Coupling Wearable Devices and Decision Theory in the United States Emergency Department Triage Process: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shortcomings of the US Triage Process
2.1.1. Patient Monitoring
2.1.2. Subjective Triage Assessment and Uncertainty
2.1.3. First-Come-First-Serve Prioritization within Groups
2.1.4. Nurse Decision Making and Lack of Feedback
2.2. Continuous Patient Monitoring
2.3. Wearable Devices
2.4. Patient Prioritization Systems
3. Results
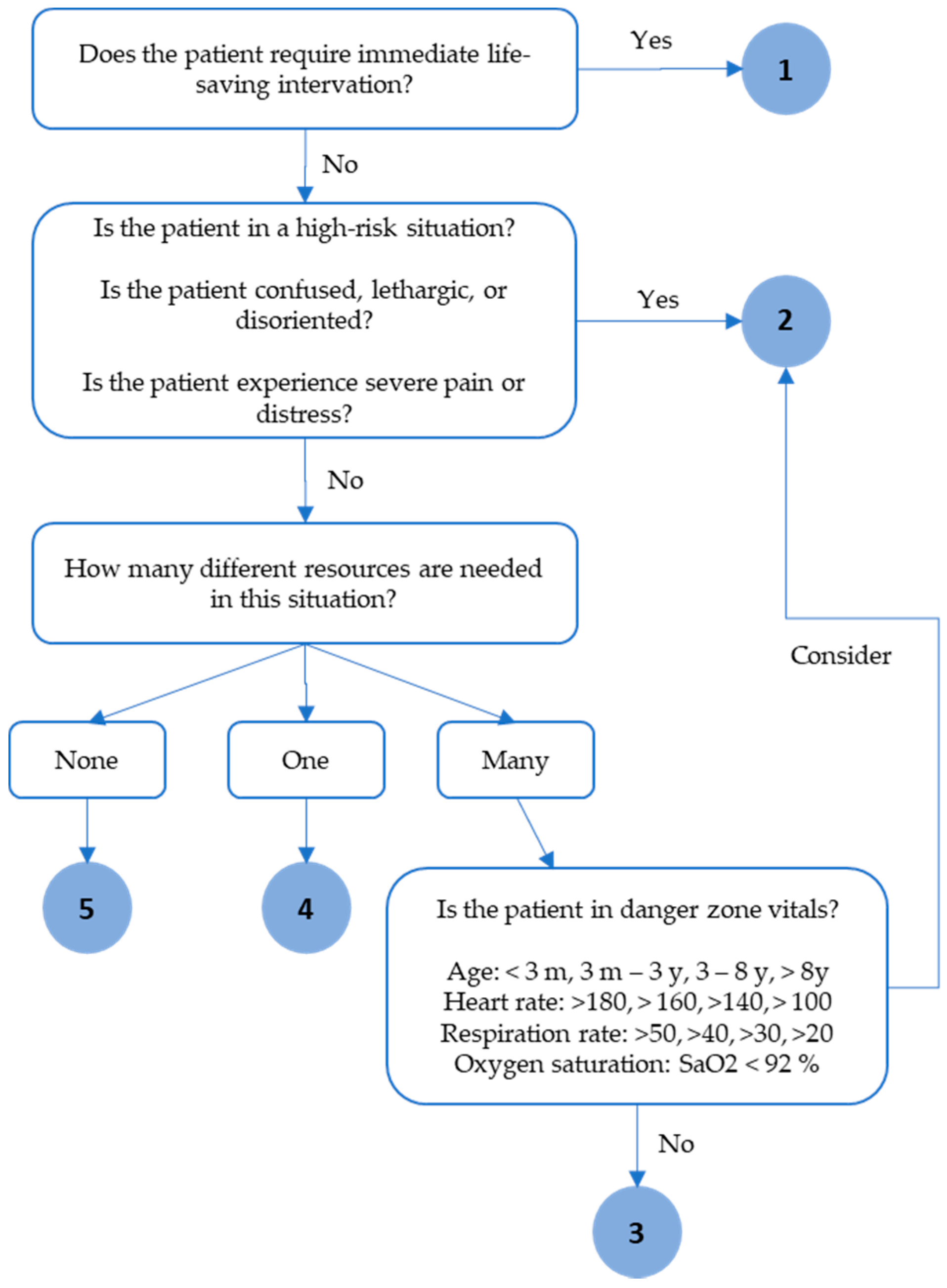
3.1. Proposed Clinical Decision Support Model
3.1.1. Proposed System Architecture
3.1.2. Proposed Mathematical Decision Model
4. Discussion
4.1. Feasibility of the Proposed Clinical Decision Support Model
4.1.1. Hospital Liability
4.1.2. Quality of Data
4.1.3. Response to Data and Workload Impacts
4.1.4. Technology Acceptance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dugas, A.F.; Kirsch, T.D.; Toerper, M.; Korley, F.; Yenokyan, G.; France, D.; Hager, D.; Levin, S. An electronic emergency triage system to improve patient distribution by critical outcomes. J. Emerg. Med. 2016, 50, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Ashour, O.; Kremer, G. Dynamic patient grouping and prioritization: A new approach to emergency department flow improvement. Health Care Manag. Sci. 2016, 19, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Eitel, D.R.; Travers, D.A.; Rosenau, A.M.; Gilboy, N.; Wuerz, R.C. The emergency severity index triage algorithm version 2 is reliable and valid. Acad. Emerg. Med. 2003, 10, 1070–1080. [Google Scholar] [CrossRef]
- Considine, J.; LeVasseur, S.A.; Charles, A. Development of physiological discriminators for the Australasian Triage Scale. Accid. Emerg. Nurs. 2002, 10, 221–234. [Google Scholar] [CrossRef]
- Beveridge, R. The Canadian Emergency Department Triage and Acuity Scale: A new and critical element in health care reform. J. Emerg. Med. 1998, 16, 507–511. [Google Scholar] [PubMed]
- Group, M.T. Emergency Triage; BMJ Publishing Group: Manchester, UK, 1997. [Google Scholar]
- Gilboy, N.; Tanabe, P.; Travers, D.; Rosenau, A.M. Emergency Severity Index (ESI): A Triage Tool for Emergency Department Care, Version 4; Implementation handbook 2012 ed.; AHRQ: Rockville, MD, USA, 2011.
- Tanabe, P.; Gimbel, R.; Yarnold, P.R.; Kyriacou, D.N.; Adams, J.G. Reliability and validity of scores on The Emergency Severity Index version 3. Acad. Emerg. Med. 2004, 11, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Travers, D.A.; Waller, A.E.; Bowling, J.M.; Flowers, D.; Tintinalli, J. Five-level triage system more effective than three-level in tertiary emergency department. J. Emerg. Nurs. 2002, 28, 395–400. [Google Scholar] [CrossRef]
- Johnson, K.D.; Mueller, L.; Winkelman, C. The nurse response to abnormal vital sign recording in the emergency department. J. Clin. Nurs. 2017, 26, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Gilboy, N.; Tanabe, P.; Travers, D.; Rosenau, A.M.; Eitel, D.R. Emergency Severity Index, Version 4: Implementation Handbook; AHRQ Publication No. 05-0046-2; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2005.
- Singer, R.F.; Infante, A.A.; Oppenheimer, C.C.; West, C.A.; Siegel, B. The Use of and Satisfaction with the Emergency Severity Index. J. Emerg. Nurs. 2012, 38, 120–126. [Google Scholar] [CrossRef]
- Claudio, D.; Kremer, G.E.O.; Bravo-Llerena, W.; Freivalds, A. A dynamic multi-attribute utility theory–based decision support system for patient prioritization in the emergency department. IIE Trans. Healthc. Syst. Eng. 2014, 4, 1–15. [Google Scholar] [CrossRef]
- Claudio, D. Utility function-based patient prioritization in the emergency department. Eur. J. Ind. Eng. 2010, 4, 59–77. [Google Scholar] [CrossRef]
- Ryynänen, O.-P.; Myllykangas, M.; Kinnunen, J.; Takala, J. Attitudes to health care prioritisation methods and criteria among nurses, doctors, politicians and the general public. Soc. Sci. Med. 1999, 49, 1529–1539. [Google Scholar] [CrossRef]
- Dong, S.L.; Bullard, M.J.; Meurer, D.P.; Blitz, S.; Holroyd, B.R.; Rowe, B.H. The effect of training on nurse agreement using an electronic triage system. Can. J. Emerg. Med. 2007, 9, 260–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loken, E.; Botterud, A.; Holen, A.T. Decision Analysis and Uncertainties in Planning Local Energy Systems. In Proceedings of the 2006 International Conference on Probabilistic Methods Applied to Power Systems, Stockholm, Sweden, 11–15 June 2006; pp. 1–8. [Google Scholar]
- Hellinger, F.J. Expected Utility Theory and Risky Choices with Health Outcomes. Med. Care 1989, 27, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M. Triage and emergency department services. Ann. Emerg. Med. 1996, 27, 506–508. [Google Scholar] [CrossRef]
- Gurney, D. Exercises in Critical Thinking at Triage: Prioritizing Patients With Similar Acuities. J. Emerg. Nurs. 2004, 30, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Claudio, D.; Ricondo, L.; Freivalds, A.; Okudan, G. Physiological and descriptive variables as predictors for the Emergency Severity Index. In Trans. Healthc. Syst. Eng. 2012, 2, 131–141. [Google Scholar] [CrossRef]
- Lake, S.; Moss, C.; Duke, J. Nursing prioritization of the patient need for care: A tacit knowledge embedded in the clinical decision-making literature. Int. J. Nurs. Pract. 2009, 15, 376–388. [Google Scholar] [CrossRef]
- Patel, V.L.; Gutnik, L.A.; Karlin, D.R.; Pusic, M. Calibrating urgency: Triage decision-making in a pediatric emergency department. Adv. Health Sci. Educ. 2008, 13, 503–520. [Google Scholar] [CrossRef]
- Andersson, A.-K.; Omberg, M.; Svedlund, M. Triage in the emergency department—A qualitative study of the factors which nurses consider when making decisions. Nurs. Crit. Care 2006, 11, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Benner, P.; Tanner, C. Clinical judgment: How expert nurses use intuition. Ajn Am. J. Nurs. 1987, 87, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Cone, K.J.; Murray, R. Characteristics, insights, decision making, and preparation of ED triage nurses. J. Emerg. Nurs. 2002, 28, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Pott, C.; Cnossen, F.; Ballast, A. More than psychologists’ chitchat: The importance of cognitive modelling for requirements engineering in complex and dynamic environments. Situat. Requir. Eng. Process. 2005, 163–175. [Google Scholar]
- Maningas, P.A.; Hime, D.A.; Parker, D.E.; McMurry, T.A. The Soterion Rapid Triage System: Evaluation of inter-rater reliability and validity. J. Emerg. Med. 2006, 30, 461–469. [Google Scholar] [CrossRef]
- Jepson, Z.K.; Darling, C.E.; Kotkowski, K.A.; Bird, S.B.; Arce, M.W.; Volturo, G.A.; Reznek, M.A. Emergency department patient safety incident characterization: An observational analysis of the findings of a standardized peer review process. BMC Emerg. Med. 2014, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Berk, W.A.; Welch, R.D.; Levy, P.D.; Jones, J.T.; Arthur, C.; Kuhn, G.J.; King, J.J.; Bock, B.F.; Sweeny, P.J. The Effect of Clinical Experience on the Error Rate of Emergency Physicians. Ann. Emerg. Med. 2008, 52, 497–501. [Google Scholar] [CrossRef]
- Morby, S.K.; Skalla, A. A Human Care Approach to Nursing Peer Review. Nurs. Sci. Q. 2010, 23, 297–300. [Google Scholar] [CrossRef]
- George, V.; Haag-Heitman, B. Nursing peer review: The manager’s role. J. Nurs. Manag. 2011, 19, 254–259. [Google Scholar] [CrossRef]
- Corner, J.L.; Buchanan, J.T. Capturing decision maker preference: Experimental comparison of decision analysis and MCDM techniques. Eur. J. Oper. Res. 1997, 98, 85–97. [Google Scholar] [CrossRef]
- McGrath, S.P.; Grigg, E.; Wendelken, S.; Blike, G.; De Rosa, M.; Fiske, A.; Gray, R. ARTEMIS: A Vision for Remote Triage and Emergency Management Information Integration. Dartm. Univ. 2003, 9, 1–9. [Google Scholar]
- Killeen, J.P.; Chan, T.C.; Buono, C.; Griswold, W.G.; Lenert, L.A. A Wireless First Responder Handheld Device for Rapid Triage, Patient Assessment and Documentation during Mass Casualty Incidents. Amia Annu. Symp. Proc. 2006, 2006, 429–433. [Google Scholar]
- Lenert, L.A.; Palmer, D.A.; Chan, T.C.; Rao, R. An Intelligent 802.11 Triage Tag For Medical Response to Disasters. Amia Annu. Symp. Proc. 2005, 2005, 440–444. [Google Scholar]
- Palmer, D.A.; Rao, R.; Lenert, L.A. An 802.11 wireless blood pulse-oximetry system for medical response to disasters. In AMIA 2005 Annual Symposium Proceedings; AMIA Symposium: Washington, DC, USA, 2005; p. 1072. [Google Scholar]
- Smalls, J.; Yue, W.; Xi, L.; Zehuang, C.; Tang, K.W. Health monitoring systems for massive emergency situations. In Proceedings of the 2009 IEEE Long Island Systems, Applications and Technology Conference, Farmingdale, NY, USA, 1–1 May 2009; pp. 1–11. [Google Scholar]
- Gao, T.; Greenspan, D.; Welsh, M.; Juang, R.R.; Alm, A. Vital Signs Monitoring and Patient Tracking Over a Wireless Network. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 1–4 September 2005; pp. 102–105. [Google Scholar]
- Booth, P.; Frisch, P.H.; Miodownik, S. Application of RFID in an Integrated Healthcare Environment. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York City, NY, USA, 30 August–3 September 2006; pp. 117–119. [Google Scholar]
- Curtis, D.W.; Pino, E.J.; Bailey, J.M.; Shih, E.I.; Waterman, J.; Vinterbo, S.A.; Stair, T.O.; Guttag, J.V.; Greenes, R.A.; Ohno-Machado, L. SMART—An Integrated Wireless System for Monitoring Unattended Patients. J. Am. Med. Inform. Assoc. 2008, 15, 44–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.J.; Ferrin, D.M.; Flynn, T.; Ashby, M.; White, K.P.; Mauer, M.G. Using RFID technologies to capture simulation data in a hospital emergency department. In Proceedings of the 38th conference on Winter simulation, Monterey, CA, USA, 3–6 December 2006; pp. 1365–1370. [Google Scholar]
- Choi, J.H.; Kim, D.K. A Remote Compact Sensor for the Real-Time Monitoring of Human Heartbeat and Respiration Rate. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 181–188. [Google Scholar] [CrossRef]
- Dae Cha, Y.; Yoon, G. Ubiquitous Health Monitoring System for Multiple Users Using a ZigBee and WLAN Dual-Network. Telemed. E-Health 2009, 15, 891–897. [Google Scholar] [CrossRef]
- Ichihashi, F.; Sankai, Y. Development of a Portable Vital Sensing System for Home Telemedicine. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; pp. 5872–5877. [Google Scholar]
- Mendoza, P.; Gonzalez, P.; Villanueva, B.; Haltiwanger, E.; Nazeran, H. A Web-based vital sign telemonitor and recorder for telemedicine applications. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–4 September 2004; pp. 2196–2199. [Google Scholar]
- Tay, F.E.H.; Guo, D.G.; Xu, L.; Nyan, M.N.; Yap, K.L. MEMSWear-biomonitoring system for remote vital signs monitoring. J. Frankl. Inst. 2009, 346, 531–542. [Google Scholar] [CrossRef]
- Wenxi, C.; Wei, D.; Xin, Z.; Uchida, M.; Ding, S.; Cohen, M. A mobile phone-based wearable vital signs monitoring system. In Proceedings of the Fifth International Conference on Computer and Information Technology (CIT’05), Shanghai, China, 21–23 September 2005; pp. 950–955. [Google Scholar]
- Dittmar, A.; Meffre, R.; Oliveira, F.D.; Gehin, C.; Delhomme, G. Wearable Medical Devices Using Textile and Flexible Technologies for Ambulatory Monitoring. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 31 August–3 September 2005; pp. 7161–7164. [Google Scholar]
- Baldassarre, A.; Mucci, N.; Lecca, L.I.; Tomasini, E.; Parcias-do-Rosario, M.J.; Pereira, C.T.; Arcangeli, G.; Oliveira, P.A.B. Biosensors in Occupational Safety and Health Management: A Narrative Review. Int. J. Environ. Res. Public Health 2020, 17, 2461. [Google Scholar] [CrossRef] [Green Version]
- Schall, M.C.; Sesek, R.F.; Cavuoto, L.A. Barriers to the Adoption of Wearable Sensors in the Workplace: A Survey of Occupational Safety and Health Professionals. Hum. Factors 2018, 60, 351–362. [Google Scholar] [CrossRef]
- Tom, P. A Forecast of the Adoption of Wearable Technology. Int. J. Technol. Diffus. 2015, 6, 12–29. [Google Scholar] [CrossRef] [Green Version]
- Pantelopoulos, A.; Bourbakis, N.G. A Survey on Wearable Sensor-Based Systems for Health Monitoring and Prognosis. IEEE Trans. Syst. Manand Cybern. Part C 2010, 40, 1–12. [Google Scholar] [CrossRef] [Green Version]
- DeVaul, R.; Sung, M.; Gips, J.; Pentland, A. MIThril 2003: Applications and architecture. In Proceedings of the ISWC 2003: Seventh IEEE International Symposium on Wearable Computers, White Plains, NY, USA, 21–23 October 2003; pp. 4–11. [Google Scholar]
- Anliker, U.; Ward, J.A.; Lukowicz, P.; Troster, G.; Dolveck, F.; Baer, M.; Keita, F.; Schenker, E.B.; Catarsi, F.; Coluccini, L.; et al. AMON: A wearable multiparameter medical monitoring and alert system. IEEE Trans. Inf. Technol. Biomed. 2004, 8, 415–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K. A Black Box for people. Available online: https://www.nasa.gov/vision/space/livinginspace-/07apr_blackbox.html (accessed on 22 June 2017).
- Habetha, J. MyHeart—Fighting Cardio-Vascular Diseases by Prevention & Early Diagnosis. Available online: http://ec.europa.eu/information_society/doc/015-ist-myheart.pdf (accessed on 22 June 2017).
- Paradiso, R. WEALTHY-a wearable healthcare system: New frontier on e-textile. J. Telecommun. Inf. Technol. 2005, 4, 105–113. [Google Scholar]
- Rienzo, M.D.; Rizzo, F.; Parati, G.; Brambilla, G.; Ferratini, M.; Castiglioni, P. MagIC System: A New Textile-Based Wearable Device for Biological Signal Monitoring. Applicability in Daily Life and Clinical Setting. In Proceedings of 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 31 August–3 September 2005; pp. 7167–7169. [Google Scholar]
- Bernard, F.X. Final Report Summary—MERMOTH (Medical Remote Monitoring of Clothes). Available online: http://cordis.europa.eu/result/rcn/51735_en.html (accessed on 22 June 2017).
- Malan, D.; Fulford-Jones, T.; Welsh, M.; Moulton, S. Code blue: An ad hoc sensor network infrastructure for emergency medical care. Int. Workshop Wearable Implant. Body Sens. Netw. 2004, 5, 1–5. [Google Scholar]
- Lee, S.; Chung, W. A robust wearable u-healthcare platform in wireless sensor network. J. Commun. Netw. 2014, 16, 465–474. [Google Scholar] [CrossRef]
- Pop, V.; de Francisco, R.; Pflug, H.; Santana, J.; Visser, H.; Vullers, R.; de Groot, H.; Gyselinckx, B. Human++: Wireless autonomous sensor technology for body area networks. In Proceedings of the 16th Asia and South Pacific Design Automation Conference, Yokohama, Japan, 25–28 January 2011; pp. 561–566. [Google Scholar]
- Zhanpeng, J.; Oresko, J.; Shimeng, H.; Cheng, A.C. HeartToGo: A Personalized medicine technology for cardiovascular disease prevention and detection. In Proceedings of the 2009 IEEE/NIH Life Science Systems and Applications Workshop, Bethesda, MA, USA, 8–9 November 2007; pp. 80–83. [Google Scholar]
- Katsis, C.D.; Ganiatsas, G.; Fotiadis, D.I. An integrated telemedicine platform for the assessment of affective physiological states. Diagn. Pathol. 2006, 1, 16. [Google Scholar] [CrossRef] [Green Version]
- Heilman, K.J.; Porges, S.W. Accuracy of the LifeShirt® (Vivometrics) in the detection of cardiac rhythms. Biol. Psychol. 2007, 75, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Zephyr™ Performance Systems: Overview. Available online: https://www.zephyranywhere.com/sys-tem/overview (accessed on 22 June 2017).
- ViSi Mobile—For Clinicians. Available online: http://www.soterawireless.com/visi-mobile/for-clinicians/ (accessed on 22 June 2017).
- EquiVital—Sense&Transmit. Available online: http://www.equivital.co.uk/products/tnr/sense-and-transmit (accessed on 22 June 2017).
- Intelesens—Hospital Monitoring. Available online: http://intelesens.com/hospital-monitoring (accessed on 22 June 2017).
- Donnelly, N.; Harper, R.; McCAnderson, J.; Branagh, D.; Kennedy, A.; Caulfield, M.; McLaughlin, J. Development of a ubiquitous clinical monitoring solution to improve patient safety and outcomes. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 6068–6073. [Google Scholar]
- Catherwood, P.A.; Donnelly, N.; Anderson, J.; McLaughlin, J. ECG motion artefact reduction improvements of a chest-based wireless patient monitoring system. In Proceedings of the 2010 Computing in Cardiology, Belfast, UK, 26–29 September 2010; pp. 557–560. [Google Scholar]
- Matthews, R.; McDonald, N.J.; Hervieux, P.; Turner, P.J.; Steindorf, M.A. A Wearable Physiological Sensor Suite for Unobtrusive Monitoring of Physiological and Cognitive State. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; pp. 5276–5281. [Google Scholar]
- Alive Bluetooth Heart & Activity Monitor. Available online: http://www.alivetec.com/alive-bluetooth-heart-activity-monitor/ (accessed on 22 June 2017).
- BodyGuardian Heart. Available online: http://www.preventicesolutions.com/services/body-guardian-heart.html (accessed on 22 June 2017).
- Caretaker—The World’s Most Innovative Wireless Patient Monitoring System. Available online: http://www.caretakermedical.net/caretaker/ (accessed on 22 June 2017).
- Kohli, R.; Piontek, F. DSS in healthcare: Advances and opportunities. In Handbook on Decision Support Systems 2; Springer: Berlin, Germany, 2008; pp. 483–497. [Google Scholar]
- Claudio, D.; Bravo-Llerena, W.; Okudan, G.; Freivalds, A. Waiting in the Emergency Department: Dynamics of Patients’ Vital Signs. In Proceedings of the IIE Annual Conference, Nevada, NV, USA, 21–25 May 2011; pp. 1–7. [Google Scholar]
- Day, A.; Oldroyd, C. The Use of Early Warning Scores in the Emergency Department. J. Emerg. Nurs. 2010, 36, 154–155. [Google Scholar] [CrossRef]
- Wolf, L.D.; Potter, P.; Sledge, J.A.; Boxerman, S.B.; Grayson, D.; Evanoff, B. Describing Nurses’ Work: Combining Quantitative and Qualitative Analysis. Hum. Factors 2006, 48, 5–14. [Google Scholar] [CrossRef]
- Fernandes, C.M.B.; Tanabe, P.; Gilboy, N.; Johnson, L.A.; McNair, R.S.; Rosenau, A.M.; Sawchuk, P.; Thompson, D.A.; Travers, D.A.; Bonalumi, N.; et al. Five-Level Triage: A Report from the ACEP/ENA Five-Level Triage Task Force. J. Emerg. Nurs. 2005, 31, 39–50. [Google Scholar] [CrossRef]
- Wuerz, R.C.; Milne, L.W.; Eitel, D.R.; Travers, D.; Gilboy, N. Reliability and Validity of a New Five-level Triage Instrument. Acad. Emerg. Med. 2008, 7, 236–242. [Google Scholar] [CrossRef]
- IOM Report: The Future of Emergency Care in the United States Health System. Acad. Emerg. Med. 2008, 13, 1081–1085. [CrossRef] [Green Version]
- Dying Man Robbed in Philadelphia er Waiting Room; The Associated Press: New York, NY, USA, 2009.
- Lei, H.-H. Woman Dies of Heart Attack in ER Waiting Room; ABC News: Lake County, IL, USA, 2006. [Google Scholar]
- Derlet, R.W. Triage and ED Overcrowding: Two Cases of Unexpected Outcome. Calif. J. Emerg. Med. 2002, 3, 8–9. [Google Scholar]
- Keeney, R.L.; Raiffa, H. Decisions with Multiple Objectives: Preferences and Value Trade-Offs; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Chan, C.-L.; Chang, C.-C. A method combining MAU and fuzzy logic for cooperative decision making. Comput. Ind. Eng. 1998, 35, 291–294. [Google Scholar] [CrossRef]
- Thevenot, H.J.; Steva, E.D.; Okudan, G.E.; Simpson, T.W. A Multiattribute Utility Theory-Based Method for Product Line Selection. J. Mech. Des. 2007, 129, 1179–1184. [Google Scholar] [CrossRef]
- Mohamedpour, M.; Asgharizadeh, E.; Mohamedpour, K. Comparison of the research units of ICT using MAUT via MBSC. In Proceedings of the IEEE International Conference of Information and Comunication Technology, Damascus, Syria, 7–11 April 2008; pp. 1–5. [Google Scholar]
- Cuthbertson, B.H.; Boroujerdi, M.; McKie, L.; Aucott, L.; Prescott, G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit. Care Med. 2007, 35, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Ashour, O.; Okudan, G. A simulation analysis of the impact of FAHP-MAUT triage algorithm on the Emergency Department performance measures. Expert Syst. Appl. 2013, 40, 177–187. [Google Scholar] [CrossRef]
- Ashour, O.; Okudan, G. Fuzzy AHP and utility theory based patient sorting in emergency departments. Int. J. Collab. Enterp. 2010, 1, 332. [Google Scholar] [CrossRef]
- Kulak, O.; Durmusoglu, M.B.; Tufekci, S. A complete cellular manufacturing system design methodology based on axiomatic design principles. Comput Ind. Eng. 2005, 48, 765–787. [Google Scholar] [CrossRef]
- Kwong, C.K.; Bai, H. A fuzzy AHP approach to the determination of importance weights of customer requirements in quality function deployment. J. Intell. Manuf. 2002, 13, 367–377. [Google Scholar] [CrossRef]
- Lee, W.B.; Lau, H.; Liu, Z.-Z.; Tam, S. A fuzzy analytic hierarchy process approach in modular product design. Expert Syst. 2002, 18, 32–42. [Google Scholar] [CrossRef]
- Kirkwood, C.W. Notes on attitude toward risk taking and the exponential utility function. In Working Paper; Department of Management, Arizona State University: Tempe, AZ, USA, 1997; pp. 1–25. [Google Scholar]
- Steuer, R.E. Multiple Criteria Optimization. Theory, Computation and Applications. 1986. Available online: https://ci.nii.ac.jp/naid/10011751184/en/ (accessed on 21 December 2020).
- Velazquez, M.A.; Claudio, D.; Ravindran, A.R. Experiments in multiple criteria selection problems with multiple decision makers. Int. J. Oper. Res. 2010, 7, 413–428. [Google Scholar] [CrossRef]
- Liu, N.T.; Holcomb, J.B.; Wade, C.E.; Darrah, M.I.; Salinas, J. Data quality of a wearable vital signs monitor in the pre-hospital and emergency departments for enhancing prediction of needs for life-saving interventions in trauma patients. J. Med. Eng. Technol. 2015, 39, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Despins, L.A.; Scott-Cawiezell, J.; Rouder, J.N. Detection of patient risk by nurses: A theoretical framework. J. Adv. Nurs. 2010, 66, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Wickens, T.D. Elementary Signal Detection Theory; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Jerison, H.J.; Pickett, R.M. Vigilance: The Importance of the Elicited Observing Rate. Science 1964, 143, 970–971. [Google Scholar] [CrossRef]
- Lisper, H.-O.; Kjellberg, A.; Melin, L. Effects of Signal Intensity on Increase of Reaction Time on an Auditory Monitoring Task. Percept. Mot. Ski. 1972, 34, 439–444. [Google Scholar] [CrossRef]
- Claudio, D.; Velázquez, M.A.; Bravo-Llerena, W.; Okudan, G.E.; Freivalds, A. Perceived usefulness and ease of use of wearable sensor-based systems in emergency departments. Trans. Occup. Ergon. Hum. Factors 2015, 3, 177–187. [Google Scholar] [CrossRef]




| Device | Manufacturer | Status | Type |
|---|---|---|---|
| MIT | Unreleased/Project Finished | Wearable Platform/Package-Multiuse |
| EUIST | Unreleased/Project Finished | Wrist-worn Multisensor |
| Stanford/NASA | Unreleased/Project Finished | Custom Chest Device and commercial sensors |
| EUIST | Unreleased/Project Finished | Smart Textiles |
| EUIST | Unreleased/Project Finished | Smart Textiles |
| University of Milan | Unreleased/Project Finished | Smart Textiles/Sensorized Vest |
| EUIST | Unreleased/Project Finished | Smart Textiles |
| Harvard | Architecture | Architecture for Ad Hoc Sensor networks |
| Dongseo University | Routing Protocol | Routing protocol for data from wearables |
| IMEC | Commercial | ULP circuits, custom hardware, commercial wearable marked |
| University of Pittsburgh | Architecture | Architecture uses Alive Tec chest band |
| Dept. Medical Physics Greece | Unreleased/Project Finished | Mask/Glove for emotional state detection in stressful environments |
| Vivometrics | Commercial | Shirt for all day monitoring |
| Medtronic | Commercial | Shirt, strap, harness for performance monitoring |
| Sotera Wireless | Commercial | Wrist cuff, wires, stick-on electrodes |
| Hidalgo | Commercial | Strap, extra peripherals |
| Intelesens | Commercial | Device, electrodes |
| Intelesens | Unreleased/Project Finished | |
| Wearable Sensing | Commercial | Headset, EEG |
| Alive Technologies | Commercial | Electrodes, bodypack |
| Preventive | Commercial | Patch and module |
| CareTaker Medical | Commercial | Wrist cuff and finger cuff |
| Device Name | Heart Rate | Pulse Oxi- MeterSpO2 | Systolic and Diastolic Blood Pressure | Blood Pressure Automatic Measure | Telemetry | Respiration Rate | Pain Level | Temperature | Activity |
|---|---|---|---|---|---|---|---|---|---|
| x | x | x | Assumed | ECG, EMG | x | x | ||
| x | x | x | x | ECG | x | x | ||
| x | x | x | x | ECG | x | x | x | |
| x | ECG | x | x | |||||
| x | ECG, EMG | x | x | x | ||||
| ECG | x | x | ||||||
| ECG | x | x | x | |||||
| x | x | Assumed | ECG | x | ||||
| |||||||||
| |||||||||
| |||||||||
| ECG, EMG | x | |||||||
| |||||||||
| ECG | x | x | x | |||||
| x | x | x | cNIBP | x | x | |||
| x | x | ECG | x | x | x | |||
| x | ECG | x | x | |||||
| |||||||||
| ECG | x | x | ||||||
| x | ECG | x | ||||||
| x | ECG | |||||||
| x | x | x | cNIBP | x | Can add | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nino, V.; Claudio, D.; Schiel, C.; Bellows, B. Coupling Wearable Devices and Decision Theory in the United States Emergency Department Triage Process: A Narrative Review. Int. J. Environ. Res. Public Health 2020, 17, 9561. https://doi.org/10.3390/ijerph17249561
Nino V, Claudio D, Schiel C, Bellows B. Coupling Wearable Devices and Decision Theory in the United States Emergency Department Triage Process: A Narrative Review. International Journal of Environmental Research and Public Health. 2020; 17(24):9561. https://doi.org/10.3390/ijerph17249561
Chicago/Turabian StyleNino, Valentina, David Claudio, Christie Schiel, and Brendan Bellows. 2020. "Coupling Wearable Devices and Decision Theory in the United States Emergency Department Triage Process: A Narrative Review" International Journal of Environmental Research and Public Health 17, no. 24: 9561. https://doi.org/10.3390/ijerph17249561







