Long-Term Benefits of Tailored Exercise in Severe Sarcoidosis: A Case Report
Abstract
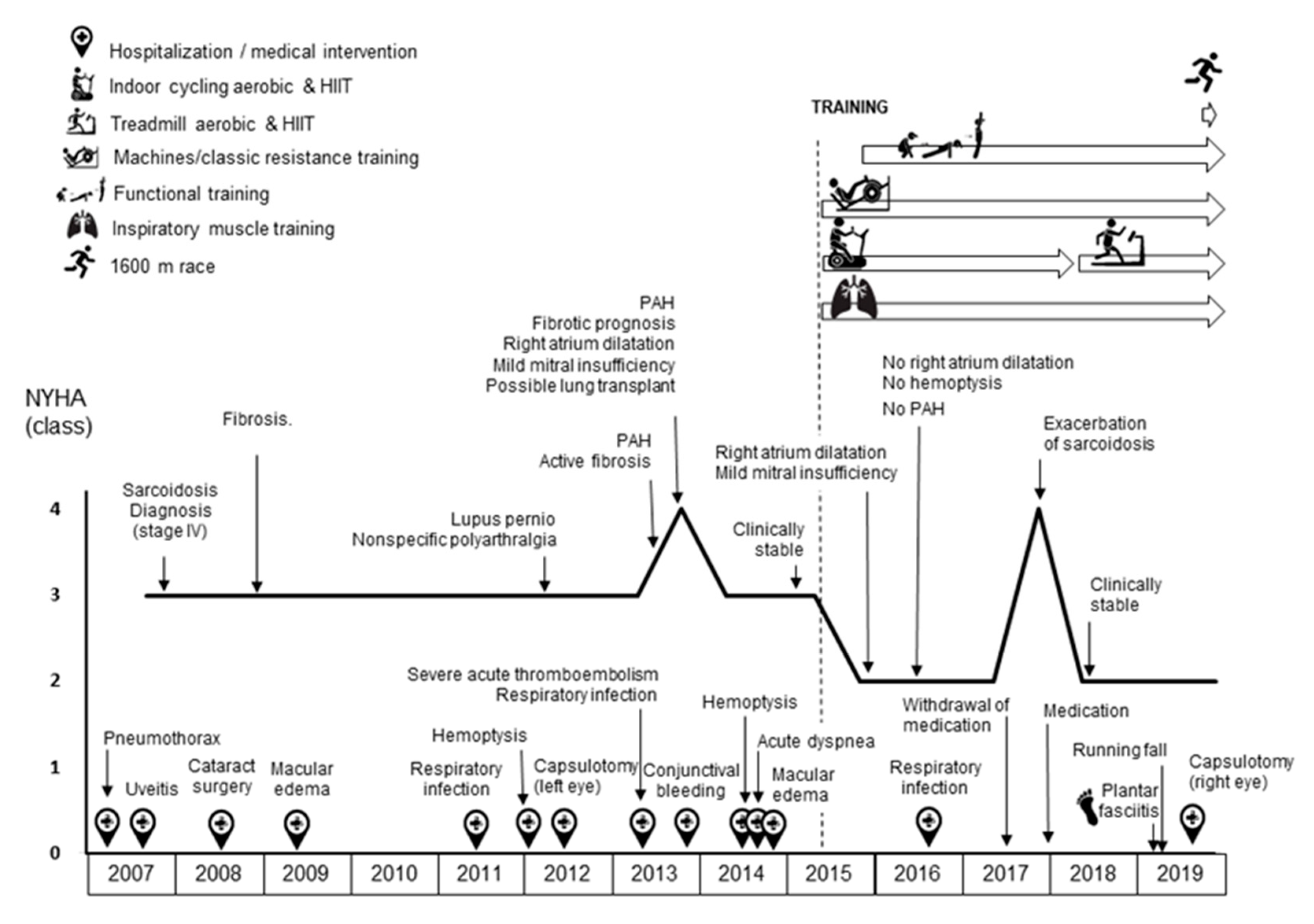
:1. Introduction
2. Experimental Section (Case Report)
2.1. Assessments
2.2. Exercise Training Intervention
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arkema, E.V.; Cozier, Y.C. Epidemiology of sarcoidosis: Current findings and future directions. Ther. Adv. Chronic Dis. 2018, 9, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Tavana, S.; Alizadeh, M.; Mohajerani, S.; Hashemian, S. Pulmonary and extra-pulmonary manifestations of sarcoidosis. Niger. Med. J. 2015, 56, 258. [Google Scholar] [CrossRef] [PubMed]
- Voortman, M.; Hendriks, C.M.R.; Elfferich, M.D.P.; Bonella, F.; Møller, J.; de Vries, J.; Costabel, U.; Drent, M. The burden of sarcoidosis symptoms from a patient perspective. Lung 2019, 197, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judson, M.A. The clinical features of sarcoidosis: A comprehensive review. Clin. Rev. Allergy Immunol. 2015, 49, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Boucly, A.; Cottin, V.; Nunes, H.; Jaïs, X.; Tazi, A.; Prévôt, G.; Reynaud-Gaubert, M.; Dromer, C.; Viacroze, C.; Horeau-Langlard, D.; et al. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Moor, C.C.; Gür-Demirel, Y.; Wijsenbeek, M.S. Feasibility of a comprehensive home monitoring program for sarcoidosis. J. Personal. Med. 2019, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Cho, P.S.P.; Vasudevan, S.; Maddocks, M.; Spinou, A.; Mitchell, S.C.; Wood, C.; Jolley, C.J.; Birring, S.S. Physical inactivity in pulmonary sarcoidosis. Lung 2019, 197, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Froidure, S.; Kyheng, M.; Grosbois, J.M.; Lhuissier, F.; Stelianides, S.; Wemeau, L.; Wallaert, B. Daily life physical activity in patients with chronic stage IV sarcoidosis: A multicenter cohort study. Health Sci. Rep. 2019, 2, e109. [Google Scholar] [CrossRef] [Green Version]
- Bahmer, T.; Watz, H.; Develaska, M.; Waschki, B.; Rabe, K.F.; Magnussen, H.; Kirsten, D.; Kirsten, A.-M. Physical activity and fatigue in patients with sarcoidosis. Respiration 2018, 95, 18–26. [Google Scholar] [CrossRef]
- Strookappe, B.; Saketkoo, L.A.; Elfferich, M.; Holland, A.; de Vries, J.; Knevel, T.; Drent, M. Physical activity and training in sarcoidosis: Review and experience-based recommendations. Expert Rev. Respir. Med. 2016, 10, 1057–1068. [Google Scholar] [CrossRef] [Green Version]
- Strookappe, B.; Elfferich, M.; Swigris, J.; Verschoof, A.; Veschakelen, J.; Knevel, T.; Drent, M. Benefits of physical training in patients with idiopathic or end-stage sarcoidosis-related pulmonary fibrosis: A pilot study. Sarcoidosis Vasc. Diffuse Lung Dis. 2015, 32, 43–52. [Google Scholar] [PubMed]
- Marcellis, R.; van der Veeke, M.; Mesters, I.; Drent, M.; de Bie, R.; de Vries, G.; Lenssen, A. Does physical training reduce fatigue in sarcoidosis? Sarcoidosis Vasc. Diffuse Lung Dis. 2015, 32, 53–62. [Google Scholar] [PubMed]
- Kullberg, S.; Rivera, N.; Eriksson, M.J.; Grunewald, J.; Eklund, A. High-intensity resistance training in newly diagnosed sarcoidosis- an exploratory study of effects on lung function, muscle strength, fatigue, dyspnea, health-related quality of life and lung immune cells. Eur. Clin. Respir. J. 2020, 7, 1730137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strookappe, B.; Swigris, J.; de Vries, J.; Elfferich, M.; Knevel, T.; Drent, M. Benefits of physical training in sarcoidosis. Lung 2015, 193, 701–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naz, I.; Ozalevli, S.; Ozkan, S.; Sahin, H. Efficacy of a structured exercise program for improving functional capacity and quality of life in patients with stage 3 and 4 sarcoidosis: A randomized controlled trial. J. Cardiopulm. Rehabil. Prev. 2018, 38, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Grongstad, A.; Vøllestad, N.; Oldervoll, L.; Spruit, M.; Edvardsen, A. The effects of high- versus moderate-intensity exercise on fatigue in sarcoidosis. J. Clin. Med. 2019, 8, 460. [Google Scholar] [CrossRef] [Green Version]
- Gangemi, A.; Myers, C.N.; Zheng, M.; Brown, J.; Butler-Lebair, M.; Cordova, F.; Marchetti, N.; Criner, G.J.; Gupta, R.; Mamary, A.J. Mortality for sarcoidosis patients on the transplant wait list in the Lung Allocation Score era: Experience from a high volume center. Respir. Med. 2019, 157, 69–76. [Google Scholar] [CrossRef]
- Marcellis, R.G.J.; Lenssen, A.F.; de Vries, J.; Drent, M. Reduced muscle strength, exercise intolerance and disabling symptoms in sarcoidosis. Curr. Opin. Pulm. Med. 2013, 19, 524–530. [Google Scholar] [CrossRef]
- Brancaleone, P.; Perez, T.; Robin, S.; Neviere, R.; Wallaert, B. Clinical impact of inspiratory muscle impairment in sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2004, 21, 219–227. [Google Scholar]
- Hunninghake, G.W.; Costabel, U.; Ando, M.; Baughman, R.; Cordier, J.F.; Bois, R.; Eklund, A.; Kitaichi, M.; Lynch, J.; Rizzto, G.; et al. Statement on sarcoidosis. Am. J. Respir. Crit. Care Med. 1999, 160, 736–755. [Google Scholar] [CrossRef]
- González-Saiz, L.; Fiuza-Luces, C.; Sanchis-Gomar, F.; Santos-Lozano, A.; Quezada-Loaiza, C.A.; Flox-Camacho, A.; Munguía-Izquierdo, D.; Ara, I.; Santalla, A.; Morán, M.; et al. Benefits of skeletal-muscle exercise training in pulmonary arterial hypertension: The WHOLEi + 12 trial. Int. J. Cardiol. 2017, 231, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zach, M.S. The physiology of forced expiration. Paediatr. Respir. Rev. 2000, 1, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.; Menezes, S.L.S.; Dias, C.M.; Oliveira, J.F.; Mainenti, M.R.M.; Guimarães, F.S. Cardiopulmonary exercise testing variables as predictors of long-term outcome in thoracic sarcoidosis. Braz. J. Med. Biol. Res. 2012, 45, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucía, A.; Hoyos, J.; Chicharro, J.L. Preferred pedalling cadence in professional cycling. Med. Sci. Sports Exerc. 2001, 33, 1361–1366. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Gómez, I.; Santalla, A.; Díez-Bermejo, J.; Munguía-Izquierdo, D.; Alegre, L.M.; Nogales-Gadea, G.; Arenas, J.; Martín, M.A.; Lucia, A.; Ara, I. A new condition in McArdle disease: Poor bone HealthBenefits of an active lifestyle. Med. Sci. Sports Exerc. 2018, 50, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.A.; Brooke-Wavell, K. Optimum frequency of exercise for bone health: Randomised controlled trial of a high-impact unilateral intervention. Bone 2010, 46, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Kemmler, W.; Kohl, M.; von Stengel, S. Long-term effects of exercise in postmenopausal women: 16-year results of the Erlangen Fitness and Osteoporosis Prevention Study (EFOPS). Menopause 2017, 24, 45–51. [Google Scholar] [CrossRef]
- Lagally, K.M.; Robertson, R.J. Construct validity of the OMNI resistance exercise scale. J. Strength Cond. Res. 2006, 20, 252–256. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and hypertrophy adaptations between low- vs. High-load resistance training: A systematic review and meta-analysis. J. Strength Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef]
- Nunes, P.R.; Barcelos, L.C.; Oliveira, A.A.; Furlanetto, R.; Martins, F.M.; Resende, E.A.; Orsatti, F.L.; Júnior, R.F. Muscular strength adaptations and hormonal responses after two different multiple-set protocols of resistance training in postmenopausal women. J. Strength Cond. Res. 2019, 33, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Maffiuletti, N.A.; Joyner, M.J.; Lucia, A.; Lepers, R. Lifelong endurance exercise as a countermeasure against age-related V˙ O 2 max decline: Physiological overview and insights from masters athletes. Sports Med. 2020, 50, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Ozemek, C.; Laddu, D.R.; Lavie, C.J.; Claeys, H.; Kaminsky, L.A.; Ross, R.; Wisloff, U.; Arena, R.; Blair, S.N. An update on the role of cardiorespiratory fitness, structured exercise and lifestyle physical activity in preventing cardiovascular disease and health risk. Prog. Cardiovasc. Dis. 2018, 61, 484–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, J.N.; Joyner, M.J. Physical activity and cardiovascular risk: 10 metabolic equivalents or bust. Mayo Clin. Proc. 2013, 88, 1353–1355. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.M.; Armstrong, H.F.; Baldwin, M.R.; Podolanczuk, A.J.; Pieszchata, N.M.; Singer, J.P.; Arcasoy, S.M.; Meza, K.S.; D’Ovidio, F.; Lederer, D.J. Frailty and maximal exercise capacity in adult lung transplant candidates. Respir. Med. 2017, 131, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [Green Version]
- Bloor, C.M. Angiogenesis during exercise and training. Angiogenesis 2005, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Habedank, D.; Reindl, I.; Vietzke, G.; Bauer, U.; Sperfeld, A.; Wernecke, K.D.; Kleber, F.X. Ventilatory efficiency and exercise tolerance in 101 healthy volunteers. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 421–426. [Google Scholar] [CrossRef]
- Karadall, M.N.; Boşnak-Güçlü, M.; Camcıoğlu, B.; Kokturk, N.; Tüktaş, H. Effects of inspiratory muscle training in subjects with sarcoidosis: A randomized controlled clinical trial. Respir. Care 2016, 61, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Souza, H.; Rocha, T.; Pessoa, M.; Rattes, C.; Brandão, D.; Fregonezi, G.; Campos, S.; Aliverti, A.; Dornelas, A. Effects of inspiratory muscle training in elderly women on respiratory muscle strength, diaphragm thickness and mobility. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1545–1553. [Google Scholar] [CrossRef] [Green Version]
- Shei, R.J.; Paris, H.L.R.; Wilhite, D.P.; Chapman, R.F.; Mickleborough, T.D. The role of inspiratory muscle training in the management of asthma and exercise-induced bronchoconstriction. Phys. Sportsmed. 2016, 44, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Romer, L.; Rodman, J.; Miller, J.; Smith, C. Consequences of exercise-induced respiratory muscle work. Respir. Physiol. Neurobiol. 2006, 151, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Hoff, J.; Gran, A.; Helgerud, J. Maximal strength training improves aerobic endurance performance. Scand. J. Med. Sci. Sports 2002, 12, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Heggelund, J.; Fimland, M.S.; Helgerud, J.; Hoff, J. Maximal strength training improves work economy, rate of force development and maximal strength more than conventional strength training. Eur. J. Appl. Physiol. 2013, 113, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Miyakoshi, N.; Kasukawa, Y.; Akagawa, M.; Kimura, R.; Nagahata, I.; Yuasa, Y.; Sato, C.; Shimada, Y. Diagnosis of presarcopenia using body height and arm span for postmenopausal osteoporosis. Clin. Interv. Aging. 2020, 15, 357–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanbury, R.M.; Graham, E.M. Systemic corticosteroid therapy side effects and their management. Br. J. Ophthalmol. 1998, 82, 704–708. [Google Scholar] [CrossRef] [Green Version]
- Owczarek, J.; Jasińska, M.; Orszulak-Michalak, D. Drug-induced myopathies. An overview of the possible mechanisms. Pharmacol. Rep. 2005, 57, 23–34. [Google Scholar]
- Kamen, G. Aging, resistance training, and motor unit discharge behavior. Can. J. Appl. Physiol. 2005, 30, 341–351. [Google Scholar] [CrossRef]
- Jenkins, N.D.; Miramonti, A.A.; Hill, E.C.; Smith, C.M.; Cochrane-Snyman, K.C.; Housh, T.J.; Cramer, J.T. Greater neural adaptations following high- vs. low-load resistance training. Front. Physiol. 2017, 8, 331. [Google Scholar] [CrossRef]
- Sipilä, S.; Törmäkangas, T.; Sillanpää, E.; Aukee, P.; Kujala, U.M.; Kovanen, V.; Laakkonen, E.K. Muscle and bone mass in middle-aged women: Role of menopausal status and physical activity. J. Cachexia Sarcopenia Muscle. 2020, 11, 698–709. [Google Scholar] [CrossRef]
- García-Bustínduy, M.; Gantes, M.A. Corticoides y osteoporosis. Actas Dermo Sifiliogr. 2007, 98, 526–530. [Google Scholar] [CrossRef]
- Greendale, G.A.; Sternfeld, B.; Huang, M.; Han, W.; Karvonen-Gutierrez, C.; Ruppert, K.; Cauley, J.A.; Finkelstein, J.S.; Jiang, S.-F.; Karlamangla, A.S. Changes in body composition and weight during the menopause transition. JCI Insight. 2019, 4, e124865. [Google Scholar] [CrossRef] [PubMed]
| Period | Days/Week | Exercise Mode | Total Session Duration (Min) | Between Intervals | Intervals/Sprints | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Internal Load | Average HR (Beats·Min−1) | Average %HRmax | Average SpO2 (%) | Number × Duration/Recovery | RPE (0–10) | Average HR (Beats·Min−1) | HR (%HRmax) | Average SPO2MIN (%) | ||||
| July–September 2015 | 4 | Cycling | 30 | 50% (VT-RCP) | 125 | 74 | 96 | 10–15 × 8 s/2–3 min | 9 | 130 | 77 | 93 |
| October–December 2015 | 3–4 | Cycling | 40–60 | 50%(VT-RCP) | 132 | 79 | 94 | 14–30 × 8 s/2–3 min | 8 | 135 | 80 | 93 |
| January–March 2016 | 3–4 | Cycling | 60 | 50% (VT-RCP) | 135 | 81 | 95 | 30 × 5–10 s/1 min | 7 | 141 | 84 | 91 |
| April–June 2016 | 3 | Cycling | 60 | 50% (VT-RCP) | 125 | 75 | 94 | 30 × 5 s/1 min | 7 | 135 | 81 | 91 |
| July–September 2016 | 3 | Cycling | 60–45 | 50% (VT-RCP) | 125 | 75 | 95 | 30–23 × 10 s/2 min | 7 | 135 | 81 | 90 |
| October–November 2016 | 3–4 | Cycling | 45 | 50% (VT-RCP) | 125 | 75 | 94 | 23 × 10 s/2 min | 7 | 135 | 81 | 90 |
| December 2016 | Decrease in medication (corticosteroids) | |||||||||||
| January–March 2017 | 3 | Cycling | 45–60 | 50% (VT-RCP) | 135 | 94 | 23–30 × 10 s/2 min | 8 | 145 | 87 | 90 | |
| April 2017 | 2 | Cycling | 60 | 50% (VT-RCP) | 135 | 81 | 94 | 30 × 10 s/2 min | 8 | 145 | 87 | 89 |
| May–June 2017 | Progressive withdrawal of medication | |||||||||||
| July–September 2017 | 2–3 | Cycling | 30–60 | 50% (VT-RCP) | 130 | 78 | 94 | 15–30 × 10 s/2 min | 7 | 145 | 87 | 90 |
| October–November 2017 | 3 | Cycling | 45–60 | 50% (VT-RCP) | 135 | 81 | 94 | 23–30 × 10 s/2 min | 7 | 145 | 87 | 90 |
| December 2017 | Exacerbation of sarcoidosis | |||||||||||
| January–March 2018 | 2 | Cycling | 60 | 50% (VT-RCP) | 135 | 82 | 94 | 30 × 10 s/2 min | 6 | 143 | 87 | 89 |
| April–June 2018 | 3 | Running | 30 | 7 km·h−1 | 135 | 82 | 90 | 12 × 15 s (15 km·h−1)/2.5 min | 9 | 140 | 85 | 89 |
| July–September 2018 | 2–3 | Running | 40–60 | 7 km·h−1 | 140 | 85 | 92 | 16–24 × 15 s (15 km·h−1)/2.5 min | 9 | 155 | 94 | 91 |
| October–December 2018 | 2 | Running | 40–60 | 7 km·h−1 | 142 | 86 | 93 | 16–24 × 15 s (15–18 km·h−1)/2.5 min | 9 | 165 | 100 | 89 |
| January–March 2019 | Plantar fasciitis | |||||||||||
| April–June 2019 | 2 | Cycling | 30–45 | 50% (VT-RCP) | 135 | 82 | 93 | 10–23 × 8 s/2–3 min | 5 | 145 | 88 | 89 |
| July–September 2019 | 2 | Running | 45 | 7 km·h−1 | 135 | 82 | 94 | 23 × 10 s (15 km·h−1)/2 min * | 9 | 155 | 95 | 85 |
| October–December 2019 | 2 | Running | 50–45 | 7 km·h−1 | 135 | 82 | 94 | 25–23 × 10 s (15 km·h−1)/2 min ** | 9 | 155 | 95 | 85 |
| Period | Days/Week | Classic Resistance Training | Days/Week | Functional Training | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modified Chest Fly Machine * | Leg Press | Modified Behind the Neck Lat Pulldown ** | Planks *** | Russian Belt Squat | Clean & Jerk/Split | Step-Full Squat | Lunges | Squat Jumps | Burpees | |||
| July–September 2015 | 2 | 3 × 5–10 kg (8–92%) | 3 × 6–10/30 kg (6–94%) | 3 × 5–15 kg (7–88%) | 2/4 × 6 (8–94%) | |||||||
| October–December 2015 | 2 | 4 × 7–10 kg (8–91%) | 4 × 7–30/40 kg (6–94%) | 4 × 7–15 kg (7–88%) | 4 × 7 (7–92%) | |||||||
| January–March 2016 | 2 | 4 × 8−15 kg (9–91%) | 4 × 8−50 kg (6–95%) | 4 × 8–25 kg (8–88%) | 4 × 8 (7–94%) | 2 | 3 × 15 (8–90%) | 3 × 15 (5–93%) | 3 × 1/12 (7–90%) | |||
| April–June 2016 | 2 | 4 × 8−15 kg (9–92%) | 4 × 8−50 kg (6–95%) | 4 × 8–25 kg (8–88%) | 4 × 8 (7–94%) | 4 × 8–10 kg (9–90%) | 2 | 3 × 15 (6–90%) | 3 × 15 (5–93%) | 3 × 12 (8–90%) | 3 × 8 (9–89%) | |
| July–September 2016 | 2 | 4 × 8−15 kg (8–92%) | 4 × 8 −50/60 kg (7–95%) | 4 × 8–25 kg (7–88%) | 4 × 8 (7–94%) | 4 × 8–10 kg (8–90%) | 2 | 3 × 15 (5–90%) | 3 × 15 (5–94%) | 3 × 12 (7–88%) | 3 × 8 (9–88%) | |
| October–December 2016 | Decrease in medication (corticosteroids) | |||||||||||
| January–March 2017 | 2 | 4 × 8−15 kg (8–92%) | 4 × 8 −50/60 kg (8–95%) | 4 × 8–25 kg (8–85%) | 4 × 8 (7–94%) | 2 | 0/3 × 15 (6–90%) | 0/15 (5–92%) | 0/3 × 12 (8–88%) | 0/3 × 0/8 (9–85%) | ||
| April 2017 | 2 | 4 × 8−20 kg (9–90%) | 4 × 8 −60 kg (8–94%) | 4 × 8–25 kg (8–85%) | 4 × 8 (7–94%) | 4 × 8–10 kg (8–88%) | 2 | 3 × 15 (6–90%) | 15 (5–92%) | 3 × 12 (8–88%) | ||
| May–June 2017 | Progressive withdrawal of medication | |||||||||||
| July–September 2017 | 0–2 | 4 × 8−20 kg (8–90%) | 4 × 8 −60 kg (7–94%) | 4 × 8–25 kg (8–85%) | 4 × 8 (7–95%) | 4 × 8–10 kg (8–88%) | 0–2 | 0/3 × 12 (8–88%) | 0/3 × 12 (9–88%) | 0/3 × 10 (9–88%) | ||
| October–December 2017 | Exacerbation of sarcoidosis | |||||||||||
| January–March 2018 | 2 | 4 × 8−20/15 kg (7–90%) | 4 × 8 −60/70 kg (7–94%) | 4 × 8–20 kg (7–89%) | 4 × 8 (7–95%) | 4 × 8 –10 kg (8–90%) | 4 × 6–7.5 kg (9–88%) | 2 | 3 × 15 (6–94%) | 3 × 15 (5–94%) | 3 × 12 (6–88%) | |
| April–June 2018 | 2 | 4 × 8−15 kg (8–88%) | 4 × 8−60 kg (7–94%) | 4 × 8–20 kg (7–89%) | 4 × 8 (7–95%) | 4 × 8–8 kg (8–89%) | 4 × 6–7.5 kg (9–88%) | 2 | 3 × 15 (5–94%) | 2 × 15 (5–94%) | 2 × 12 (5–90%) | |
| July–September 2018 | 0–2 | 2/4 × 8−20 kg (7–92%) | 4 × 8−70 kg (7–92%) | 2/4 × 8–25 kg (8–88%) | 2/4 × 8 (7–95%) | 2/4 × 8–8 kg (7–92%) | 4 v 6–7.5 kg (9–88%) | 1–2 | 3 × 15 (5–94%) | 0/2 × 15 (5–94%) | 0/2 × 12 (5–90%) | |
| October–December 2018 | 0–2 | 2 × 8−20 kg (7–92%) | 4 × 8−70 kg (7–94%) | 2 × 8–20 kg (7–88%) | 2 × 8 (7–95%) | 2 × 8–8 kg (7–92%) | 4 × 6–7.5 kg (9–86%) | 1–2 | 3 × 15 (5–94%) | 0/3 × 15 (5–95%) | 0/3 × 12 (5–90%) | 0/3 × 10 (9–85%) |
| January–March 2019 | 1–2 | 2/3 × 8−20 kg (9–88%) | 2/3 × 8−70 kg (9–93%) | 2/3 × 8–25 kg (9–88%) | 2/3 × 8 (8–95%) | 2/3 × 8–8 kg (8–90%) | Plantar fasciitis | |||||
| April–June 2019 | 1 | 2/3 × 8−20 kg (9–88%) | 2/3 × 8−70 kg (8–93%) | 2/4 × 8–25 kg (9–85%) | 2/4 × 8 (8–93%) | 2/3 × 8–8 kg (9–90%) | 1 | 3 × 15 (8–90%) | 3 × 15 (8–92%) | 3 × 12 (7–90%) | 4 static planks | |
| July–September 2019 | 1 | 4 × 8−20 kg (8–92%) | 4 × 8−70 kg (7–94%) | 4 × 8–25 kg (7–85%) | 4 × 8 (9–88%) | 1 | 3 × 15 (8–90%) | 3 × 15 (8–90%) | 3 × 12 (6–90%) | 4 static planks | ||
| October–December 2019 | 1 | 4 × 8−20 kg (7–92%) | 4 × 8−70 kg (7–95%) | 4 × 8–25 kg (7–85%) | 4 × 8 (9–88%) | 1 | 3 × 15 (9–90%) | 3 × 15 (8–90%) | 3 × 12 (6–90%) | 4 static planks | ||
| Variables | Pretraining | December 2015 | June 2016 | December 2016 | June 2017 | December 2017 | June 2018 | December 2018 | June 2019 | December 2019 | Change from Baseline to 4.5 Years Later (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak values | |||||||||||
| VO2peak (mL·kg−1·min−1) | 20.1 | 22.4 | 30.2 | 23.6 | 24.4 | 25.2 | 28.9 | 28.3 | 26.6 | 28.9 | +44% |
| VO2peak (mL·min−1) | 1086 | 1212 | 1631 | 1275 | 1316 | 1360 | 1560 | 1550 | 1502 | 1561 | +44% |
| PPO (watts) | 100 | 119 | 123 | 130 | 125 | 120 | 130 | 127 | 130 | 124 | +24% |
| HRpeak (bpm) | 166 | 164 | 164 | 167 | 162 | 170 | 170 | 166 | 170 | 172 | +4% |
| HR (% HRmax) | 99 | 98 | 98 | 100 | 98 | 102 | 103 | 100 | 104 | 105 | +6% |
| VEpeak (L/min) | 62 | 59 | 73 | 65 | 83 | 87 | 81 | 97 | 77 | 99 | +60% |
| SpO2peak (%) | 93 | 91 | 91 | 88 | 89 | 88 | 89 | 89 | 89 | 91 | −2% |
| VT | |||||||||||
| VO2 (mL·kg−1·min−1) | 11.7 | 14.2 | 18.7 | 11.2 | 12.8 | 15.4 | 17.9 | 11.7 | 15.6 | 15.1 | +29% |
| PO (watts) | 47 | 57 | 66 | 35 | 47 | 50 | 47 | 41 | 60 | 42 | −11% |
| VE·VO2−1 | 38 | 29 | 31 | 28 | 31 | 27 | 29 | 25 | 28 | 26 | −32% |
| VE·VCO2−1 | 39 | 39 | 35 | 35 | 39 | 33 | 38 | 35 | 34 | 38 | −3% |
| RCP | |||||||||||
| VO2 (mL·kg−1·min−1) | 17.4 | 18.1 | 26.6 | 19.8 | 19.3 | 21.2 | 24.1 | 22.3 | 18.3 | 23.9 | +37% |
| PO (watts) | 77 | 87 | 103 | 106 | 89 | 87 | 83 | 102 | 95 | 90 | +17% |
| VE·VO2−1 | 42 | 42 | 35 | 36 | 36 | 35 | 35 | 36 | 32 | 36 | −14% |
| VE·VCO2−1 | 36 | 37 | 33 | 32 | 35 | 32 | 36 | 32 | 33 | 37 | +3% |
| Variables | Oct. | June | December | June | December | June | December | June | December | Change from Baseline to 3.5 Years Later (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2016 | 2016 | 2017 | 2017 | 2018 | 2018 | 2019 | 2019 | ||
| BMD (g·cm−2) | ||||||||||
| Whole body | 1.11 | 1.07 | 1.07 | 1.05 | 1.06 | 1.04 | 1.02 | 1.06 | −4.5 | |
| Subtotal body | 0.91 | 0.89 | 0.89 | 0.87 | 0.88 | 0.86 | 0.86 | 0.89 | −2.2 | |
| Pelvic | 1.09 | 1.06 | 1.06 | 1.06 | 1.07 | 1.01 | 1.05 | 1.10 | 0.9 | |
| Arms (mean) | 0.67 | 0.65 | 0.65 | 0.64 | 0.65 | 0.65 | 0.64 | 0.65 | −3.0 | |
| Legs (mean) | 1.10 | 1.09 | 1.08 | 1.06 | 1.04 | 1.06 | 1.06 | 1.06 | −3.6 | |
| Lumbar (mean L1–L4) | 1.24 | 0.98 | 0.97 | 0.96 | 0.94 | 0.92 | 0.92 | 0.93 | 0.96 | −2.0 |
| T-Score spine | 0.4 | −0.6 | −0.7 | −0.8 | −0.9 | −1.2 | −1.1 | −1.1 | −0.8 | |
| Z-Score spine | 1.1 | 0.4 | 0.3 | 0.3 | 0.1 | −0.1 | 0.0 | 0.1 | 0.4 | |
| Femoral neck | 1.01 | 0.83 | 0.76 | 0.81 | 0.84 | 0.76 | 0.73 | 0.74 | 0.73 | −12.0 |
| T-Score femoral neck | 0.2 | −0.1 | −0.8 | −0.3 | −0.1 | −0.8 | −1.2 | −1.0 | −1.1 | |
| Z-Score femoral neck | 0.7 | 0.8 | 0.2 | 0.7 | 1.0 | 0.2 | 0.1 | 0.1 | 0.1 | |
| Lean mass (kg) | ||||||||||
| Whole body | 40.4 | 38.5 | 39.6 | 38.5 | 40.4 | 39.2 | 38.4 | 35.8 | −11.4 | |
| Subtotal body | 35.6 | 33.6 | 34.6 | 33.7 | 35.5 | 34.4 | 35.4 | 33.0 | −7.3 | |
| Trunk | 19.0 | 17.7 | 18.6 | 17.5 | 18.6 | 18.9 | 19.4 | 17.2 | −9.5 | |
| Arms (mean) | 2.0 | 1.9 | 2.0 | 1.9 | 2.1 | 1.9 | 1.8 | 1.9 | −5.0 | |
| Legs (mean) | 6.3 | 6.1 | 6.1 | 6.2 | 6.4 | 5.9 | 6.3 | 6.0 | −4.8 | |
| Fat mass (kg) | ||||||||||
| Whole body | 10.3 | 12.9 | 12.9 | 12.1 | 13.9 | 14.1 | 16.3 | 15.9 | 54.4 | |
| Subtotal body | 9.6 | 12.2 | 12.1 | 11.4 | 13.2 | 13.3 | 15.6 | 15.2 | 58.3 | |
| Trunk | 3.7 | 4.9 | 4.7 | 4.6 | 5.3 | 5.4 | 6.2 | 6.3 | 70.3 | |
| Arms (mean) | 0.3 | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 | 0.7 | 0.6 | 100 | |
| Legs (mean) | 2.6 | 3.2 | 3.3 | 3.0 | 3.5 | 3.4 | 4.0 | 3.9 | 50 | |
| Fat mass (%) | 20.3 | 26.0 | 24.6 | 23.9 | 25.6 | 26.4 | 28.8 | 29.7 | 46.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Olivares, A.M.; García-Manso, J.M.; Rodríguez-Gómez, I.; Ara, I.; Lucia, A.; Santalla, A. Long-Term Benefits of Tailored Exercise in Severe Sarcoidosis: A Case Report. Int. J. Environ. Res. Public Health 2020, 17, 9512. https://doi.org/10.3390/ijerph17249512
Herrera-Olivares AM, García-Manso JM, Rodríguez-Gómez I, Ara I, Lucia A, Santalla A. Long-Term Benefits of Tailored Exercise in Severe Sarcoidosis: A Case Report. International Journal of Environmental Research and Public Health. 2020; 17(24):9512. https://doi.org/10.3390/ijerph17249512
Chicago/Turabian StyleHerrera-Olivares, Alba M., Juan M. García-Manso, Irene Rodríguez-Gómez, Ignacio Ara, Alejandro Lucia, and Alfredo Santalla. 2020. "Long-Term Benefits of Tailored Exercise in Severe Sarcoidosis: A Case Report" International Journal of Environmental Research and Public Health 17, no. 24: 9512. https://doi.org/10.3390/ijerph17249512








