United States. Ann. Allergy Asthma Immunol. 2019, 123, 564–572.e3. [Google Scholar] [CrossRef]
- Anderson, W.C.; Szefler, S.J. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma. Ann. Allergy Asthma Immunol. 2019, 122, 367–372. [Google Scholar] [CrossRef]
- Rogliani, P.; Calzetta, L.; Matera, M.G.; Laitano, R.; Ritondo, B.L.; Hanania, N.A.; Cazzola, M. Severe Asthma and Biological Therapy: When, Which, and for Whom. Pulm. Ther. 2020, 6, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Sokol, K.C.; Sharma, G.; Lin, Y.-L.; Goldblum, R.M. Choosing Wisely: Adherence by Physicians to Recommended Use of Spirometry in the Diagnosis and Management of Adult Asthma. Am. J. Med. 2015, 128, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Luks, V.P.; Vandemheen, K.L.; Aaron, S.D. Confirmation of asthma in an era of overdiagnosis. Eur. Respir. J. 2010, 36, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Aaron, S.D.; Vandemheen, K.; Fitzgerald, J.M.; Ainslie, M.; Gupta, S.; Lemiere, C.; Field, S.K.; McIvor, A.; Hernandez, P.; Mayers, I.; et al. Reevaluation of Diagnosis in Adults with Physician-Diagnosed Asthma. JAMA 2017, 317, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Israel, E.; Reddel, H.K. Severe and Difficult-to-Treat Asthma in Adults. N. Engl. J. Med. 2017, 377, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2013, 43, 343–373. [Google Scholar] [CrossRef]
- Gherasim, A.; Dao, A.; Bernstein, J.A. Confounders of severe asthma: Diagnoses to consider when asthma symptoms persist despite optimal therapy. World Allergy Organ. J. 2018, 11, 29. [Google Scholar] [CrossRef]
- Backer, V.; Sverrild, A.; Ulrik, C.S.; Bødtger, U.; Seersholm, N.; Porsbjerg, C. Diagnostic work-up in patients with possible asthma referred to a university hospital. Eur. Clin. Respir. J. 2015, 2, 331–341. [Google Scholar] [CrossRef]
- Schneider, A.; Gindner, L.; Tilemann, L.; Schermer, T.; Dinant, G.-J.; Meyer, F.J.; Szecsenyi, J. Diagnostic accuracy of spirometry in primary care. BMC Pulm. Med. 2009, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Yurdakul, A.S.; Dursun, B.; Canbakan, S.; Cakaloğlu, A.; Capan, N. The assessment of validity of different asthma diagnostic tools in adults. J. Asthma Off. J. Assoc. Care Asthma 2005, 42, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Sumino, K.; Sugar, E.A.; Irvin, C.G.; Kaminsky, D.A.; Shade, D.; Wei, C.Y.; Holbrook, J.T.; Wise, R.A.; Castro, M. Methacholine challenge test: Diagnostic characteristics in asthmatic patients receiving controller medications. J. Allergy Clin. Immunol. 2012, 130, 69–75.e6. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.J.; Brightling, C.; Woltmann, G.; Wardlaw, A.J.; Pavord, I. A comparison of the validity of different diagnostic tests in adults with asthma. Chest 2002, 121, 1051–1057. [Google Scholar] [CrossRef]
- Dean, B.W.; Birnie, E.E.; Whitmore, G.A.; Vandemheen, K.; Boulet, L.-P.; Fitzgerald, J.M.; Ainslie, M.; Gupta, S.; Lemiere, C.; Field, S.K.; et al. Between-Visit Variability in FEV1 as a Diagnostic Test for Asthma in Adults. Ann. Am. Thorac. Soc. 2018, 15, 1039–1046. [Google Scholar] [CrossRef]
- Joos, G.; O’Connor, B. Indirect airway challenges. Eur. Respir. J. 2003, 21, 1050–1068. [Google Scholar] [CrossRef]
- Hallstrand, T.S.; Leuppi, J.D.; Joos, G.; Hall, G.L.; Carlsen, K.-H.; Kaminsky, D.A.; Coates, A.L.; Cockcroft, D.W.; Culver, B.H.; Diamant, Z.; et al. ERS technical standard on bronchial challenge testing: Pathophysiology and methodology of indirect airway challenge testing. Eur. Respir. J. 2018, 52, 1801033. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Rodway, G.W.; Choi, J.; Hoffman, L.A.; Sethi, J.M. Exhaled nitric oxide in the diagnosis and management of asthma: Clinical implications. Chronic Respir. Dis. 2009, 6, 19–29. [Google Scholar] [CrossRef]
- Duong-Quy, S. Clinical Utility of the Exhaled Nitric Oxide (NO) Measurement with Portable Devices in the Management of Allergic Airway Inflammation and Asthma. J. Asthma Allergy 2019, 12, 331–341. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Asthma: Diagnosis, Monitoring and Chronic Asthma Management. Available online: https://www.nice.org.uk/guidance/ng80 (accessed on 11 December 2020).
- Bumbacea, D.; Campbell, D.; Nguyen, L.; Carr, D.; Barnes, P.; Robinson, D.; Chung, K. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur. Respir. J. 2004, 24, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Beuther, D.A.; Sutherland, E.R. Overweight, Obesity, and Incident Asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Vortmann, M.; Eisner, M.D. BMI and Health Status among Adults with Asthma. Obesity 2008, 16, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Salome, C.M.; King, G.G.; Berend, N. Physiology of obesity and effects on lung function. J. Appl. Physiol. 2010, 108, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Nacaroglu, H.; Gayret, O.; Erol, M.; Buke, O.; Zengi, O.; Tasdemir, M.; Yigit, O. Biomarkers of airway and systemic inflammation in obese asthmatic paediatric patients. Allergol. Immunopathol. 2017, 45, 534–540. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Swern, A.; Bird, S.S.; Hustad, C.M.; Grant, E.; Edelman, J.M. Influence of body mass index on the response to asthma controller agents. Eur. Respir. J. 2006, 27, 495–503. [Google Scholar] [CrossRef]
- Boulet, L.-P.; Franssen, E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir. Med. 2007, 101, 2240–2247. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Goleva, E.; Strand, M.; Beuther, D.A.; Leung, D.Y.M. Body Mass and Glucocorticoid Response in Asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 682–687. [Google Scholar] [CrossRef]
- Okayama, M.; Yafuso, N.; Nogami, H.; Lin, Y.; Horio, S.; Hida, W.; Inoue, H.; Takishima, T. A new method of inhalation challenge with propranolol: Comparison with methacholine-induced bronchoconstriction and role of vagal nerve activity. J. Allergy Clin. Immunol. 1987, 80, 291–299. [Google Scholar] [CrossRef]
- Patakas, D.; Argiropoulou, V.; Louridas, G.; Tsara, V. Beta-blockers in bronchial asthma: Effect of propranolol and pindolol on large and small airways. Thorax 1983, 38, 108–112. [Google Scholar] [CrossRef]
- Morales, D.R.; Jackson, C.; Lipworth, B.J.; Donnan, P.T.; Guthrie, B. Adverse Respiratory Effect of Acute β-Blocker Exposure in Asthma. Chest 2014, 145, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Covar, R.A.; Macomber, B.A.; Szefler, S.J. Medications as asthma triggers. Immunol. Allergy Clin. N. Am. 2005, 25, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Schiavetti, I.; Rindone, E.; Ricciardolo, F.L. The impact of anxiety and depression on outpatients with asthma. Ann. Allergy Asthma Immunol. 2015, 115, 408–414. [Google Scholar] [CrossRef]
- Lavoie, K.L.; Cartier, A.; Labrecque, M.; Bacon, S.L.; Lemiere, C.; Malo, J.-L.; Lacoste, G.; Barone, S.; Verrier, P.; Ditto, B. Are psychiatric disorders associated with worse asthma control and quality of life in asthma patients? Respir. Med. 2005, 99, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Crespo, A.; Fernandez-Sanchez, A.; Rial, M.; Plaza, V.; González, F.C.; López, J.J.; Riaza, M.M.; Orenes, M.M.; Montaño, P.P.; et al. Anxiety, Depression, and Asthma Control: Changes After Standardized Treatment. J. Allergy Clin. Immunol. Pr. 2018, 6, 1953–1959. [Google Scholar] [CrossRef]
- Wichmann, D.; Campos, C.E.B.; Ehrhardt, S.; Kock, T.; Weber, C.; Rohde, H.; Kluge, S. Efficacy of introducing a checklist to reduce central venous line associated bloodstream infections in the ICU caring for adult patients. BMC Infect. Dis. 2018, 18, 267. [Google Scholar] [CrossRef]
- Haynes, A.B.; Weiser, T.G.; Berry, W.R.; Lipsitz, S.R.; Breizat, A.-H.S.; Dellinger, E.P.; Herbosa, T.; Joseph, S.; Kibatala, P.L.; Lapitan, M.C.M.; et al. A Surgical Safety Checklist to Reduce Morbidity and Mortality in a Global Population. N. Engl. J. Med. 2009, 360, 491–499. [Google Scholar] [CrossRef]
- Torr, J.; Iacono, T.; Graham, M.J.; Galea, J. Checklists for general practitioner diagnosis of depression in adults with intellectual disability. J. Intellect. Disabil. Res. 2008, 52, 930–941. [Google Scholar] [CrossRef]
- Ely, J.W.; Graber, M.L.; Croskerry, P. Checklists to Reduce Diagnostic Errors. Acad. Med. 2011, 86, 307–313. [Google Scholar] [CrossRef]

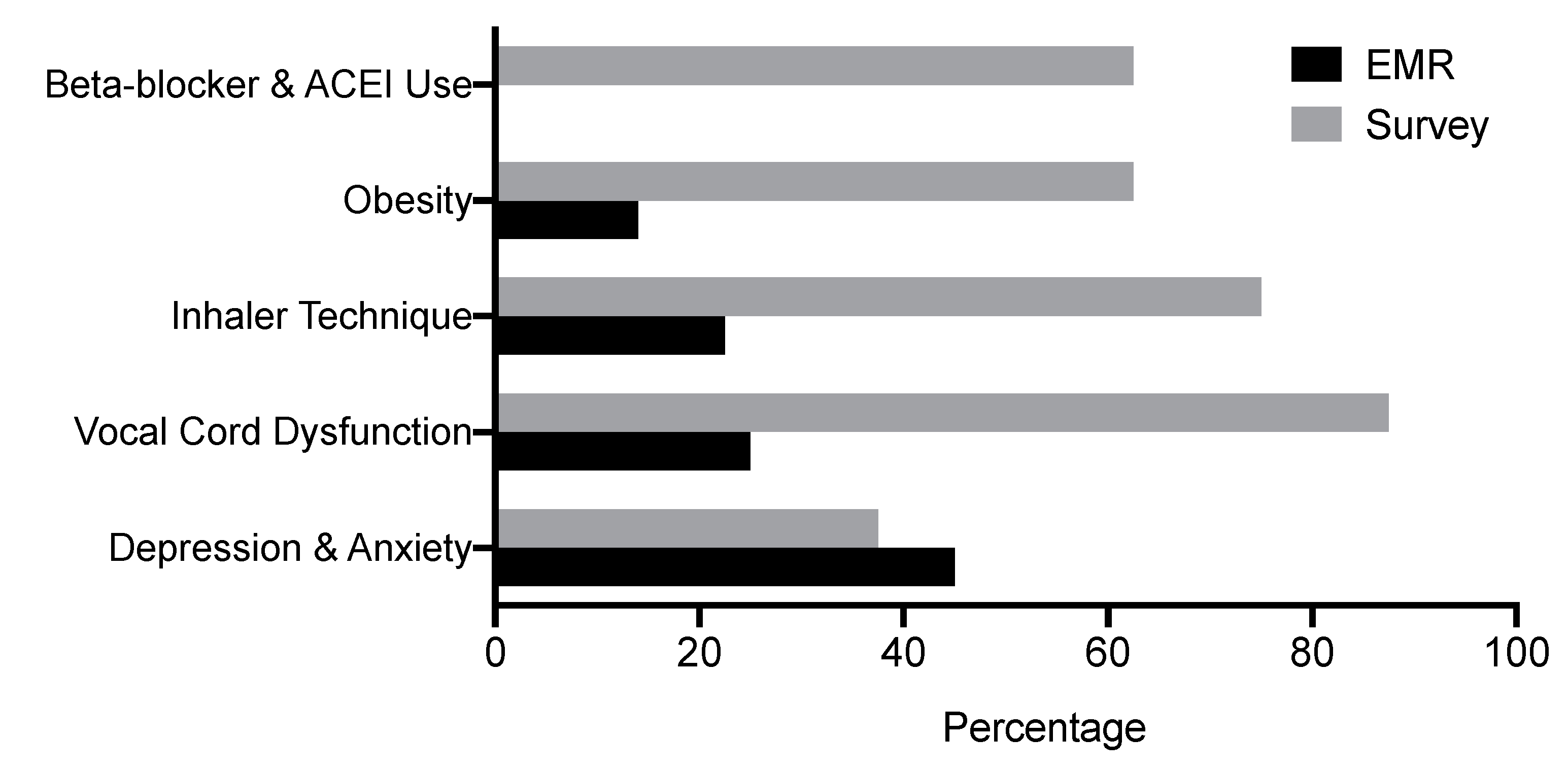
| Characteristics | Value † |
|---|---|
| Age, (years) | 55.5 (50–64) |
| Gender | |
| Female | 26 (65) |
| Male | 14 (35) |
| Biologic used | |
| Omalizumab | 29 (72.5) |
| Benralizumab | 6 (15) |
| Mepolizumab | 4 (10) |
| Dupilumab | 1 (2.5) |
| Asthma Therapy Optimized | 38 (95) |
| Asthma Diagnosis Confirmed | 23 (58) |
| BMI (kg/m2) | 33.8 (28–40) |
| Obesity | 29 (73) |
| FEV1 % predicted | 61.5 (47.5–76.5) |
| Comorbidities addressed | |
| Gastroesophageal reflux disease (GERD) | 28 (70) |
| Vocal cord dysfunction | 10 (25) |
| Beta-blocker and angiotensin-converting enzyme inhibitor (ACE-I) | 0 (0) |
| Depression and anxiety | 18 (45) |
| Obesity | 4 (14) |
| Inhaler technique | 9 (22.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziewa, I.; Craig, T.; Al-Shaikhly, T. How Frequently Is Asthma Objectively Demonstrated before Starting a Biologic? Quality Assessment of a Group Practice of Allergists and Immunologists. Int. J. Environ. Res. Public Health 2020, 17, 9482. https://doi.org/10.3390/ijerph17249482
Dziewa I, Craig T, Al-Shaikhly T. How Frequently Is Asthma Objectively Demonstrated before Starting a Biologic? Quality Assessment of a Group Practice of Allergists and Immunologists. International Journal of Environmental Research and Public Health. 2020; 17(24):9482. https://doi.org/10.3390/ijerph17249482
Chicago/Turabian StyleDziewa, Iwona, Timothy Craig, and Taha Al-Shaikhly. 2020. "How Frequently Is Asthma Objectively Demonstrated before Starting a Biologic? Quality Assessment of a Group Practice of Allergists and Immunologists" International Journal of Environmental Research and Public Health 17, no. 24: 9482. https://doi.org/10.3390/ijerph17249482
APA StyleDziewa, I., Craig, T., & Al-Shaikhly, T. (2020). How Frequently Is Asthma Objectively Demonstrated before Starting a Biologic? Quality Assessment of a Group Practice of Allergists and Immunologists. International Journal of Environmental Research and Public Health, 17(24), 9482. https://doi.org/10.3390/ijerph17249482






