An Appraisal of Potential for Sowing of Nasturtium officinale into Streams to Mitigate Nutrient Pollution in Eastern Scotland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area for Watercress Seeding Trial
2.2. Estimation of Nutrient Retention by Ditches
2.3. Estimation of Plant Nutrient Uptake
2.4. Appraisal of Potential for Watercress Seeding in the Lunan Water Catchment
3. Results
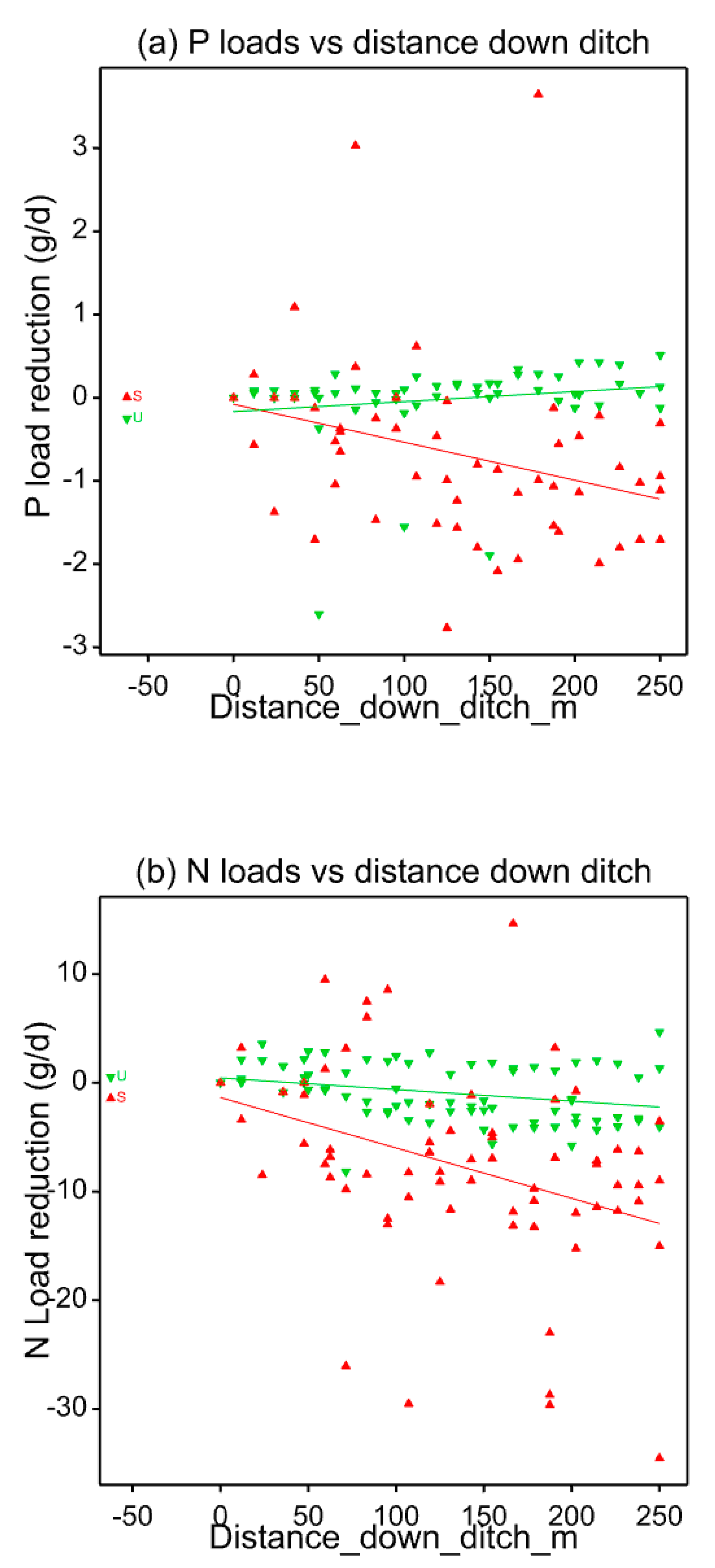
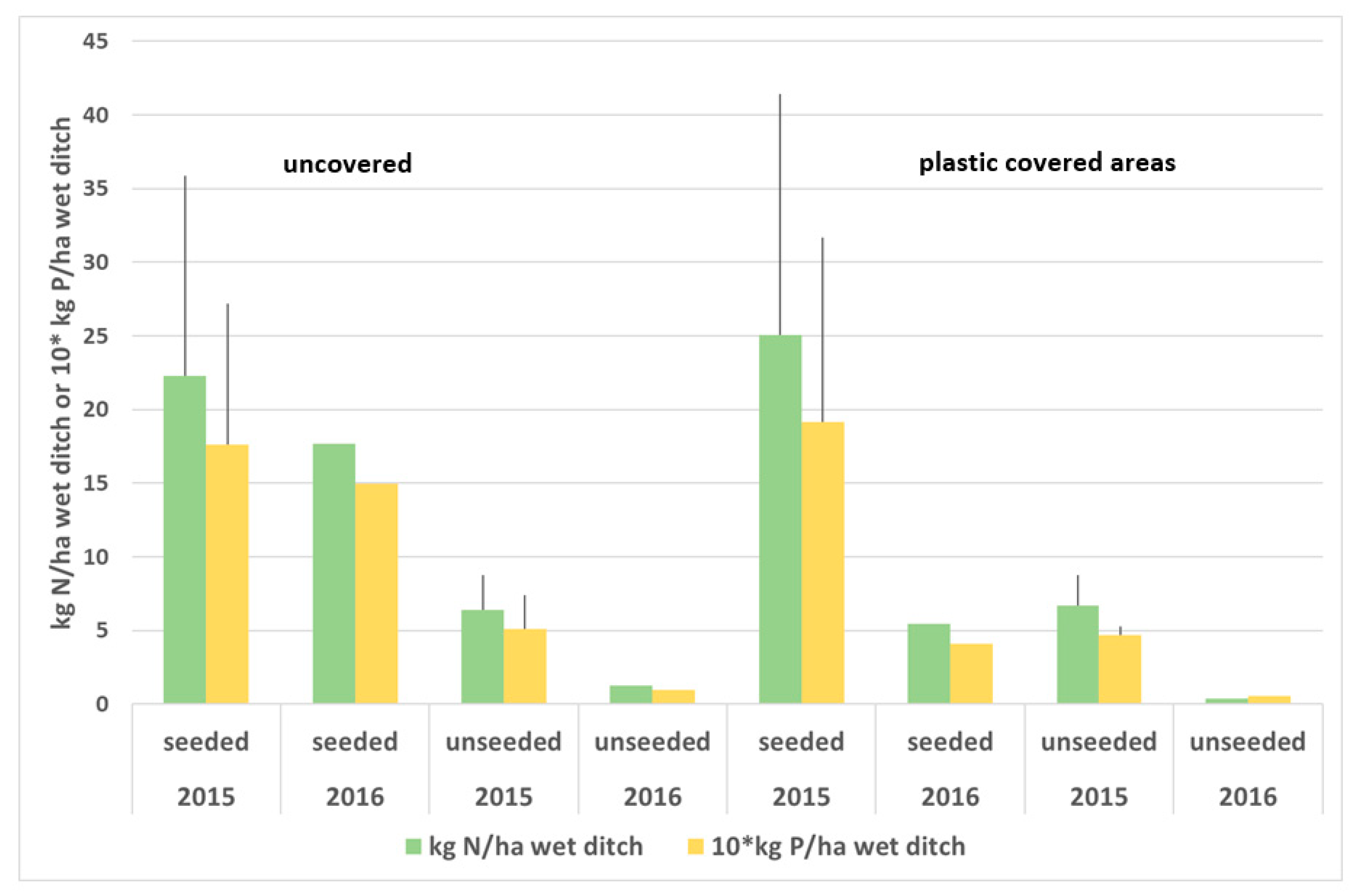
3.1. Nutrient Retention
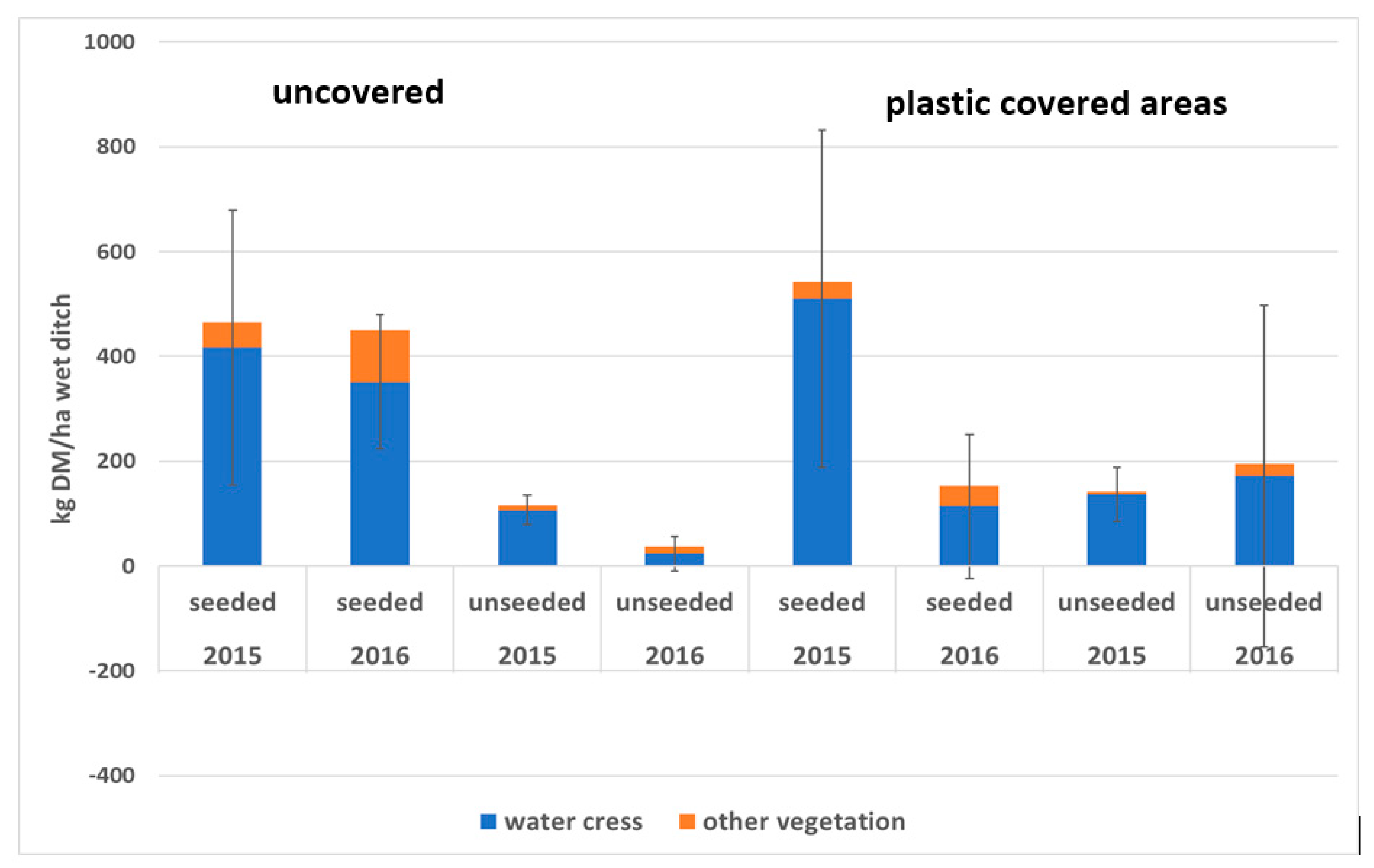
3.2. Effect of Plastic Covering
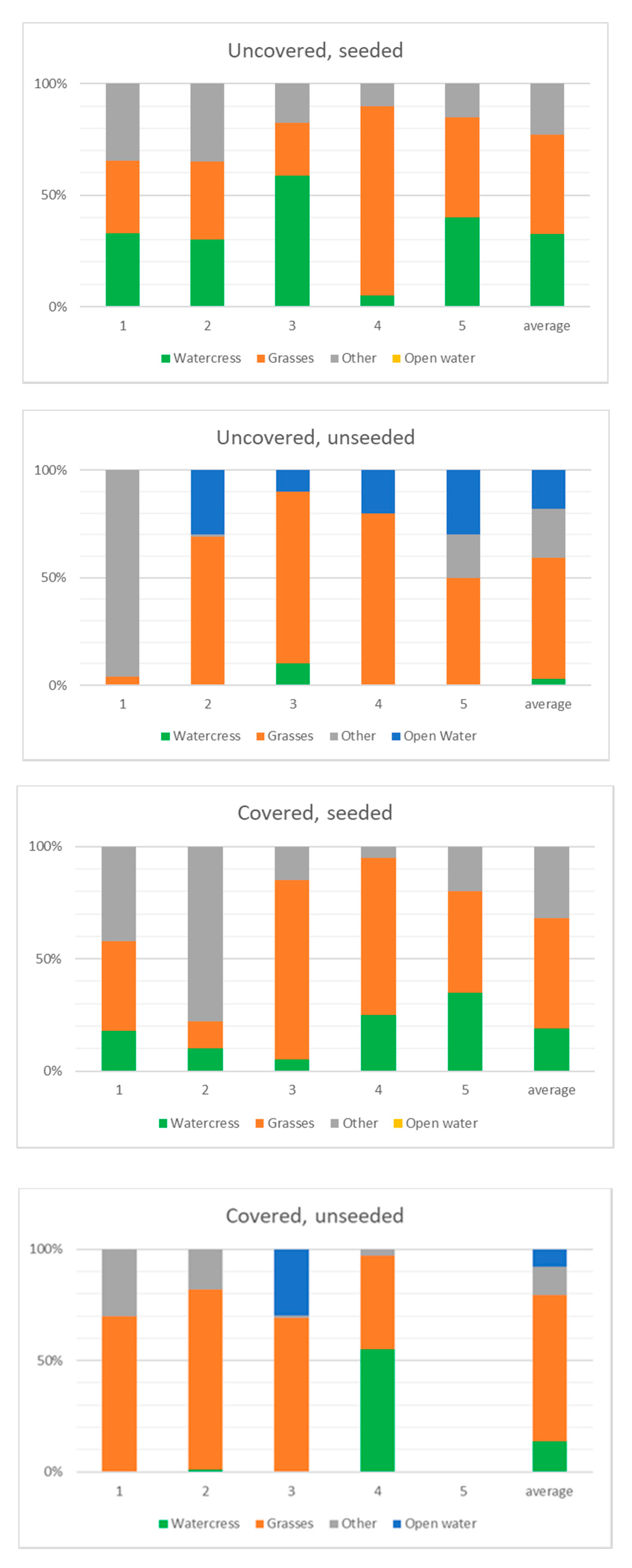
3.3. Growth and Nutrient Uptake
3.4. Potential Impact of Watercress Seeding in Upper Lunan Water Catchment
4. Discussion
4.1. Comparison with Other Work on Nutrient Retention by Macrophytes
4.2. Factors Affecting Nutrient Retention
4.3. Other Management Considerations
5. Conclusions
- Watercress planting in drainage ditches on grassland under Scottish lowland conditions enhanced the retention of N and P during the growing period between April and August, compared with natural ditch vegetation (by 0.092 g N/m2 wetted ditch/d and 0.0092 g P/m2 wetted ditch/d). Only part of this retention can be explained by plant nutrient removal.
- Plastic covering of ditch sections had a negative impact on watercress growth. The high air temperature (up to >30 °C) promoted greater colonisation by opportunist species such as nettles (Urtica), rush (Juncus), and grasses. This way of enhancing spring aquatic plant growth is not recommended.
- Estimates of potential watercress-mediated N and P retention for the Lunan Water catchment showed that loading into the eutrophic Rescobie and Balgavies Lochs in the upper catchment could be reduced by about 26% of the required load reductions, by targeting first and second order streams for watercress seeding. Seeding of streams is most suitable for shallow slopes with steady water flow.
- Other factors need to be considered before adopting the practice of watercress seeding in ditches and streams for water quality enhancement, including potential re-release in periods of high flow, impact on stream ecology.
- Ecology of release of natural mustard oils, impact on upstream water levels, and cost-effectiveness compared with other mitigation methods.
- The farmer-led approach to use watercress-seeded ditches to mitigate diffuse pollution, supported by science-based evidence, has the potential to facilitate its adoption on a wider scale.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iseyemi, O.O.; Farris, J.L.; Moore, M.T.; Choi, S.-E. Nutrient Mitigation Efficiency in Agricultural Drainage Ditches: An Influence of Landscape Management. Bull. Environ. Contam. Toxicol. 2016, 96, 750–756. [Google Scholar] [CrossRef]
- Quilliam, R.S.; van Niekerk, M.A.; Chadwick, D.R.; Cross, P.; Hanley, N.; Jones, D.L.; Vinten, A.J.A.; Willby, N.; Oliver, D.M. Can macrophyte harvesting from eutrophic water close the loop on nutrient loss from agricultural land? J. Environ. Manag. 2015, 152, 210–217. [Google Scholar] [CrossRef]
- Levi, P.S.; Riis, T.; Alnøe, A.B.; Peipoch, M.; Maetzke, K.; Bruus, C.; Baattrup-Pedersen, A. Macrophyte Complexity Controls Nutrient Uptake in Lowland Streams. Ecosystems 2015, 18, 914–931. [Google Scholar] [CrossRef]
- Nikolakopoulou, M.; Argerich, A.; Drummond, J.D.; Gacia, E.; Martí, E.; Sorolla, A.; Sabater, F. Emergent Macrophyte Root Architecture Controls Subsurface Solute Transport. Water Resour. Res. 2018, 54, 5958–5972. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Lessard, J.L.; Plew, D.; Graham, S.E.; McIntosh, A.R. Aquatic Macrophytes Alter Metabolism and Nutrient Cycling in Lowland Streams. Ecosystems 2014, 17, 405–417. [Google Scholar] [CrossRef]
- Zak, D.; Stutter, M.; Jensen, H.; Egemose, S.; Vodder Carstensen, M.; Audet, J.; Strand, J.; Feuerbach, P.; Hoffmann, C.; Christen, B.; et al. An Assessment of the Multifunctionality of Integrated Buffer Zones in Northwestern Europe. J. Environ. Qual. 2019, 48, 362–375. [Google Scholar] [CrossRef]
- Vincent, W.F.; Downes, M.T. Variation in nutrient removal from a stream by watercress (Nasturtium officinale R. Br.). Aquat. Bot. 1980, 9, 221–235. [Google Scholar] [CrossRef]
- Cox, T.J.; Rutherford, J.C. Nitrogen fate and transport in a watercress-dominated stream. N. Z. J. Mar. Freshw. Res. 2012, 46, 191–205. [Google Scholar] [CrossRef]
- Nitrogen Management by Watercress (Nasturtium officinale) in Hydroponic Conditions. Available online: http://www.massey.ac.nz/~flrc/workshops/12/Manuscripts/Robertson_2012.pdf (accessed on 30 January 2020).
- Conrad, C.C.; Hilchey, K.G. A review of citizen science and community-based environmental monitoring: Issues and opportunities. Environ. Monit. Assess. 2011, 176, 273–291. [Google Scholar] [CrossRef]
- Okumah, M.; Chapman, J.P.; Martin-Ortega, J.; Novo, P. Mitigating Agricultural Diffuse Pollution: Uncovering the Evidence Base of the Awareness–Behaviour–Water Quality Pathway. Water 2018, 11, 29. [Google Scholar] [CrossRef]
- Sidemo-Holm, W.; Smith, H.G.; Brady, M.V. Improving agricultural pollution abatement through result-based payment schemes. Land Use Policy 2018, 77, 209–219. [Google Scholar] [CrossRef]
- RSPB. Giving Nature a Home. Available online: https://www.rspb.org.uk/our-work/conservation/conservation-and-sustainability/farming/case-studies/scotland/ (accessed on 30 January 2020).
- Water Framework Directive in Scotland (WFD). Available online: http://www.gov.scot/Topics/Environment/Water/15561/WFD (accessed on 30 January 2020).
- Dunn, S.M.; Sample, J.; Potts, J.; Abel, C.; Cook, Y.; Taylor, C.; Vinten, A.J.A. Recent trends in water quality in an agricultural catchment in Eastern Scotland: Elucidating the roles of hydrology and land use. Environ. Sci. Process. Impacts 2014, 16, 1659–1675. [Google Scholar] [CrossRef]
- Vinten, A.; Sample, J.; Ibiyemi, A.; Abdul-Salam, Y.; Stutter, M. A tool for cost-effectiveness analysis of field scale sediment-bound phosphorus mitigation measures and application to analysis of spatial and temporal targeting in the Lunan Water catchment, Scotland. Sci. Total Environ. 2017, 586, 631–641. [Google Scholar] [CrossRef]
- Balana, B.B.; Lago, M.; Baggaley, N.; Castellazzi, M.; Sample, J.; Stutter, M.; Slee, B.; Vinten, A. Integrating Economic and Biophysical Data in Assessing Cost-Effectiveness of Buff er Strip Placement. J. Environ. Qual. 2012, 41, 380–388. [Google Scholar] [CrossRef]
- National River Flow Archive. Available online: https://nrfa.ceh.ac.uk/ (accessed on 30 January 2020).
- Gianfagna, C.C.; Johnson, C.E.; Chandler, D.G.; Hofmann, C. Watershed area ratio accurately predicts daily streamflow in nested catchments in the Catskills, New York. J. Hydrol. Reg. Stud. 2015, 4, 583–594. [Google Scholar] [CrossRef]
- Birkel, C.; Tetzlaff, D.; Dunn, S.M.; Soulsby, C. Using lumped conceptual rainfall–runoff models to simulate daily isotope variability with fractionation in a nested mesoscale catchment. Adv. Water Resour. 2011, 34, 383–394. [Google Scholar] [CrossRef]
- Stutter, M.; Dawson, J.J.C.; Glendell, M.; Napier, F.; Potts, J.M.; Sample, J.; Vinten, A.; Watson, H. Evaluating the use of in-situ turbidity measurements to quantify fluvial sediment and phosphorus concentrations and fluxes in agricultural streams. Sci. Total Environ. 2017, 607, 391–402. [Google Scholar] [CrossRef]
- De Klein, J.J.M.; Koelmans, A.A. Quantifying seasonal export and retention of nutrients in West European lowland rivers at catchment scale. Hydrol. Process. 2011, 25, 2102–2111. [Google Scholar] [CrossRef]
- Eugene Turner, R.; Bodker, J.E.; Schulz, C. The belowground intersection of nutrients and buoyancy in a freshwater marsh. Wetl. Ecol. Manag. 2018, 26, 151–159. [Google Scholar] [CrossRef]
- Washbourne, I.J.; Crenshaw, C.; Baker, M. Dissimilatory nitrate reduction pathways in an oligotrophic freshwater ecosystem: Spatial and temporal trends. Aquat. Microb. Ecol. 2011, 65, 55–64. [Google Scholar] [CrossRef]
- Tank, J.L.; Martí, E.; Riis, T.; von Schiller, D.; Reisinger, A.J.; Dodds, W.K.; Whiles, M.R.; Ashkenas, L.R.; Bowden, W.B.; Collins, S.M.; et al. Partitioning assimilatory nitrogen uptake in streams: An analysis of stable isotope tracer additions across continents. Ecol. Monogr. 2018, 88, 120–138. [Google Scholar] [CrossRef]
- Going, B.; Simpson, J.; Even, T. The influence of light on the growth of watercress (Nasturtium officinale R. Br.). Hydrobiologia 2008, 607, 75–85. [Google Scholar] [CrossRef]
- Newman, R.M.; Kerfoot, W.C.; Hanscom, Z. Watercress Allelochemical Defends High-Nitrogen Foliage Against Consumption: Effects on Freshwater Invertebrate Herbivores. Ecology 1996, 77, 2312–2323. [Google Scholar] [CrossRef]
- Cox, J. Watercress Growing and Its Environmental Impacts on Chalk Rivers in England, NECR ed.; NECR027; Natural England: York, UK, 2009. [Google Scholar]
- Cotter, S. Impact of Watercress Farming on Stream Ecosystem Functioning and Community Structure. Ph.D. Thesis, Queen Mary University of London, University of London, London, UK, September 2012. [Google Scholar]
- Riis, T.; Suren, A.; Clausen, B.; Sand-Jensen, K.A.J. Vegetation and flow regime in lowland streams. Freshw. Biol. 2008, 53, 1531–1543. [Google Scholar] [CrossRef]
- Vinten, A.; Kuhfuss, L.; Shortall, O.; Stockan, J.; Ibiyemi, A.; Pohle, I.; Gabriel, M.; Gunn, I.; May, L. Water for all: Towards an integrated approach to wetland conservation and flood risk reduction in a lowland catchment in Scotland. J. Environ. Manag. 2019, 246, 881–896. [Google Scholar] [CrossRef]
- Okumah, M.; Yeboah, A.S. Exploring stakeholders’ perceptions of the quality and governance of water resources in the Wenchi municipality. J. Environ. Plan. Manag. 2019, 1–29. [Google Scholar] [CrossRef]
- Withanachchi, S.S.; Ghambashidze, G.; Kunchulia, I.; Urushadze, T.; Ploeger, A. Water Quality in Surface Water: A Preliminary Assessment of Heavy Metal Contamination of the Mashavera River, Georgia. Int. J. Environ. Res. Public Health 2018, 15, 621. [Google Scholar] [CrossRef]
- Withanachchi, S.; Ploeger, A.; Al Sidawi, R.; Kunchulia, I.; Ghambashidze, G.; Urushadze, T. Farmers’ Perception of Water Quality and Risks in the Mashavera River Basin, Georgia: Analyzing the Vulnerability of the Social-Ecological System through Community Perceptions. Sustainability 2018, 10, 3062. [Google Scholar] [CrossRef]
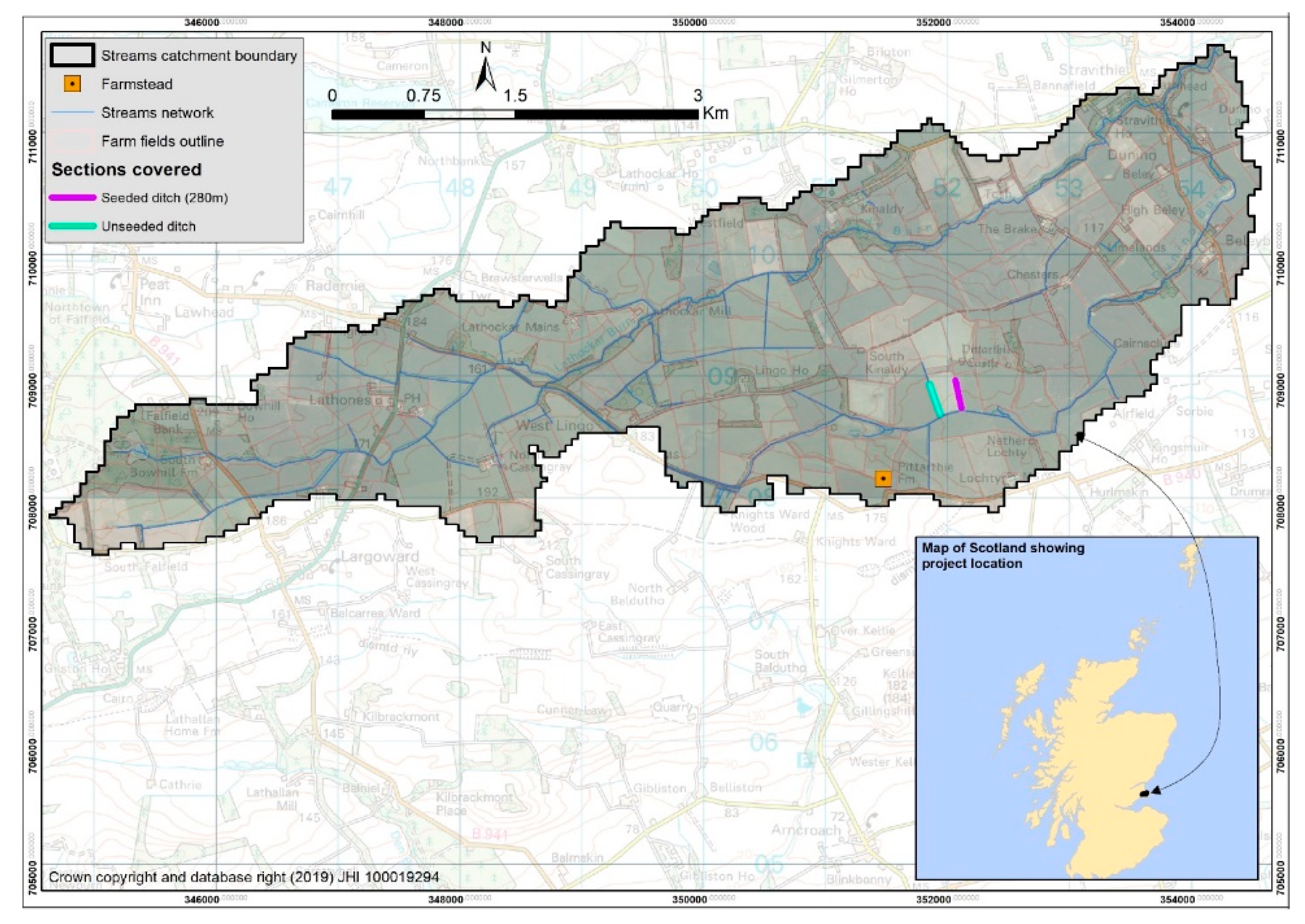

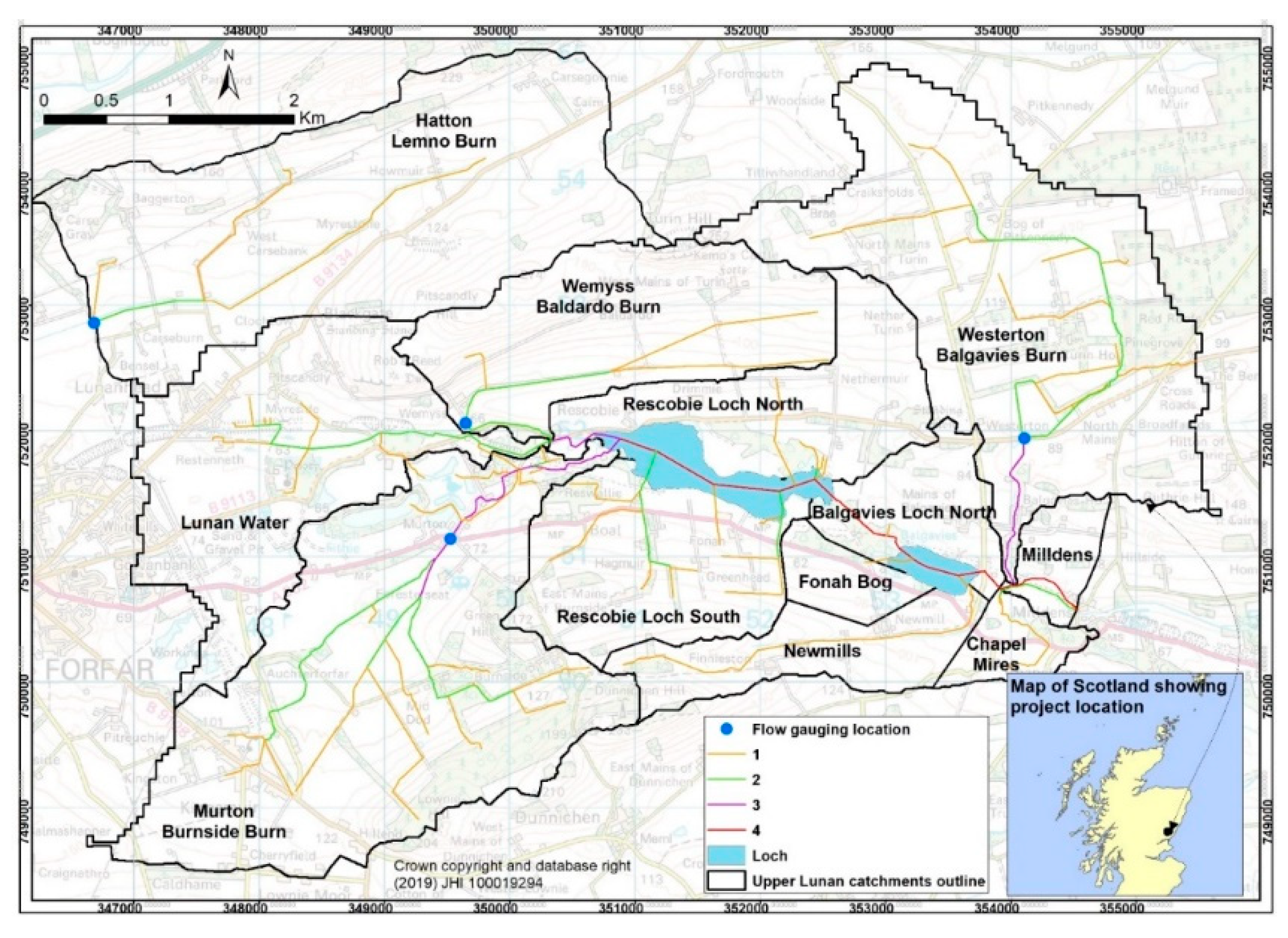
| Year/Month | Water Samples | No. of Samples along Seeded, Unseeded Ditches | Plastic in Covered Sections | Vegetation Samples | |
|---|---|---|---|---|---|
| 2014 | March | 13/03/2014 | 5,6 | ||
| 2014 | May | 30/05/14 | 5,6 | Covered on 26/5/14 | |
| 2014 | August | 14/08/14 | 5,6 | ||
| 2015 | June | 10/06/15 | 18,20 | Covers removed after sampling | 10/06/2015 |
| 2016 | April | 28/04/16 | 22,22 | Covered on 28/4/16 | |
| 2016 | May | 30/05/16 | 20,20 | Covers removed after sampling | 30/05/2016 |
| SRP (μg/L) | 2014 | 2015 | 2016 | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Seeded ditch | 42 | 52 | 30 | 30 | 52 | 26 |
| Unseeded ditch | 49 | 38 | 27 | 19 | 33 | 6 |
| Receiving stream | 28 | 10 | 15 | 2 | ||
| NO3-N(mg/L) | ||||||
| Seeded ditch | 1.6 | 0.7 | 1.5 | 0.3 | 4.7 | 0.2 |
| Unseeded ditch | 2.0 | 1.2 | 0.6 | 0.1 | 1.5 | 0.3 |
| Receiving stream | 1.0 | 0.6 | 1.7 | 0.6 | ||
| June 2015 | May 2016 | |||||
|---|---|---|---|---|---|---|
| Air | Water | Air | Water | |||
| SC | Seeded | Covered | 31.7 | 11.4 | 16.0 | 9.1 |
| SU | Seeded | Uncovered | 22.5 | 11.2 | 14.7 | 8.9 |
| UC | Unseeded | Covered | 29.0 | 16.4 | 17.0 | 11.1 |
| UU | Unseeded | Uncovered | n/a | 17.1 | 16.4 | 11.0 |
| Sub- Catchment | Season | TP Load kg/ha | % Reduction in P Loads by Seeding | NO3-N Load Kg/ha | % Reduction in N Loads | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2010–2011 | 2011–2012 | 2010–2011 | 2011–2012 | 2010–2011 | 2011–2012 | 2010–2011 | 2011–2012 | ||
| Lemno | autumn | 0.20 | 0.25 | 0.0% | 0.0% | 7.7 | 9.7 | 0.0% | 0.0% |
| winter | 0.59 | 0.13 | 0.0% | 0.0% | 17.6 | 9.6 | 0.0% | 0.0% | |
| spring | 0.12 | 0.40 | 26.1% | 7.9% | 7.3 | 4.9 | 4.4% | 6.5% | |
| summer | 1.32 | 0.20 | 0.9% | 5.8% | 24.8 | 11.3 | 0.5% | 1.0% | |
| annual | 2.24 | 0.99 | 1.9% | 4.4% | 57.4 | 35.5 | 0.8% | 1.2% | |
| Baldardo | autumn | 0.19 | 0.08 | 0.0% | 0.0% | 8.7 | 6.9 | 0.0% | 0.0% |
| winter | 0.27 | 0.06 | 0.0% | 0.0% | 17.8 | 6.5 | 0.0% | 0.0% | |
| spring | 0.04 | 0.12 | 83.0% | 25.9% | 4.5 | 4.5 | 7.2% | 7.1% | |
| summer | 0.11 | 0.07 | 10.4% | 17.3% | 9.2 | 8.5 | 1.2% | 1.4% | |
| annual | 0.60 | 0.33 | 7.3% | 13.1% | 40.1 | 26.4 | 1.1% | 1.7% | |
| Balgavies | autumn | 0.12 | 0.04 | 0.0% | 0.0% | 7.3 | 4.3 | 0.0% | 0.0% |
| winter | 0.18 | 0.02 | 0.0% | 0.0% | 23.6 | 5.3 | 0.0% | 0.0% | |
| spring | 0.01 | 0.03 | 298.4% | 93.9% | 4.5 | 2.8 | 7.1% | 11.4% | |
| summer | 0.07 | 0.03 | 15.7% | 34.5% | 7.8 | 10.3 | 1.5% | 1.1% | |
| annual | 0.39 | 0.13 | 11.3% | 34.8% | 43.2 | 22.7 | 1.0% | 1.9% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinten, A.; Bowden-Smith, P. An Appraisal of Potential for Sowing of Nasturtium officinale into Streams to Mitigate Nutrient Pollution in Eastern Scotland. Int. J. Environ. Res. Public Health 2020, 17, 895. https://doi.org/10.3390/ijerph17030895
Vinten A, Bowden-Smith P. An Appraisal of Potential for Sowing of Nasturtium officinale into Streams to Mitigate Nutrient Pollution in Eastern Scotland. International Journal of Environmental Research and Public Health. 2020; 17(3):895. https://doi.org/10.3390/ijerph17030895
Chicago/Turabian StyleVinten, Andy, and Patrick Bowden-Smith. 2020. "An Appraisal of Potential for Sowing of Nasturtium officinale into Streams to Mitigate Nutrient Pollution in Eastern Scotland" International Journal of Environmental Research and Public Health 17, no. 3: 895. https://doi.org/10.3390/ijerph17030895
APA StyleVinten, A., & Bowden-Smith, P. (2020). An Appraisal of Potential for Sowing of Nasturtium officinale into Streams to Mitigate Nutrient Pollution in Eastern Scotland. International Journal of Environmental Research and Public Health, 17(3), 895. https://doi.org/10.3390/ijerph17030895




