Abstract
The association between vitamins and oral health have recently been discussed, yielding increased attention from medical and dental perspectives. The present review aimed to systematically evaluate and appraise the most recently scientific papers investigating the role of vitamins in the prevention and treatment of the main oral diseases as hard dental pathological processes and gum/periodontal disease. Randomized controlled trials, cross-sectional studies, cohort studies, comparative studies, validation studies and evaluation studies, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, reporting associations between vitamins and oral diseases or the use of vitamins to prevent or treat oral diseases in patients of any age were included. PubMed, Embase and Scopus were searched to November 2019 using an ad hoc prepared search string. All the papers meeting the inclusion criteria were subjected to a quality assessment. The search identified 1597 papers; 741 were selected after removing duplicates. A total of 334 articles were excluded after title and abstract evaluation; 407 were assessed and 73 papers were full-text assessed; other 14 papers were discharged after full text evaluation, leaving finally 58 papers included. In general, there is weak evidence supporting the association between vitamins and both gingival/periodontal disease and hard dental pathological processes.
1. Introduction
The role of vitamins is well known in a medical perspective, but the scientific evidence regarding the oral health perspective is still not fully clarified [].
Vitamins are catalysts for all metabolic reactions, using proteins, fats and carbohydrates for energy, growth and cell maintenance. As only small amounts of these fundamental substances are obtained from food, vitamins are often administered though food supplements []. Fat-soluble vitamins such as A, C, D, E and K can be stored in the liver and fat tissues as reserves, while water-soluble vitamins as B and C are expelled if not absorbed.
It is general knowledge that vitamins play a significant effect on oral and general health where its imbalance leads to malnutrition. The process of chewing allows one to extract the greatest possible amount of nutrients and the number and distribution of teeth influence the chewing efficacy. The available literature on the role of vitamins toward oral health is really scarce with no available data on the prevalence of oral disease related to vitamin deficiencies. Teeth loss affects dietary choice and nutritional status []. A significant improvement of vitamin D levels was obtained in partially dentate patients aged ≥ 65 years after the replacement of lost teeth using prosthetic solutions []; still, no strong evidence on the effect of tooth loss on nutritional status was found in a recent review [].
Vitamin deficiency prompted several non-specific oral conditions as glossitis, stomatitis and mucosal ulceration. Glossitis with linear lesions was postulated to be an early sign of vitamin B12 paucity [].
Vitamin D deficiency leads to reduced bone density, osteoporosis, and, as consequence, to the progression of periodontal disease; on the other hand, sufficient levels of this vitamin might reduce the risk of gingivitis and periodontitis; the vitamin acting as immunomodulator, anti-inflammatory and antiproliferative agent [].
In the developmental phases, hard dental tissues are strongly influenced by nutritional status and consequently to vitamin deficiency []. A positive relationship between malnutrition, enamel hypoplasia and caries in the primary dentition was postulated in children [,].
The frequent and prolonged exposure to acidic agents contained in food, beverages, drugs or food supplements can lead to significant tooth wear []. Chewable vitamin C tablets have been reported to have a pH of about 2, lower than the critical pH value (5.5) for enamel dissolution, postulating an association between vitamin C and erosion with an odds ratio of 1.16 [].
The aim of the present study was to perform a systematic review and meta-analysis of the scientific papers published during the last 20 years, investigating the association between vitamins and gingival/periodontal disease and hard dental pathological processes such as dental caries, tooth wear and developmental defects.
2. Materials and Methods
Reporting of this review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline []. The review protocol was registered with the International prospective register of systematic reviews (PROSPERO) system (ID 150613, 12 September 2019).
2.1. Eligibility Criteria
The review included randomized controlled trials (RCTs), cross-sectional studies, comparative studies, validation studies and evaluation studies, reporting vitamins supplementary (foods, tablets etc) or vitamin serum levels in patients of any age. Only papers in English published from 1 January 2000 to 30 November 2019 were collected. Electronically published articles and paper-based article were taken into consideration.
2.2. Information Sources
Electronic databases Medline via PubMed, Embase via Ovid and Scopus were screened for articles.
2.3. Information Sources and Search Strategy
Several search strategies were used. The first included a combination of Medical Subject Headings (MeSH) terms and key words: Vitamin OR Vitamins OR oral health, OR caries OR dental caries OR periodontal disease OR dental erosion OR gingivitis. The second strategy included the search string “Vitamins OR Vitamin OR vitamin A OR Vitamin B OR Vitamin C OR Vitamin D OR Vitamins B OR Vitamin E OR Vitamin K” and “Oral health OR oral health OR caries OR dental caries OR root caries OR tooth diseases OR salivation OR saliva OR periodontal diseases OR ‘dental erosion’ OR tooth erosion OR tooth erosion OR ‘cariogenic bacteria’ OR biofilms OR biofilm OR periodontitis OR periodontitis OR gingivitis OR gingivitis OR dental plaque OR plaque”. Cross-referencing was performed using the bibliographies of full-text articles. Grey literature was also retrieved via opengrey.eu (http://www.opengrey.eu).
2.4. Study Selection
Repeated or duplicate papers were excluded after comparing the results from the different research strategies. Three authors (T.G.W., M.G.C., and N.C.) independently examined all the abstracts of the papers. All the papers meeting the inclusion criteria were obtained in the full-text format. The authors independently assessed the papers to establish whether each paper should or should not be included in the systematic review.
2.5. Data Collection, Summary Measures and Synthesis of Results
Data collection and synthesis was independently carried out by three authors (G.C., M.G.C. and N.C.) using an ad hoc designed data extraction form, without masking journal title or authors. Different studies outcomes were compared on the use of vitamins to prevent or treat oral diseases per different diseases and publication years. To facilitate the synthesis, the results were summarised in tables where each selected paper was included and the main aspects presented (i.e., vitamin and oral disease studies, sample, age, healthy subjects or affected by systemic diseases effect on the disease, statistically significance). For each paper, the following data were searched and recorded when available: a) publication year and study duration; b) details/characteristics of the participants at baseline; c) oral data, including gingival or periodontal conditions or gingival bleeding or pocket dept or gingival recession or loss of clinical attachment level; actual caries status, caries experience and caries increment measured through DMFT/S or dmft/s (for decayed, missing, filled teeth/surfaces in permanent and primary teeth indexes) or ICDAS (for International Caries Detection and Assessment System), or other detection systems; the presence of tooth wear; the presence of developmental enamel defect.
The ProMeta 3 Software (IdoStatistics https://idostatistics.com/prometa3/, Cesena, Italy: Internovi) was used for the meta-analysis of the data. Mean difference (MD) and odds ratio (OR) were chosen for calculating the effect size. The analysis was computed on the different vitamins used. Associations between vitamins and gingivitis, periodontitis, caries and enamel defects were computed separately. The I² statistic was calculated to describe the percentage of variation across studies due to heterogeneity rather than chance []. The heterogeneity was categorized as follows: <30% not significant; 30–50% moderate; 51–75% substantial, and 76–100% considerable. Whether homogeneity was obtained or not, the random effects model (REM) with 95% confidence intervals was chosen as the meta-analysis model. Potential moderators as publication type, publication year, age groups, vitamins were evaluated and analysed to explain which factors might affect heterogeneity. The funnel plot method was used to assess the potential role of publication bias []. The significance levels of the effect sizes were determined based on the two-tailed test. In all tests, the level of significance was set at p < 0.05.
2.6. Assessment of Bias across Studies
The risk of bias assessment was conducted by two authors (C.T., T.G.W.). The methodological quality of the included RCTs was scored according to the customized quality assessment tool developed by the National Heart, Lung, and Blood Institute and Research Triangle Institute International for Observational Cohort and Cross-Sectional Studies and Study Quality Assessment Tools Guidance for Assessing the Quality of Controlled Intervention Studies www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools]. The tools included items for evaluating potential flaws in study methods or implementation, including sources of bias (e.g., patient selection, performance, attrition, and detection), confounding, study power, the strength of causality in the association between interventions and outcomes and other factors. For each item, “yes,” “no,” or “cannot determine/not reported/not applicable” was selected. Each study was finally scored as “good” when it has the least risk of bias, “fair” if it is susceptible to some bias and "poor" when significant risk of bias is conceivable.
Disagreements between authors were resolved by discussion. Where this was not possible, another author was consulted (M.G.C.).
3. Results
The search identified 1597 papers; 741 were selected after removing duplicates. A total of 334 articles were excluded after title and abstract evaluation; 407 were assessed and 73 papers were full-text assessed (Table S1. List of excluded papers); the quality assessment scores of the papers included is presented in the Supplementary Materials (Table S2. Quality assessment), other 14 papers were discharged after full text evaluation (Table S3. List of excluded papers after full text evaluation), leaving 58 included papers (Figure 1).
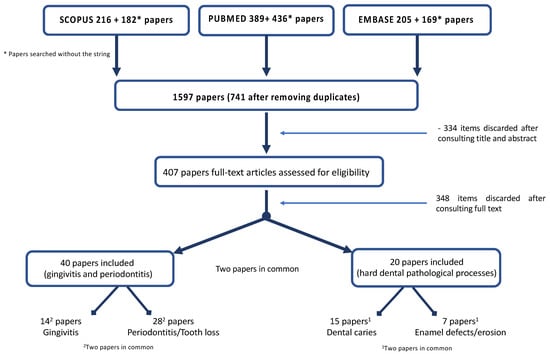
Figure 1.
Flow chart of the search.
Forty papers concerned on gingival/periodontal disease and 20 (two papers were in common) on hard dental pathological processes were included. Regarding gingival/periodontal disease, 26 papers were ranked of as being of good quality, 12 were classified of fair quality and only two of poor quality. Regarding hard dental tissues, 16 papers were ranked of as being of good quality, four were classified of fair quality and only two of poor quality (Table 1).

Table 1.
General characteristics of the studies included: (a) Gingivitis and periodontitis; (b) Hard dental tissues (dental caries, enamel defects).
Funnel plot analysis (Figure 2) showed that for gingivitis, caries and enamel defects no study was trimmed, and the overall effect sizes observed and estimated were the same 0.81, (95% CI ranging from 0.33 to 1.29; p = 0.06) and 1.04, (95% CI ranging from 0.92 to 1.18; p = 0.52) and 0.27 (95% CI ranging from −0.04 to 0.57; p = 0.09) respectively. Furthermore, no significant publication bias existed based on the Egger regression analysis (p = 0.109, 0.79 and 0.19, respectively). Regarding periodontal disease, six studies were trimmed, the observed effect size was 0.97, (95% CI ranging from 0.78 to 1.219; p = 0.78) while the estimated one was 0.76, (95% CI ranging from 0.60 to 0.97; p = 0.03) with no statistically significant publication bias (p = 0.91).
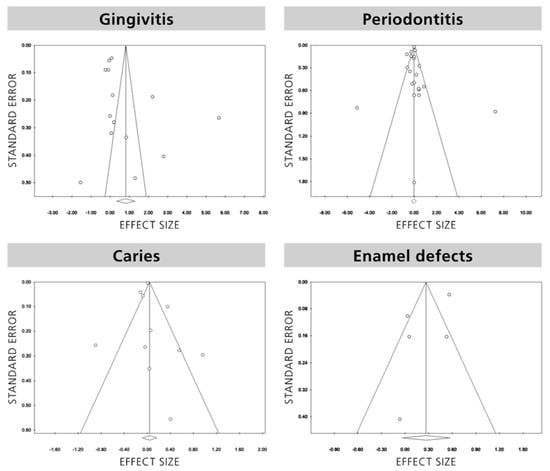
Figure 2.
Funnel plots of publication bias.
Due to the low numbers of studies for each vitamin, the heterogeneity was very high for all vitamins ranging from 83.68% for vitamin B to 99.13% for vitamin C (Figure 3). Regarding gingivitis, the heterogeneity analysis was measured as considerable with the highest value observed for vitamin C. Heterogeneity analysis for periodontal disease revealed the highest value for vitamin B (97.39%) followed by vitamin D (84.39%) and then vitamin C (13.27%). Heterogeneity analysis for caries showed the highest value for vitamin C (95.50%) while a substantial I2 value was observed for vitamin D (70.06%). Considering enamel defects, there were not enough data levels for performing this analysis.
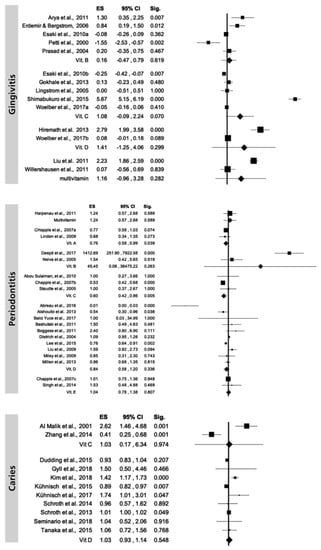
Figure 3.
Random-effects model overall level of studies included, categorized by vitamins.
3.1. Gingival/Periodontal Disease
The main characteristics of the included studies regarding gingivitis and periodontitis/tooth loss are reported in Table 2.

Table 2.
Main characteristics of the studies included regarding gingivitis and periodontitis/tooth loss. (a) Gingivitis, (b) Periodontitis, (c) Tooth loss.
Four studies were conducted to evaluate the effect of vitamin D on gingivitis; in one study vitamin D was given alone [], while in the other three it was administered in combination with vitamin C [] or vitamin B and E [] or vitamin A, B1, B2, B6, B9, C, E [] through the diet. A dose-dependent effect was found on gingival scores, showing the supplementation of 2000 International Unit (IU) of vitamin D obtained a greater improvement in gingival parameters compared to lower amount (1000 IU and 500 IU). A similar effect was obtained with a 4-week diet rich in vitamin C, D, Omega-3 fatty acids and antioxidants. All inflammatory parameters (gingival index, bleeding on probing and the total periodontal inflamed surface area) were halved compared to baseline. The administration of a dietary supplement containing different micronutrients (including vitamin D, C, E, B complex) for 3 months produced a slight improvement of the gingival inflammation in students under stress with poor oral hygiene, compared to students also under stress but not provided with the dietary supplement. The 6-month administration of a dietary supplement containing vitamin A, B1, B2, B6, B9, C, D, E in Type 2 diabetic adults, reduced gingivitis and oral ulcers incidence compared to placebo (p < 0.05).
Five studies analyzed the effect of vitamin C on gingival parameters, three of them considering vitamin C as the only variable [,,] and two on vitamin C combined with other vitamins [,]. All these studies used different administration modalities, including toothpaste, dietary supplement, chewing gum and foods. In the first three studies, vitamin C showed to reduce gingival scores of inflammation. vitamin C and B9 levels were statistically associated to bleeding on probing (p < 0.01) []; vitamin A was also associated (p < 0.05), while vitamin B1 and B2 levels were found to be associated to gingivitis presence in adolescent girls, while vitamin A and B3 resulted in not being associated []. Three studies investigated the effects of vitamin B9 on gingival scores, two with the vitamin as the only variable [,] and one with vitamin B9 combined with vitamin B12 []. Vitamin B9 was administered in patients with epilepsy to reduced Phenytoin-induced gingival overgrowth (PIGO) [,,,,,,,,,]. In both studies vitamin B9 administration reduced the development of PIGO or delayed its onset. A statistically significantly association between vitamin B9 and gingival index was found in smokers (p < 0.01) compared to non-smokers, while vitamin B12 resulted not associated []. Finally, a fluoridated toothpaste containing vitamin B3 and pro-vitamin B5 provided a statistically significantly reduction in calculus presence compared to a fluoridated toothpaste not containing vitamins (p = 0.01) [].
Twelve papers were concerned on the effect of vitamin D on periodontitis. A reduction of the clinical disease level (i.e., clinical attachment level and/or probing pocket depth) was described in five papers [,,,,], while in four papers [,,,] vitamin D levels had no statistically significant impact on clinical attachment level and probing pocket depth improvements in teriparatide patients. Low serum vitamin D levels were not statistically associated to periodontitis and tooth loss in pregnant and post-menopausal women [,,].
Four papers concerned on the effect of vitamin C on periodontitis. Two papers [,] underlined the reduction of gingival bleeding consequent to use of vitamin C in patients affected by chronic periodontitis. The use of fruit or vegetables rich in vitamin C was statistically significantly lower in subjects affected by chronic periodontitis respect to healthy subjects []. Serum concentrations of vitamin C, bilirubin, and total antioxidant capacity were inversely associated with periodontitis, the association being stronger in severe disease []. Vitamin B-complex supplement resulted in statistically significantly superior clinical attachment gains and reduction of inflammatory mediators respect to placebo [,]. The use of a standard multivitamin formula provided modest benefits in reducing periodontal inflammation [].
Four studies reported on gingivitis/periodontitis/tooth loss and vitamin D during particular periods of a woman’s life, pregnancy [,], menopause [,]. Low vitamin D levels in saliva and serum were statistically associated with gingivitis and periodontitis during pregnancy [,]. vitamin D in post-menopausal was statistically associated with periodontitis [], but the association with tooth loss failed [,].
3.2. Hard Dental Pathological Processes
The main characteristics of the studies included regarding hard tooth tissues (caries and enamel defects) are reported in Table 3.

Table 3.
Main characteristics of the studies included regarding hard dental pathological processes ((a) caries and (b) enamel defects).
Eleven papers were focused on vitamin D and caries; six of them [,,,,] were observational studies showing a statistically significantly association between vitamin D serum level and caries level and/or experience. Five papers [,,,] were on children (age range 1–11 years).
Vitamin D treatment in children or in mothers during pregnancy were associated to caries incidence or experience in five papers [,,,,]. Two cross-sectional studies [,] were done associating vitamin C intake and caries (levels and experience) and erosion in children. Multivitamins intake was related to caries in two papers [,]; vitamin B2, B7, B12 were associated to caries [], while vitamin A was not statistically significantly correlated and vitamin C and vitamin E. statistically significantly correlated to caries []. In early childhood (up to 8 years), serum levels of vitamin D seem to be associated with DMFT and caries risk in the following years [,,]. In early teenagers (10–11 years old) a significantly less caries experience of the first molars was found, when serum vitamin D levels are higher than 50 nmol []. Another study found a direct correlation of serum vitamin D levels in children, 6–17 years of age. The authors found a drop in DMFT of 0.66 at each 10 ng/ml increase of vitamin D []. Regarding the correlation of vitamin C and the occurrence of caries lesions there are controversial results. One study found vitamin C supplementation, but also soft drink consumption, to be positively correlated to caries in 12-year-old children []. Another study including 6- to 13-year-old children found negative correlations between vitamins C and E and caries risk. Salivary vitamin A levels are not to be statistically significantly associated to caries risk []. High intake of especially vitamin B12, riboflavin, pantothenic acid and nicotinic acid seem to be correlated to lower caries rates in 5 years old children, but the association seems not clinically significant [].
Despite caries, the occurrence of enamel hypoplasia seems to be associated with low blood levels of vitamin D during pregnancy [], whereas the occurrence of MIH seems not affected by fetal, postnatal and early childhood levels of vitamin D []. One study found no association between the occurrence of enamel defects and vitamin D in 1- and 2-year-old children born preterm [].
Newly erupted permanent teeth of children have immature enamel, which is more susceptible to acid attack of nutritional acids, e.g. soft drinks or fruit juices. The intake of vitamin C supplements was found to be associated with the incidence of erosive tooth wear in early childhood. In 2- to 5-year-old children, vitamin C supplementation significantly reduced the incidence of erosive tooth wear []. A study on 10- to 12-year-old children found that an intake of vitamin supplements (not specified) seems not to affect the incidence of erosive tooth wear, but decreased their progression significantly []. In general, malnutrition and associated deficiency in vitamin intake increases the occurrence of enamel hypoplasia in children [].
4. Discussion
There is no clear scientific evidence on the role played by vitamins on oral health. There is a general consensus on the effect of vitamins deficiencies or supplementation on oral health but without a substantial scientific evidence.
The aim of this systematic and metanalysis review was to evaluate if there were associations between vitamin intake (supplementation or diet intake or saliva/serum level) and gingival/periodontitis and hard dental pathological processes (dental caries, tooth wear and enamel defects).
The lack of convincing associations and the relative dearth of possible associations suggest that the evidence for oral health benefits of vitamins that may be reaped from population-wide vitamin supplementation is weak. The issues to attain positive outcomes from experimental clinical trials are linked to the dosages of vitamins or more effective treatments that might act as confounding factors, thereby camouflaging the effect of the vitamins. Probable associations, where highly significant effects appear in randomised trials, hold the most promise for clinical translation; however, studies pertain to specific populations (children, pregnant women, patients with systemic diseases), and even in these cases the evidence is not sufficient to make universal recommendations about daily intake. Multivitamin supplement or a combination of two or more vitamins adds more biases as it is not possible to identify the single benefit of each vitamin.
Moreover, the majority of papers are short-term papers. Hence, it was not possible to provide clear scientific evidence for the role played by vitamins. Concerning observational studies, there was a wide variety in the use of dietary supplement and clinical parameters used, which could explain the differences found among their results.
Until at least the middle of the 18th century, several oral diseases like periodontitis were considered a manifestation of vitamin deficiency [,,], but there is no sufficient data supporting the need for vitamin supplementation for oral health. Vitamin D has been related to gingival inflammation [] and tooth loss [,,,,,,]. Moreover, vitamins and in particular vitamin D as a promising oral health-preventive agent were the object of several previous reviews [,,,,], systematic [,,] and narrative [,] leading to a low-certainty conclusion that vitamins may reduce the incidence of caries and periodontitis.
5. Conclusions
In general, although the existing literature suggests that vitamins are important in the prevention and treatment of oral diseases, there is weak evidence supporting the association between vitamins and both gingival/periodontal disease and hard dental pathological processes.
Overall, future longitudinal studies of the oral outcomes associated with vitamins and focused research on the detailed biological mechanisms will have broader applications in dentistry and medicine.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/3/938/s1: Table S1: List of excluded papers, Table S2: Quality Assessment, Table S3: List of excluded papers after full text evaluation.
Author Contributions
Conceptualization, T.G.W. and G.C.; methodology, M.G.C., N.C. and G.C.; formal analysis, T.G.W. and C.T.; data curation, G.C.; supervision, P.L.; validation, K.R.E.; original draft preparation, M.G.C. and C.G.; writing—review and editing K.R.E. and P.L. All authors have read and agreed to the published version of the manuscript.
Funding
All person that had taken part in the study are mentioned as authors. This research received no specific grant from any funding agency in public, commercial or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Varela-López, A.; Navarro-Hortal, M.D.; Giampieri, F.; Bullón, P.; Battino, M.; Quiles, J.L. Nutraceuticals in Periodontal Health: A Systematic Review on the Role of Vitamins in Periodontal Health Maintenance. Molecules 2018, 20, 23. [Google Scholar]
- Dickinson, A.; MacKay, D. Health habits and other characteristics of dietary supplement users: A review. Nutr. J. 2014, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, P.J. The relationship between nutrition and systemic and oral well-being in older people. J. Am. Dent. Assoc. 2007, 138, 493–497. [Google Scholar] [CrossRef] [PubMed]
- McKenna, G.; Allen, P.F.; O’Mahony, D.; Flynn, A.; Cronin, M.; Da Mata, C.; Woods, N. Comparison of functionally orientated tooth replacement and removable partial dentures on the nutritional status of partially dentate older patients: A randomised controlled clinical trial. J. Dent. 2014, 42, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Gaewkhiew, P.; Sabbah, W.; Bernabé, E. Does tooth loss affect dietary intake and nutritional status? A systematic review of longitudinal studies. J. Dent. 2017, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Graells, J.; Ojeda, R.M.; Muniesa, C.; Gonzalez, J.; Saavedra, J. Glossitis with linear lesions: An early sign of vitamin B12 deficiency. J. Am. Acad. Dermatol. 2009, 60, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Jagelavičienė, E.; Vaitkevičienė, I.; Šilingaitė, D.; Šinkūnaitė, E.; Daugėlaitė, G. The Relationship between Vitamin D and Periodontal Pathology. Medicina 2018, 54, 3. [Google Scholar] [CrossRef]
- Psoter, W.J.; Reid, B.C.; Katz, R.V. Malnutrition and dental caries: A review of the literature. Caries Res. 2005, 39, 441–447. [Google Scholar] [CrossRef]
- Kanchanakamol, U.; Tuongratanaphan, S.; Tuongratanaphan, S.; Lertpoonvilaikul, W.; Chittai-Song, C.; Pattanaporn, K.; Navia, J.M.; Davies, G.N. Prevalence of developmental enamel defects and dental caries in rural pre-school Thai children. Commun. Dent. Health 1996, 13, 204–207. [Google Scholar]
- Gyll, J.; Ridell, K.; Öhlund, I.; Karlsland Åkeson, P.; Johansson, I.; Lif Holgerson, P. Vitamin D status and dental caries in healthy Swedish children. Nutr. J. 2018, 17, 11. [Google Scholar]
- Lussi, A.; Carvalho, T.S. Erosive tooth wear: A multifactorial condition of growing concern and increasing knowledge. Monogr. Oral. Sci. 2014, 25, 1–15. [Google Scholar] [PubMed]
- Li, H.; Zou, Y.; Ding, G. Dietary factors associated with dental erosion: A meta-analysis. PLoS ONE 2012, 7, e42626. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Egger, M.; Sterne, J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, L.; Lin, Y.F.; Xie, G.F. Role of vitamin C in wound healing after dental implant surgery in patients treated with bone grafts and patients with chronic periodontitis. Clin. Implant Dent. Relat. Res. 2018, 20, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Balci Yuce, H.; Gokturk, O.; Aydemir Turkal, H.; Inanir, A.; Benli, I.; Demir, O. Assessment of local and systemic 25-hydroxy-vitamin D, RANKL, OPG, and TNF levels in patients with rheumatoid arthritis and periodontitis. J. Oral. Sci. 2017, 59, 397–404. [Google Scholar] [CrossRef]
- Deepti, J.; Tewari, S.; Narula, S.C.; Singhal, S.R.; Sharma, R.K. Effect of non-surgical periodontal therapy along with myo-inositol on high-sensitivity c-reactive protein and insulin resistance in women with polycystic ovary syndrome and chronic periodontitis: A randomized controlled trial. J. Periodontol. 2017, 88, 999–1011. [Google Scholar] [CrossRef]
- Abreu, O.J.; Tatakis, D.N.; Elias-Boneta, A.R.; Lopez Del Valle, L.; Hernandez, R.; Pousa, M.S.; Palacios, C. Low vitamin D status strongly associated with periodontitis in Puerto Rican adults. BMC Oral Health 2016, 16, 89. [Google Scholar] [CrossRef]
- Adegboye, A.R.; Boucher, B.J.; Kongstad, J.; Fiehn, N.E.; Christensen, L.B.; Heitmann, B.L. Calcium, vitamin D, casein and whey protein intakes and periodontitis among Danish adults. Public Health Nutr. 2016, 19, 503–510. [Google Scholar] [CrossRef]
- Gümüş, P.; Öztürk, V.Ö.; Bozkurt, E.; Emingil, G. Evaluation of the gingival inflammation in pregnancy and postpartum via 25-hydroxy-vitamin D3, prostaglandin E2 and TNF-α levels in saliva. Arch. Oral Biol. 2016, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pavlesen, S.; Mai, X.; Wactawski-Wende, J.; LaMonte, M.J.; Hovey, K.M.; Genco, R.J.; Millen, A.E. Vitamin D Status and Tooth Loss in Postmenopausal Females: The Buffalo Osteoporosis and Periodontal Disease (OsteoPerio) Study. J. Periodontol. 2016, 87, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef]
- Shimabukuro, Y.; Nakayama, Y.; Ogata, Y.; Tamazawa, K.; Shimauchi, H.; Nishida, T.; Ito, K.; Chikazawa, T.; Kataoka, S.; Murakami, S. Effects of an ascorbic acid-derivative dentifrice in patients with gingivitis: A double-masked, randomized, controlled clinical trial. J. Periodontol. 2015, 86, 27–35. [Google Scholar] [CrossRef]
- Lee, H.J.; Je, D.I.; Won, S.J.; Paik, D.I.; Bae, K.H. Association between vitamin D deficiency and periodontal status in current smokers. Community Dent. Oral Epidemiol. 2015, 43, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chander Narula, S.; Kumar Sharma, R.; Tewari, S.; Kumar Sehgal, P. Vitamin E supplementation, superoxide dismutase status, and outcome of scaling and root planing in patients with chronic periodontitis: A randomized clinical trial. J. Periodontol. 2014, 85, 242–249. [Google Scholar] [CrossRef]
- Jimenez, M.; Giovannucci, E.; Krall Kaye, E.; Joshipura, K.J.; Dietrich, T. Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr. 2014, 17, 844–852. [Google Scholar] [CrossRef]
- Alshouibi, E.N.; Kaye, E.K.; Cabral, H.J.; Leone, C.W.; Garcia, R.I. Vitamin D and periodontal health in older men. J. Dent. Res. 2013, 92, 689–693. [Google Scholar] [CrossRef]
- Gokhale, N.H.; Acharya, A.B.; Patil, V.S.; Trivedi, D.J.; Thakur, S.L. A short-term evaluation of the relationship between plasma ascorbic acid levels and periodontal disease in systemically healthy and type 2 diabetes mellitus subjects. J. Diet Suppl. 2013, 10, 93–104. [Google Scholar] [CrossRef]
- Hiremath, V.P.; Rao, C.B.; Naik, V.; Prasad, K.V. Anti-inflammatory effect of vitamin D on gingivitis: A dose-response randomised control trial. Oral Health Prev. Dent. 2013, 11, 61–69. [Google Scholar] [CrossRef]
- Iwasaki, M.; Moynihan, P.; Manz, M.C.; Taylor, G.W.; Yoshihara, A.; Muramatsu, K.; Watanabe, R.; Miyazaki, H. Dietary antioxidants and periodontal disease in community-based older Japanese: A 2-year follow-up study. Public Health Nutr. 2013, 16, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Andrews, C.A.; LaMonte, M.J.; Hovey, K.M.; Swanson, M.; Genco, R.J.; Wactawski-Wende, J. Vitamin D status and 5-year changes in periodontal disease measures among postmenopausal women: The Buffalo OsteoPerio Study. J. Periodontol. 2014, 85, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.R.; Teles, R.P.; Martin, L.; Socransky, S.S.; Haffajee, A.D. Relationships among interleukin-6, tumor necrosis factor-α, adipokines, vitamin D, and chronic periodontitis. J. Periodontol. 2012, 83, 1183–1191. [Google Scholar] [CrossRef]
- Arya, R.; Gulati, S.; Kabra, M.; Sahu, J.K.; Kalra, V. Folic acid supplementation prevents phenytoin-induced gingival overgrowth in children. Neurology 2011, 76, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Bashutski, J.D.; Eber, R.M.; Kinney, J.S.; Benavides, E.; Maitra, S.; Braun, T.M.; Giannobile, W.V.; McCauley, L.K. The impact of vitamin D status on periodontal surgery outcomes. J. Dent. Res. 2011, 90, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Boggess, K.A.; Espinola, J.A.; Moss, K.; Beck, J.; Offenbacher, S.; Camargo, C.A., Jr. Vitamin D status and periodontal disease among pregnant women. J. Periodontol. 2011, 82, 195–200. [Google Scholar] [CrossRef]
- Harpenau, L.A.; Cheema, A.T.; Zingale, J.A.; Chambers, D.W.; Lundergan, W.P. Effects of nutritional supplementation on periodontal parameters, carotenoid antioxidant levels, and serum C-reactive protein. J. Calif. Dent. Assoc. 2011, 39, 309–312. [Google Scholar]
- Liu, Y.; Jing, H.; Wang, J.; Zhang, R.; Zhang, Y.; Zhang, Y.; Xu, Q.; Yu, X.; Xue, C. Micronutrients decrease incidence of common infections in type 2 diabetic outpatients. Asia Pac. J. Clin. Nutr. 2011, 20, 375–382. [Google Scholar]
- Willershausen, B.; Ross, A.; Försch, M.; Willershausen, I.; Mohaupt, P.; Callaway, A. The influence of micronutrients on oral and general health. Eur. J. Med. Res. 2011, 10, 514–518. [Google Scholar] [CrossRef]
- Abou Sulaiman, A.E.; Shehadeh, R.M. Assessment of total antioxidant capacity and the use of vitamin C in the treatment of non-smokers with chronic periodontitis. J. Periodontol. 2010, 81, 1547–1554. [Google Scholar] [CrossRef]
- Esaki, M.; Morita, M.; Akhter, R.; Akino, K.; Honda, O. Relationship between folic acid intake and gingival health in non-smoking adults in Japan. Oral Dis. 2010, 16, 96–101. [Google Scholar] [CrossRef]
- Liu, K.; Meng, H.; Tang, X.; Xu, L.; Zhang, L.; Chen, Z.; Shi, D.; Feng, X.; Lu, R. Elevated plasma calcifediol is associated with aggressive periodontitis. J. Periodontol. 2009, 80, 1114–1120. [Google Scholar] [CrossRef]
- Llena, C.; Forner, L.; Vento, C. Anticalculus efficacy of a new dentifrice. Quintessence Int. 2009, 40, 497–501. [Google Scholar] [PubMed]
- Linden, G.J.; McClean, K.M.; Woodside, J.V.; Patterson, C.C.; Evans, A.; Young, I.S.; Kee, F. Antioxidants and periodontitis in 60-70-year-old men. J. Clin. Periodontol. 2009, 36, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Miley, D.D.; Garcia, M.N.; Hildebolt, C.F.; Shannon, W.D.; Couture, R.A.; Anderson Spearie, C.L.; Dixon, D.A.; Langenwalter, E.M.; Mueller, C.; Civitelli, R. Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J. Periodontol. 2009, 80, 1433–1439. [Google Scholar] [CrossRef]
- Chapple, I.L.; Milward, M.R.; Dietrich, T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J. Nutr. 2007, 137, 657–664. [Google Scholar] [CrossRef]
- Dietrich, T.; Kaye, E.K.; Nunn, M.E.; Van Dyke, T.; Garcia, R.I. Gingivitis susceptibility and its relation to periodontitis in men. J. Dent. Res. 2006, 85, 1134–1137. [Google Scholar] [CrossRef]
- Erdemir, E.O.; Bergstrom, J. Relationship between smoking and folic acid, vitamin B12 and some haematological variables in patients with chronic periodontal disease. J. Clin. Periodontol. 2006, 33, 878–884. [Google Scholar] [CrossRef]
- Lingstrom, P.; Fure, S.; Dinitzen, B.; Fritzne, C.; Klefbom, C.; Birkhed, D. The release of vitamin C from chewing gum and its effects on supragingival calculus formation. Eur. J. Oral Sci. 2005, 113, 20–27. [Google Scholar] [CrossRef]
- Neiva, R.F.; Al-Shammari, K.; Nociti, F.H., Jr.; Soehren, S.; Wang, H.L. Effects of vitamin-B complex supplementation on periodontal wound healing. J. Periodontol. 2005, 76, 1084–1091. [Google Scholar] [CrossRef]
- Staudte, H.; Sigusch, B.W.; Glockmann, E. Grapefruit consumption improves vitamin C status in periodontitis patients. Br. Dent. J. 2005, 199, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Joshipura, K.J.; Dawson-Hughes, B.; Bischoff-Ferrari, H.A. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am. J. Clin. Nutr. 2004, 80, 108–113. [Google Scholar]
- Prasad, V.N.; Chawla, H.S.; Goyal, A.; Gauba, K.; Singhi, P. Folic acid and phenytoin induced gingival overgrowth--is there a preventive effect. J. Indian Soc. Pedod. Prev. Dent. 2004, 22, 82–91. [Google Scholar] [PubMed]
- Krall, E.A.; Wehler, C.; Garcia, R.I.; Harris, S.S.; Dawson-Hughes, B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am. J. Med. 2001, 111, 452–456. [Google Scholar] [CrossRef]
- Petti, S.; Cairella, G.; Tarsitani, G. Nutritional variables related to gingival health in adolescent girls. Community Dent. Oral Epidemiol. 2000, 28, 407–413. [Google Scholar] [CrossRef]
- Syed, S.; Yassin, S.M.; Dawasaz, A.A.; Amanullah, M.; Alshahrani, I.; Togoo, R.A. Salivary 1,5-Anhydroglucitol and Vitamin Levels in Relation to Caries Risk in Children. Biomed. Res. Int. 2019, 4503450. [Google Scholar] [CrossRef]
- Kim, I.J.; Lee, H.S.; Ju, H.J.; Na, J.Y.; Oh, H.W. A cross-sectional study on the association between vitamin D levels and caries in the permanent dentition of Korean children. BMC Oral Health 2018, 18, 43. [Google Scholar] [CrossRef]
- Seminario, A.L.; Jumani, K.; Velan, E.; Scott, J.M.; Latimer, J.; Schroth, R.J. Suboptimal Serum Vitamin D Associated with Early Childhood Caries in Special Health Care Needs Children. J. Dent. Child. 2018, 85, 93–101. [Google Scholar]
- van der Tas, J.T.; Elfrink, M.E.C.; Heijboer, A.C.; Rivadeneira, F.; Jaddoe, V.W.V.; Tiemeier, H.; Schoufour, J.D.; Moll, H.A.; Ongkosuwito, E.M.; Wolvius, E.B.; et al. Foetal, neonatal and child vitamin D status and enamel hypomineralization. Community Dent. Oral. Epidemiol. 2018, 46, 343–351. [Google Scholar] [CrossRef]
- Wójcik, D.; Krzewska, A.; Szalewski, L.; Pietryka-Michałowska, E.; Szalewska, M.; Krzewski, S.; Pels, E.; Beń-Skowronek, I. Dental caries and vitamin D3 in children with growth hormone deficiency: A STROBE compliant study. Medicine 2018, 97, e981. [Google Scholar]
- Kühnisch, J.; Thiering, E.; Heinrich-Weltzien, R.; Hellwig, E.; Hickel, R.; Heinrich, J. Fluoride/vitamin D tablet supplementation in infants-effects on dental health after 10 years. Clin. Oral Investig. 2017, 21, 2283–2290. [Google Scholar] [CrossRef]
- Reed, S.G.; Voronca, D.; Wingate, J.S.; Murali, M.; Lawson, A.B.; Hulsey, T.C.; Ebeling, M.D.; Hollis, B.W.; Wagner, C.L. Prenatal vitamin D and enamel hypoplasia in human primary maxillary central incisors: A pilot study. Pediatr. Dent. J. 2017, 27, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Dudding, T.; Thomas, S.J.; Duncan, K.; Lawlor, D.A.; Timpson, N.J. Re-Examining the Association between Vitamin D and Childhood Caries. PLoS ONE 2015, 10, e0143769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kühnisch, J.; Thiering, E.; Kratzsch, J.; Heinrich-Weltzien, R.; Hickel, R.; Heinrich, J.; GINI-10 Plus Study Group; LISA-10 Plus Study Group. Elevated serum 25(OH)-vitamin D levels are negatively correlated with MIH. J. Dent. Res. 2015, 94, 381–387. [Google Scholar]
- Tanaka, K.; Hitsumoto, S.; Miyake, Y.; Okubo, H.; Sasaki, S.; Miyatake, N.; Arakawa, M. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann. Epidemiol. 2015, 25, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Schroth, R.J.; Lavelle, C.; Tate, R.; Bruce, S.; Billings, R.J.; Moffatt, M.E. Prenatal vitamin D and dental caries in infants. Pediatrics 2014, 133, e1277–e1284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chau, A.M.; Lo, E.C.; Chu, C.H. Dental caries and erosion status of 12-year-old Hong Kong children. BMC Public Health 2014, 14, 7. [Google Scholar] [CrossRef]
- Schroth, R.J.; Levi, J.A.; Sellers, E.A.; Friel, J.; Kliewer, E.; Moffatt, M.E. Vitamin D status of children with severe early childhood caries: A case-control study. BMC Pediatr. 2013, 13, 174. [Google Scholar] [CrossRef]
- El Aidi, H.; Bronkhorst, E.M.; Huysmans, M.C.D.N.J.M.; Truin, G.J. Multifactorial Analysis of Factors Associated with the Incidence and Progression of Erosive Tooth Wear. Caries Res. 2011, 45, 303–312. [Google Scholar] [CrossRef]
- MacKeown, J.M.; Cleaton-Jones, P.E.; Fatti, P. Caries and micronutrient intake among urban South African children: A cohort study. Community Dent. Oral Epidemiol. 2003, 31, 213–220. [Google Scholar] [CrossRef]
- Al-Malik, M.I.; Holt, R.D.; Bedi, R. The relationship between erosion, caries and rampant caries and dietary habits in preschool children in Saudi Arabia. Int. J. Paediatr. Dent. 2001, 11, 430–439. [Google Scholar]
- Aine, L.; Backström, M.C.; Mäki, R.; Kuusela, A.L.; Koivisto, A.M.; Ikonen, R.S.; Mäki, M. Enamel defects in primary and permanent teeth of children born prematurely. J. Oral Pathol. Med. 2000, 29, 403–409. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Lingstrom, P. Nutrition, dental caries and periodontal disease: A narrative review. J. Clin. Periodontol. 2017, 44, S79–S84. [Google Scholar] [CrossRef]
- Hujoel, P.P. Vitamin D and dental caries in controlled clinical trials: Systematic review and meta-analysis. Nutrition Reviews 2012, 71, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Zuzannna Slebioda, Z.; Szponar, E.; Dorocka-Bobkowska, B. Vitamin D and Its Relevance in the Etiopathogenesis of Oral Cavity Diseases. Arch. Immunol. Ther. Exp. 2016, 64, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Seminario, A.L.; Velan, E. Vitamin D and Dental Caries in Primary Dentition. J. Dent. Child. 2016, 83, 114–119. [Google Scholar]
- Shaik, P.S.; Pachava, S. The Role of Vitamins and Trace Elements on Oral Health: A Systematic Review. Int. J. Med. Rev. 2017, 4, 22–31. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).