Long-Term Exposure to Benzo[a]Pyrene Affects Sexual Differentiation and Embryos Toxicity in Three Generations of Marine Medaka (Oryzias Melastigma)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
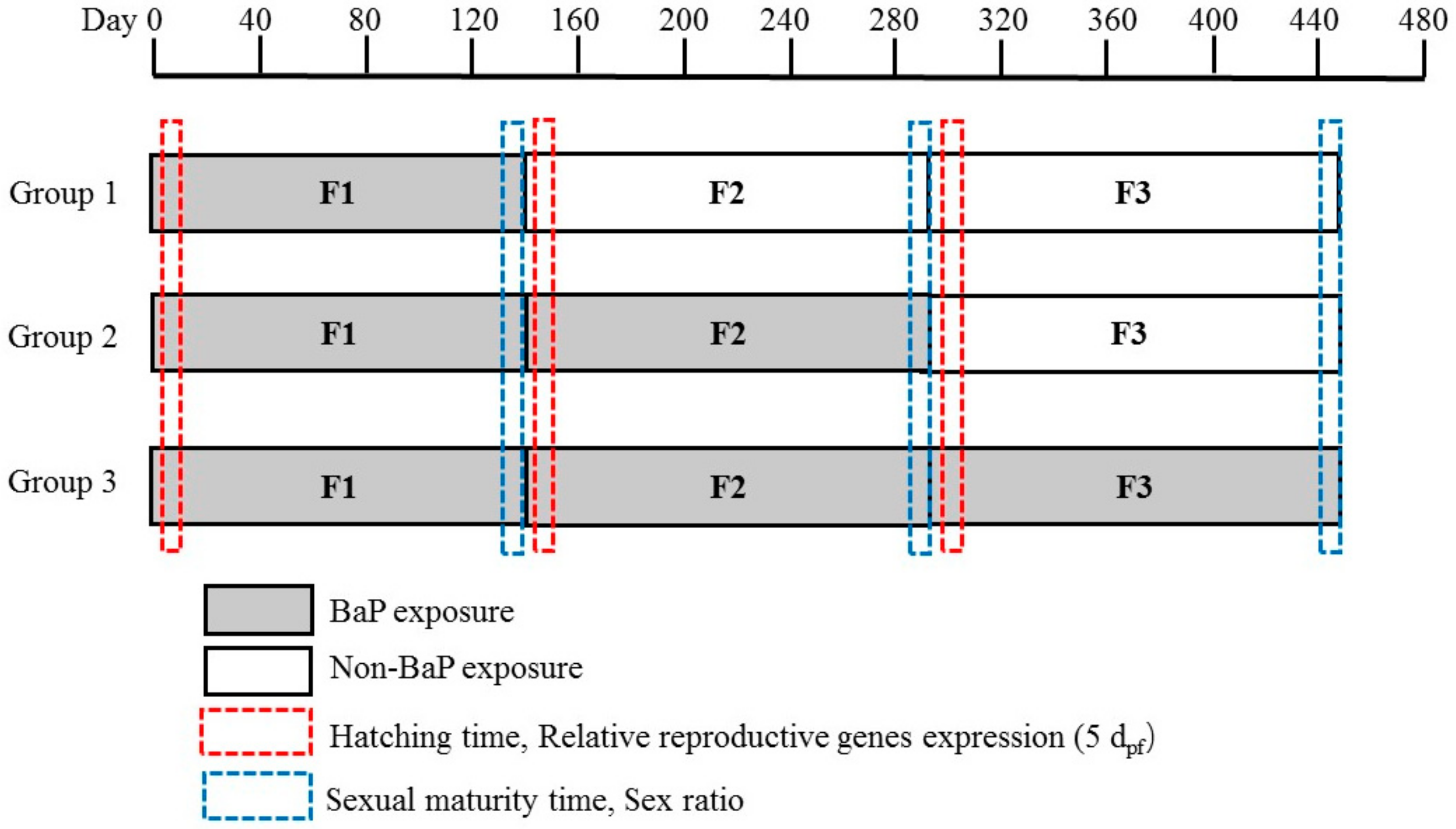
2.2. Test Fish Husbandry and Reproduction
2.3. Exposure Assays
2.4. Quantitative Real-Time PCR Analysis
2.5. Data and Statistical Analysis
3. Results
3.1. Hatching Time in the Offspring of Multigeneration Marine Medaka Exposed to BaP
3.2. Sexual Maturity Time in Multigeneration Marine Medaka Exposed to BaP
3.3. Sex Ratio in Multigeneration Marine Medaka Exposed to BaP
3.4. Gene Transcriptional Expression in Multigeneration Marine Medaka Exposed to BaP
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BaP | Benzo[a]pyrene; |
| cyp19 | cytochrome P450 19/aromatase; |
| dpf | days post fertilization; |
| EDC | endocrine disrupting chemical; |
| ERα | estrogen receptors α; |
| PAHs | polycyclic aromatic hydrocarbons; |
| vtg1 | vitellogenin. |
References
- Tamamura, S.; Sato, T.; Ota, Y.; Wang, X.L.; Tang, N.; Hayakawa, K. Long-range transport of polycyclic aromatic hydrocarbons (PAHs) from the eastern Asian continent to Kanazawa, Japan with Asian dust. Atmos. Environ. 2007, 41, 2580–2593. [Google Scholar]
- Yu, N.; Ding, Q.Q.; Li, E.C.; Qin, J.G.; Chen, L.Q.; Wang, X.D. Growth, energy metabolism and transcriptomic responses in Chinese mitten crab (Eriocheir sinensis) to benzo[alpha]pyrene (BaP) toxicity. Aquat. Toxicol. 2018, 203, 150–158. [Google Scholar]
- Tian, M.P.; Zhao, B.H.; Zhang, J.; Martin, F.L.; Huang, Q.Y.; Liu, L.P.; Shen, H.Q. Association of environmental benzo[a]pyrene exposure and DNA methylation alterations in hepatocellular carcinoma: A Chinese case-control study. Sci. Total Environ. 2016, 541, 1243–1252. [Google Scholar]
- Saunders, C.R.; Ramesh, A.; Shockley, D.C. Modulation of neurotoxic behavior in F-344 rats by temporal disposition of benzo(a)pyrene. Toxicol. Lett. 2002, 129, 33–45. [Google Scholar]
- McCallister, M.M.; Li, Z.; Zhang, T.W.; Ramesh, A.; Clark, R.S.; Maguire, M.; Hutsell, B.; Newland, M.C.; Hood, D.B. Revealing Behavioral Learning Deficit Phenotypes Subsequent to In Utero Exposure to Benzo(a)pyrene. Toxicol. Sci. 2016, 149, 42–54. [Google Scholar]
- Baan, R.; Grosse, Y.; Straif, K.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. Special Report: Policy A review of human carcinogens-Part F: Chemical agents and related occupations. Lancet Oncol. 2009, 10, 1143–1144. [Google Scholar]
- Groll, M.; Opp, C.; Kulmatov, R.; Ikramova, M.; Normatov, I. Water quality, potential conflicts and solutions—An upstream-downstream analysis of the transnational Zarafshan River (Tajikistan, Uzbekistan). Environ. Earth Sci. 2015, 73, 743–763. [Google Scholar]
- Li, J.F.; Dong, H.; Zhang, D.H.; Han, B.; Zhu, C.J.; Liu, S.P.; Liu, X.M.; Ma, Q.Y.; Li, X.G. Sources and ecological risk assessment of PAHs in surface sediments from Bohai Sea and northern part of the Yellow Sea, China. Mar. Pollut. Bull. 2015, 96, 485–490. [Google Scholar]
- Yi, Y.Y.; Ling, Y.B.; Huang, X.F. Association between air pollution and female breast cancer: A meta-analysis. Eur. J. Gynaecol. Oncol. 2017, 38, 578–583. [Google Scholar]
- Khaksar, F.; Manavi, P.N.; Ardalan, A.A.; Abedi, E.; Saleh, A. Concentration of polycyclic aromatic hydrocarbons in zooplanktons of Bushehr coastal waters (north of the Persian Gulf). Mar. Pollut. Bull. 2019, 140, 35–39. [Google Scholar]
- Regnault, C.; Willison, J.; Veyrenc, S.; Airieau, A.; Meresse, P.; Fortier, M.; Fournier, M.; Brousseau, P.; Raveton, M.; Reynaud, S. Metabolic and immune impairments induced by the endocrine disruptors benzo[a]pyrene and triclosan in Xenopus tropicalis. Chemosphere 2016, 155, 519–527. [Google Scholar]
- Yan, Z.H.; Lu, G.H.; Ye, Q.X.; Liu, J.C. Modulation of 17 beta-estradiol induced estrogenic responses in male goldfish (Carassius auratus) by benzo[a]pyrene and ketoconazole. Environ. Sci. Pollut. R. 2016, 23, 9036–9045. [Google Scholar]
- Corrales, J.; Thornton, C.; White, M.; Willett, K.L. Multigenerational effects of benzo[a]pyrene exposure on survival and developmental deformities in zebrafish larvae. Aquat. Toxicol. 2014, 148, 16–26. [Google Scholar]
- Booc, F.; Thornton, C.; Lister, A.; MacLatchy, D.; Willett, K.L. Benzo[a]pyrene Effects on Reproductive Endpoints in Fundulus heteroclitus. Toxicol. Sci. 2014, 140, 73–82. [Google Scholar]
- Knecht, A.L.; Truong, L.; Marvel, S.W.; Reif, D.M.; Garcia, A.; Lu, C.; Simbnich, M.T.; Teeguarden, J.G.; Tanguay, R.L. Transgenerational inheritance of neurobehavioral and physiological deficits from developmental exposure to benzo[a]pyrene in zebrafish. Toxicol. Appl. Pharmacol. 2017, 329, 148–157. [Google Scholar]
- Baumann, L.; Holbech, H.; Keiter, S.; Kinnberg, K.L.; Knorr, S.; Nagel, T.; Braunbeck, T. The maturity index as a tool to facilitate the interpretation of changes in vitellogenin production and sex ratio in the Fish Sexual Development Test. Aquat. Toxicol. 2013, 128, 34–42. [Google Scholar]
- Bang, H.W.; Lee, W.; Kwak, I.S. Detecting points as developmental delay based on the life-history development and urosome deformity of the harpacticoid copepod, Tigriopus japonicus sensu lato, following exposure to benzo(a)pyrene. Chemosphere 2009, 76, 1435–1439. [Google Scholar]
- Wu, Q.Y.; Wang, S.Q.; Chen, X.P.; Li, P. Reproductive toxicity assessment of benzo[a]pyrene in the marine polychaete Perinereis nuntia. Chin. J. Oceanol. Limnol. 2017, 35, 867–873. [Google Scholar]
- Chikae, M.; Hatano, Y.; Ikeda, R.; Morita, Y.; Hasan, Q.; Tamiya, E. Effects of bis(2-ethylhexyl) phthalate and benzo[a]pyrene on the embryos of Japanese medaka (Oryzias latipes). Environ. Toxicol. Pharmacol. 2004, 16, 141–145. [Google Scholar]
- Maskaoui, K.; Zhou, J.L.; Hong, H.S.; Zhang, Z.L. Contamination by polycyclic aromatic hydrocarbons in the Jiulong River Estuary and Western Xiamen Sea, China. Environ. Pollut. 2002, 118, 109–122. [Google Scholar]
- Sun, D.; Chen, Q.; He, N.; Diao, P.P.; Jia, L.X.; Duan, S.S. Effect of environmentally-relevant concentrations of nonylphenol on sexual differentiation in zebrafish: A multi-generational study. Sci. Rep. 2017, 7, 42907. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar]
- Fang, C.; Wu, X.L.; Huang, Q.S.; Liao, Y.Y.; Liu, L.P.; Qiu, L.; Shen, H.Q.; Dong, S.J. PFOS elicits transcriptional responses of the ER, AHR and PPAR pathways in Oryzias melastigma in a stage-specific manner. Aquat. Toxicol. 2012, 106, 9–19. [Google Scholar]
- Mohamed, E.S.A.; Song, W.H.; Oh, S.A.; Park, Y.J.; You, Y.A.; Lee, S.; Choi, J.Y.; Kim, Y.J.; Jo, I.; Pang, M.G. The transgenerational impact of benzo(a)pyrene on murine male fertility. Hum. Reprod. 2010, 25, 2427–2433. [Google Scholar]
- Staples, C.A.; Hall, A.T.; Friederich, U.; Caspers, N.; Klecka, G.M. Early life-stage and multigeneration toxicity study with bisphenol A and fathead minnows (Pimephales promelas). Ecotoxicol. Environ. Saf. 2011, 74, 1548–1557. [Google Scholar]
- Sternberg, R.M.; Hotchkiss, A.K.; LeBlanc, G.A. The contribution of steroidal androgens and estrogens to reproductive maturation of the eastern mud snail Ilyanassa obsoleta. Gen. Comp. Endocrinol. 2008, 156, 15–26. [Google Scholar]
- Rust, A.J.; Burgess, R.M.; Brownawell, B.J.; McElroy, A.E. Relationship between metabolism and bioaccumulation of benzo[a]pyrene in benthic invertebrates. Environ. Toxicol. Chem. 2004, 23, 2587–2593. [Google Scholar]
- Colli-Dula, R.C.; Fang, X.; Moraga-Amador, D.; Albornoz-Abud, N.; Zamora-Bustillos, R.; Conesa, A.; Zapata-Perez, O.; Moreno, D.; Hernandez-Nunez, E. Transcriptome analysis reveals novel insights into the response of low-dose benzo(a)pyrene exposure in male tilapia. Aquat. Toxicol. 2018, 201, 162–173. [Google Scholar]
- Yin, S.S.; Tang, M.L.; Chen, F.F.; Li, T.L.; Liu, W.P. Environmental exposure to polycyclic aromatic hydrocarbons (PAHs): The correlation with and impact on reproductive hormones in umbilical cord serum. Environ. Pollut. 2017, 220, 1429–1437. [Google Scholar]
- Lee, J.W.; Lee, J.W.; Shin, Y.J.; Kim, J.E.; Ryu, T.K.; Ryu, J.; Lee, J.; Kim, P.; Choi, K.; Park, K. Multi-generational xenoestrogenic effects of Perfluoroalkyl acids (PFAAs) mixture on Oryzias latipes using a flow-through exposure system. Chemosphere 2017, 169, 212–223. [Google Scholar]
- Ankley, G.T.; Jensen, K.M.; Kahl, M.D.; Korte, J.J.; Makynen, E.A. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2001, 20, 1276–1290. [Google Scholar]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar]
- Hoffmann, J.L.; Oris, J.T. Altered gene expression: A mechanism for reproductive toxicity in zebrafish exposed to benzo[a]pyrene. Aquat. Toxicol. 2006, 78, 332–340. [Google Scholar]
- Bussmann, U.A.; Saez, J.M.P.; Bussmann, L.E.; Baranao, J.L. Aryl hydrocarbon receptor activation leads to impairment of estrogen-driven chicken vitellogenin promoter activity in LMH cells. Comp. Biochem. Phys. C 2013, 157, 111–118. [Google Scholar]


| Gene | Sense Primer (5′-3′) | Antisense Primer (5′-3′) | GenBank Number | Reference |
|---|---|---|---|---|
| 18s | AACGCTGTGCTGCGTAGCCTCAATT | AGAAGAAGCCCCACTTTTCCTCGCA | DQ105650 | [23] |
| ERα | TCGCCGCTGTTGTGCTGTGATGTT | TCCTGGATCTGAGTGCGGGTCCGA | JF907629 | [23] |
| Cyp19a | ACCTCGCGTTTTGGCAGCAAACA | TTTCCACAGCGCCACGTTGTTGT | JF907625 | [23] |
| Vtg1 | TTGGCAGAGATGCAGCAGCGGT | GGAAATGCAGGACACCCCAGTAGCC | JF268651 | [23] |
| BaP Concentration (μg·L−1) | Hatching Time (dpf) | |||||
|---|---|---|---|---|---|---|
| G1F1 | G1F2 | G1F3 | G2F2 | G2F3 | G3F3 | |
| Control | 10.5 ± 0.5 | 11.33 ± 0.58 | 11.1 ± 0.17 | 10.33 ± 0.58 | 11.43 ± 0.51 | 11.16 ± 0.29 |
| S.Control | 10.67 ± 0.58 | 10.67 ± 0.58 | 11 ± 0.3 | 11.33 ± 0.58 | 10.33 ± 0.85 | 11.1 ± 1.01 |
| 2 | 10.33 ± 0.58 | 10.68 ± 0.58 | 9.9 ± 0.17 | 10.33 ± 0.58 | 10 ± 0.7 | 10.57 ± 1.25 |
| 20 | 11.16 ± 1.04 * | 12.1 ± 0.85 * | 10.67 ± 1.15 | 12.67 ± 0.58 * | 11.67 ± 0.58 | 11.43 ± 0.51 |
| 200 | 13.67 ± 0.58 * | 12.9 ± 0.85 * | 13.43 ± 1.25 * | 15.1 ± 1.15 * | 0 *a | 0 *a |
| BaP Concentration (μg·L−1) | Sexual Maturity Time (dph) | |||||
|---|---|---|---|---|---|---|
| G1F1 | G1F2 | G1F3 | G2F2 | G2F3 | G3F3 | |
| Control | 125 ± 1 | 127.33 ± 3.06 | 124.67 ± 14.47 | 121.67 ± 10.97 | 133 ± 2 | 121.33 ± 11.59 |
| S.Control | 127 ± 1.73 | 127.33 ± 7.09 | 119.33 ± 4.16 | 123.33 ± 6.66 | 131.33 ± 3.21 | 120.33 ± 2.08 |
| 2 | 131.67 ± 1.53 * | 126 ± 3.61 | 128.67 ± 5.03 | 127 ± 3.61 | 136 ± 2 | 119 ± 5 |
| 20 | 134 ± 2 * | 124 ± 5.57 | 122 ± 6.24 | 126.33 ± 6.66 | 132.33 ± 2.08 | 142.67 ± 6.11 * |
| 200 | 142 ± 2.65 * | 130 ± 6.08 | 124 ± 8 | 145 ± 6.56 * | 149.33 ± 6.66 * | 158.67 ± 8.02 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Chen, Q.; Zhu, B.; Lan, Y.; Duan, S. Long-Term Exposure to Benzo[a]Pyrene Affects Sexual Differentiation and Embryos Toxicity in Three Generations of Marine Medaka (Oryzias Melastigma). Int. J. Environ. Res. Public Health 2020, 17, 970. https://doi.org/10.3390/ijerph17030970
Sun D, Chen Q, Zhu B, Lan Y, Duan S. Long-Term Exposure to Benzo[a]Pyrene Affects Sexual Differentiation and Embryos Toxicity in Three Generations of Marine Medaka (Oryzias Melastigma). International Journal of Environmental Research and Public Health. 2020; 17(3):970. https://doi.org/10.3390/ijerph17030970
Chicago/Turabian StyleSun, Dong, Qi Chen, Bo Zhu, Yu Lan, and Shunshan Duan. 2020. "Long-Term Exposure to Benzo[a]Pyrene Affects Sexual Differentiation and Embryos Toxicity in Three Generations of Marine Medaka (Oryzias Melastigma)" International Journal of Environmental Research and Public Health 17, no. 3: 970. https://doi.org/10.3390/ijerph17030970
APA StyleSun, D., Chen, Q., Zhu, B., Lan, Y., & Duan, S. (2020). Long-Term Exposure to Benzo[a]Pyrene Affects Sexual Differentiation and Embryos Toxicity in Three Generations of Marine Medaka (Oryzias Melastigma). International Journal of Environmental Research and Public Health, 17(3), 970. https://doi.org/10.3390/ijerph17030970





