Home-Based Resistance Training for Older Subjects during the COVID-19 Outbreak in Italy: Preliminary Results of a Six-Months RCT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants’ Screening
2.3. Exercise Prescription
2.4. Clinical Assessments
2.4.1. Anthropometric Assessment
2.4.2. Whole-Body Dual Energy X-ray Absorptiometry
2.4.3. Magnetic Resonance Imaging Scan
2.4.4. Strength Assessment
2.4.5. Chair Stand Test
2.4.6. Hand Grip Strength Test (HGS)
2.4.7. Maximal Isometric Strength (MIS) of Knee Flexors and Extensors Muscles
2.4.8. Balance and Gait Assessment
2.5. Statistical Analysis
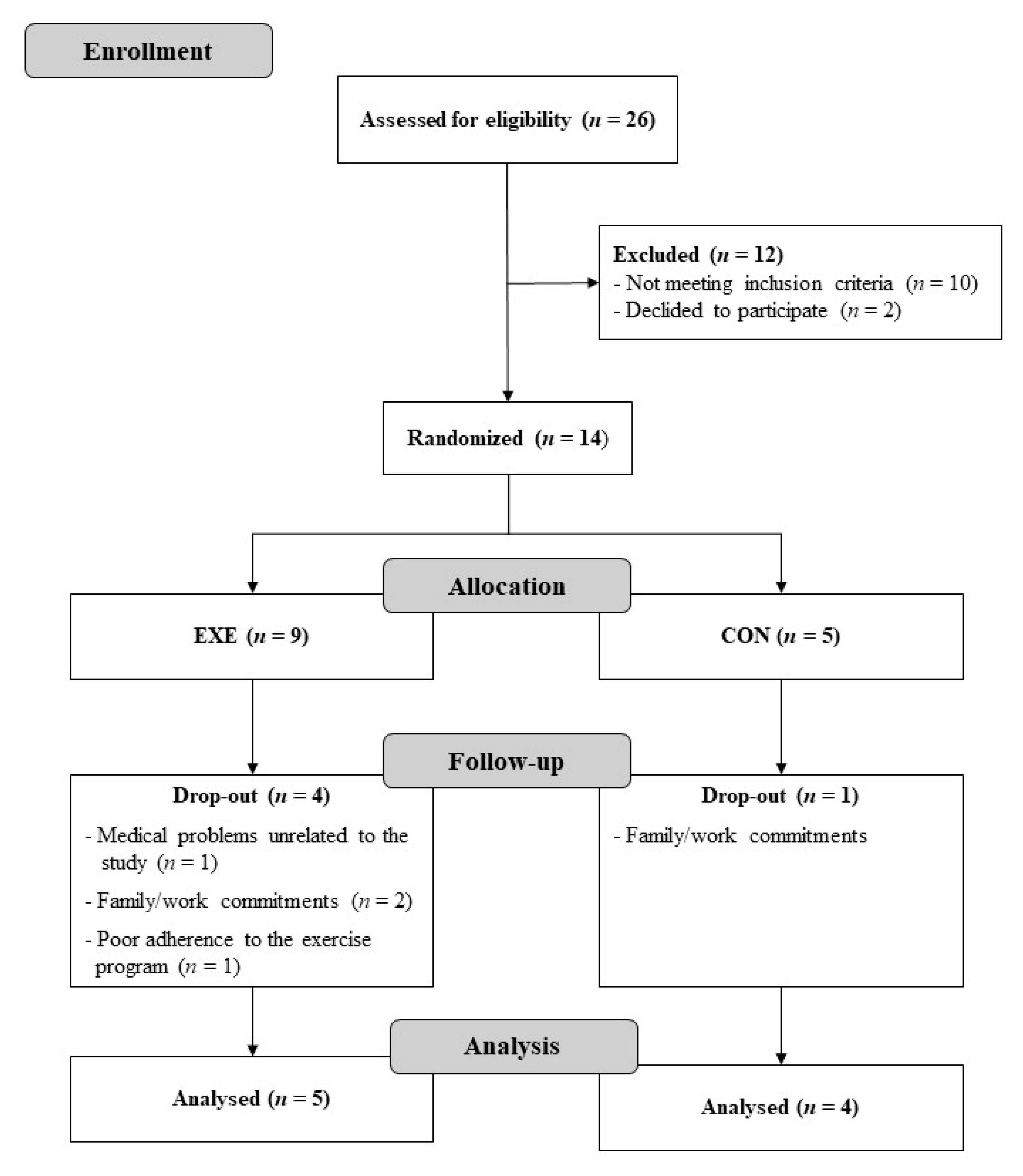
3. Results
3.1. Study Population
3.2. Physical Performance Evaluation
3.3. Body Composition Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Phu, S.; Boersma, D.; Duque, G. Exercise and Sarcopenia. J. Clin. Densitom. 2015, 18, 488–492. [Google Scholar] [CrossRef]
- Aguirre, L.E.; Villareal, D.T. Physical Exercise as Therapy for Frailty. In Frailty: Pathophysiology, Phenotype and Patient Care; Karger Publishers: Basel, Switzerland, 2016; Volume 176, pp. 139–148. [Google Scholar] [CrossRef]
- Messina, C.; Albano, D.; Gitto, S.; Tofanelli, L.; Bazzocchi, A.; Ulivieri, F.M.; Guglielmi, G.; Sconfienza, L.M. Body composition with dual energy X-ray absorptiometry: From basics to new tools. Quant. Imaging Med. Surg. 2020, 10, 1687–1698. [Google Scholar] [CrossRef]
- Blair, S.N.; Sallis, R.E.; Hutber, A.; Archer, E. Exercise therapy—The public health message. Scand. J. Med. Sci. Sport. 2012, 22, e24–e28. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.; Saad Al-Ansari, S.; Biddle, S.; Borodulin, K.; Buman, M.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.P.W.; De Lisio, M.; Parise, G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl. Physiol. Nutr. Metab. 2008, 33, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bonato, M.; Turrini, F.; Galli, L.; Banfi, G.; Cinque, P. The role of physical activity for the management of sarcopenia in people living with HIV. Int. J. Environ. Res. Public Health 2020, 17, 1283. [Google Scholar] [CrossRef] [Green Version]
- Burton, L.A.; Sumukadas, D. Optimal management of sarcopenia. Clin. Interv. Aging 2010, 5, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Vitale, J.A.; Bonato, M.; La Torre, A.; Banfi, G. The role of the molecular clock in promoting skeletal muscle growth and protecting against sarcopenia. Int. J. Mol. Sci. 2019, 20, 4318. [Google Scholar] [CrossRef] [Green Version]
- Johnston, J.D.; Massey, A.P.; Devaneaux, C.A. Innovation in weight loss programs: A 3-dimensional virtual-world approach. J. Med. Internet Res. 2012, 14, e120. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Peterson, C. Healthspan, translation, and new outcomes for animal studies of aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 209–212. [Google Scholar] [CrossRef]
- Kis, O.; Buch, A.; Stern, N.; Moran, D.S. Minimally supervised home-based resistance training and muscle function in older adults: A meta-analysis. Arch. Gerontol. Geriatr. 2019, 84, 103909. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Perazzo, P.; Silingardi, M.; Biffi, M.; Banfi, G.; Negrini, F. Is disruption of sleep quality a consequence of severe Covid-19 infection? A case-series examination. Chronobiol. Int. 2020, 37, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Watanabe, M. The COVID-19 Pandemic in Japan. Surg. Today 2020, 50, 787–793. [Google Scholar] [CrossRef]
- World Health Organization. Mental Health and Psychosocial Considerations during COVID-19 Outbreak. World Health Organ. 2020, 1, 6. [Google Scholar]
- Government of Italy Decree of the President of the Council of Ministers. Available online: https://www.gazzettaufficiale.it/eli/id/2020/03/11/20A01605/sg (accessed on 11 March 2020).
- Cellini, N.; Canale, N.; Mioni, G.; Costa, S. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J. Sleep Res. 2020, 29, e13074. [Google Scholar] [CrossRef]
- Chirico, A.; Lucidi, F.; Galli, F.; Giancamilli, F.; Vitale, J.; Borghi, S.; La Torre, A.; Codella, R. COVID-19 Outbreak and Physical Activity in the Italian Population: A Cross-Sectional Analysis of the Underlying Psychosocial Mechanisms. Front. Psychol. 2020, 11, 2100. [Google Scholar] [CrossRef]
- Machado, C.L.F.; Pinto, R.S.; Brusco, C.M.; Cadore, E.L.; Radaelli, R. COVID-19 pandemic is an urgent time for older people to practice resistance exercise at home. Exp. Gerontol. 2020, 141, 111101. [Google Scholar] [CrossRef]
- Pagano, A.F.; Brioche, T.; Arc-Chagnaud, C.; Demangel, R.; Chopard, A.; Py, G. Short-term disuse promotes fatty acid infiltration into skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 335–347. [Google Scholar] [CrossRef]
- Latham, N.K.; Bennett, D.A.; Stretton, C.M.; Anderson, C.S. Systematic Review of Progressive Resistance Strength Training in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, 48–61. [Google Scholar] [CrossRef]
- World Health Organization. WHO Announces COVID-19 Outbreak a Pandemic. Available online: http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic (accessed on 12 March 2020).
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, 332. [Google Scholar] [CrossRef] [PubMed]
- Bellg, A.J.; Resnick, B.; Minicucci, D.S.; Ogedegbe, G.; Ernst, D.; Borrelli, B.; Hecht, J.; Ory, M.; Orwig, D.; Czajkowski, S. Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004, 23, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Messina, C.; Vitale, J.; Sconfienza, L.M. Imaging of sarcopenia: Old evidence and new insights. Eur. Radiol. 2020, 30, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Maffi, G.; Vitale, J.A.; Ulivieri, F.M.; Guglielmi, G.; Sconfenza, L.M. Diagnostic imaging of osteoporosis and sarcopenia: A narrative review. Quant. Imaging Med. Surg. 2018, 8, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Sergi, G.; Trevisan, C.; Veronese, N.; Lucato, P.; Manzato, E. Imaging of sarcopenia. Eur. J. Radiol. 2016, 85, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Grimm, A.; Meyer, H.; Nickel, M.D.; Nittka, M.; Raithel, E.; Chaudry, O.; Friedberger, A.; Uder, M.; Kemmler, W.; Engelke, K.; et al. Repeatability of Dixon magnetic resonance imaging and magnetic resonance spectroscopy for quantitative muscle fat assessments in the thigh. J. Cachexia Sarcopenia Muscle 2018, 9, 1093–1100. [Google Scholar] [CrossRef]
- Grimm, A.; Meyer, H.; Nickel, M.D.; Nittka, M.; Raithel, E.; Chaudry, O.; Friedberger, A.; Uder, M.; Kemmler, W.; Quick, H.H.; et al. Evaluation of 2-point, 3-point, and 6-point Dixon magnetic resonance imaging with flexible echo timing for muscle fat quantification. Eur. J. Radiol. 2018, 103, 57–64. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Günther, C.M.; Bürger, A.; Rickert, M.; Crispin, A.; Schulz, C.U. Grip Strength in Healthy Caucasian Adults: Reference Values. J. Hand Surg. Am. 2008, 33, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Katoh, M.; Gomi, M.; Arai, S. Validity and reliability of isometric knee extension muscle strength measurements using a belt-stabilized hand-held dynamometer: A comparison with the measurement using an isokinetic dynamometer in a sitting posture. J. Phys. Ther. Sci. 2020, 32, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, R.L.; Den Hartog, R.; Yochum, K.; Pearlmutter, L.; Ruttinger, A.C.; Mooradian, A.D. A Comparison of Hand-Held Isometric Strength Measurement with Isokinetic Muscle Strength Measurement in the Elderly. J. Am. Geriatr. Soc. 1993, 41, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Banfi, G.; Belli, E.; Negrini, F.; la Torre, A. A 9-month multidisciplinary rehabilitation protocol based on early postoperative mobilization following a chronic-degenerative patellar tendon rupture in a professional soccer player. Eur. J. Phys. Rehabil. Med. 2019, 55, 676–681. [Google Scholar] [CrossRef]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The balance evaluation systems test (BESTest) to differentiate balance deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using psychometric techniques to improve the balance evaluation systems test: The mini-bestest. J. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Hamre, C.; Botolfsen, P.; Tangen, G.G.; Helbostad, J.L. Interrater and test-retest reliability and validity of the Norwegian version of the BESTest and mini-BESTest in people with increased risk of falling. BMC Geriatr. 2017, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, W.G. Spreadsheets for Analysis of Controlled Trials, with Adjustment for a Subject Characteristic. Sportscience 2006, 10, 46–50. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Italian Ministry of Health COVID-19 Situazione Italia. Available online: http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1 (accessed on 12 November 2020).
- Gutin, B.; Yin, Z.; Humphries, M.C.; Barbeau, P. Relations of moderate and vigorous physical activity to fitness and fatness in adolescents. Am. J. Clin. Nutr. 2005, 81, 746–750. [Google Scholar] [CrossRef]
- Huremović, D. Psychiatry of Pandemics: A Mental Health Response to Infection Outbreak; Springer: Berlin, Germany, 2019; ISBN 9783030153458. [Google Scholar]
- Bryson, W.J. Circadian rhythm sleep-wake disorders and the COVID-19 pandemic. J. Clin. Sleep Med. 2020, 16, 1423. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Burtscher, M. Run for your life: Tweaking the weekly physical activity volume for longevity. Br. J. Sports Med. 2020, 54, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.D.; Hunter, S.W.; Batchelor, F.A.; Cavalheri, V.; Burton, E. Individualized home-based exercise programs for older people to reduce falls and improve physical performance: A systematic review and meta-analysis. Maturitas 2015, 82, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Gates, S.; Ealing, E. Reporting and interpretation of results from clinical trials that did not claim a treatment difference: Survey of four general medical journals. BMJ Open 2019, 9, e024785. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, C.; O’Sullivan, R.; Caserotti, P.; Tully, M.A. Consequences of physical inactivity in older adults: A systematic review of reviews and meta-analyses. Scand. J. Med. Sci. Sport. 2020, 30, 816–827. [Google Scholar] [CrossRef]
- López-Sánchez, G.F.; López-Bueno, R.; Gil-Salmerón, A.; Zauder, R.; Skalska, M.; Jastrzębska, J.; Jastrzębski, Z.; Schuch, F.B.; Grabovac, I.; Tully, M.A.; et al. Comparison of physical activity levels in Spanish adults with chronic conditions before and during COVID-19 quarantine. Eur. J. Public Health 2020, 1–6. [Google Scholar] [CrossRef]

| Exercise Type | Number of Sets | Number of Repetitions | Resting Time | Exercise Progression | Load | |
|---|---|---|---|---|---|---|
| Sitting on- and standing from a chair |  | 4 | 15 | 60–90 s | increase of sets (up to n = 5) and/or repetitions (up to n = 20) and/or squat exercise (no chair) and/or use of two bottles as extra-weights | Body weight |
| Leg adduction from a standing position |  | 4 | 15 | 60–90 s | increase of sets (up to n = 5) and/or repetitions (up to n = 20) and/or use of sport anklets as extra-weights | Body weight Sport anklets |
| Leg abduction from a standing position |  | 4 | 15 | 60–90 s | increase of sets (up to n = 5) and/or repetitions (up to n = 20) and/or use of sport anklets as extra-weights | Body weight Sport anklets |
| Calf exercise from a sitting position |  | 4 | 15 | 60–90 s | increase of sets (up to n = 5) and/or repetitions (up to n = 20) and/or execution from a standing position | Body weight |
| Monopodalic balance exercise from a standing position with external support |  | 3 | 30 s | 60–90 s | increase of sets (up to n = 4) and/or time of work (up to 45 secs) and/or execution with closed eyes | Body weight |
| Side raises from a sitting position |  | 3 | 12 | 60–90 s | increase of sets (up to n= 4) and/or repetitions (up to n = 20) and/or execution from a standing position and/or use of two larger bottles as extra-weights | Bottles |
| Biceps curl from a sitting position |  | 3 | 12 | 60–90 s | increase of sets (up to n = 4) and/or repetitions (up to n = 20) and/or execution from a standing position and/or use of two larger bottles as extra-weights | Bottles |
| Triceps curl from a sitting position |  | 3 | 12 | 60–90 s | increase of sets (up to n = 4) and/or repetitions (up to n = 20) and/or execution from a standing position and/or use of two larger bottles as extra-weights | Bottles |
| Variable | Total (n = 9) | EXE (n = 5) | CON (n = 4) | p | ES |
|---|---|---|---|---|---|
| Male (n, %) | 3, 33% | 3, 60% | 0, 0% | 0.167 | n.a. |
| Age (years) | 68 ± 7 (62.9–73.1) | 66 ± 4 (60.8–71.2) | 71 ± 9 (56.5–84.5) | 0.342 | 1.25 |
| Height (m) | 1.64 ± 0.08 (1.55–1.71) | 1.67 ± 0.09 (1.56–1.78) | 1.61 ± 0.09 (1.42–1.74) | 0.196 | 0.6 |
| Body mass (kg) | 70 ± 16 (57.4–81.7) | 77 ± 17 (55.9–98.2) | 60 ± 6 (50.4–69.2) | 0.095 | 1.0 |
| BMI (kg/m2) | 26.0 ± 4.0 (22.9–29.1) | 27.5 ± 3.7 (22.9–32.1) | 24.2 ± 4.1 (17.6–30.7) | 0.237 | 0.9 |
| Handgrip strength (kg) | 27.4 ± 6.8 (22.2–32.8) | 30.4 ± 7.5 (21.17–39.7) | 23.8 ± 4.3 (16.9–30.66) | 0.236 | 0.9 |
| ASMMI (kg/m2) | 7.0 ± 1.4 (5.9–8.1) | 7.8 ± 1.3 (6.1–9.5) | 6.0 ± 0.6 (5.2–6.9) | 0.045 | 1.4 |
| Mini-BESTest | 25 ± 3 (23–27) | 25 ± 3 (22–28) | 25 ± 4 (19–30) | 0.981 | <0.2 |
| Variable | Within-Group Differences | Between-Group Differences | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EXE | CON | EXE | CON | |||||||||
| PRE | POST | p | ES | PRE | POST | p | ES | Δ ± SD | Δ ± SD | p | ES | |
| Chair-stand-test (rep) | 14 ± 2 (11–16) | 16 ± 3 (13–19) | 0.048 | 1.0 | 14 ± 4 (7–19) | 15 ± 3 (10–20) | 0.215 | 0.3 | 2.6 ± 2.1 (0.1–5.2) | 1.5 ± 1.9 (−1.5–4.5) | 0.441 | 0.5 |
| Handgrip strength (kg) | 30.4 ± 7.5 (21.2–39.7) | 28.2 ± 9.7 (16.1–40.2) | 0.417 | 0.3 | 23.8 ± 4.3 (16.9–39.7) | 23.3 ± 3.6 (17.6–29.0) | 0.468 | ≤0.2 | −2.3 ± 5.6 (−9.2–4.6) | −0.5 ± 1.3 (−2.4–1.5) | 0.556 | 0.4 |
| Mini-BESTest (score) | 25 ± 3 (22–28) | 26 ± 1 (25–27) | 0.260 | 0.3 | 25 ± 4 (18.6–30.4) | 25 ± 3 (20.2–29.3) | 0.637 | ≤0.2 | 1.2 ± 2.1 (−1.4–3.7) | 0.3 ± 0.9 (−1.3–1.8) | 0.425 | 0.4 |
| Thigh extensors strength (n) | 333 ± 96 (214–453) | 329 ± 93 (213–445) | 0.825 | ≤0.2 | 242 ± 109 (68.5–415.3) | 264 ± 102 (102.2–426.4) | 0.591 | 0.2 | −4.4 ± 42.3 (−57.0–48.2) | −1.5 ± 48.2 (−78.2–75.2) | 0.926 | ≤0.2 |
| Thigh flexors strength (n) | 176 ± 49 (115–237) | 178 ± 51 (115–241) | 0.931 | ≤0.2 | 164 ± 43 (96.1–231.3) | 140 ± 44 (70.3–208.7) | 0.09 | 0.6 | 2.1 ± 49.9 (−59.9–64.1) | −35.3 ± 14.9 (−59.1–11.6) | 0.272 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, J.A.; Bonato, M.; Borghi, S.; Messina, C.; Albano, D.; Corbetta, S.; Sconfienza, L.M.; Banfi, G. Home-Based Resistance Training for Older Subjects during the COVID-19 Outbreak in Italy: Preliminary Results of a Six-Months RCT. Int. J. Environ. Res. Public Health 2020, 17, 9533. https://doi.org/10.3390/ijerph17249533
Vitale JA, Bonato M, Borghi S, Messina C, Albano D, Corbetta S, Sconfienza LM, Banfi G. Home-Based Resistance Training for Older Subjects during the COVID-19 Outbreak in Italy: Preliminary Results of a Six-Months RCT. International Journal of Environmental Research and Public Health. 2020; 17(24):9533. https://doi.org/10.3390/ijerph17249533
Chicago/Turabian StyleVitale, Jacopo Antonino, Matteo Bonato, Stefano Borghi, Carmelo Messina, Domenico Albano, Sabrina Corbetta, Luca Maria Sconfienza, and Giuseppe Banfi. 2020. "Home-Based Resistance Training for Older Subjects during the COVID-19 Outbreak in Italy: Preliminary Results of a Six-Months RCT" International Journal of Environmental Research and Public Health 17, no. 24: 9533. https://doi.org/10.3390/ijerph17249533
APA StyleVitale, J. A., Bonato, M., Borghi, S., Messina, C., Albano, D., Corbetta, S., Sconfienza, L. M., & Banfi, G. (2020). Home-Based Resistance Training for Older Subjects during the COVID-19 Outbreak in Italy: Preliminary Results of a Six-Months RCT. International Journal of Environmental Research and Public Health, 17(24), 9533. https://doi.org/10.3390/ijerph17249533








