Modified Screen-Printed Potentiometric Sensors based on Man-Tailored Biomimetics for Diquat Herbicide Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Man-Taillored MIPs Synthesis
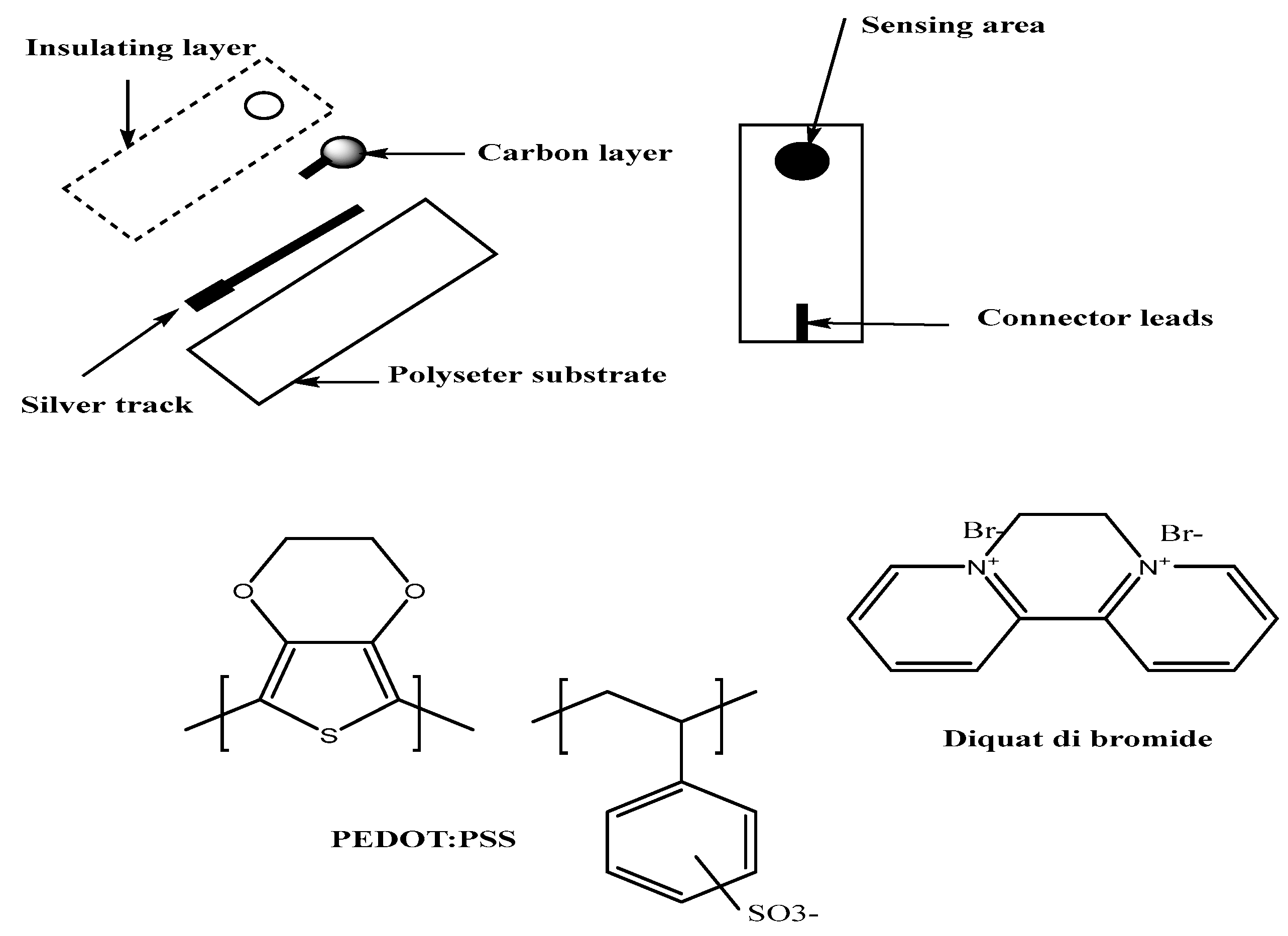
2.4. Screen-Printed Design and Sensor Fabrication
2.5. Diquat Determination in Real Samples
3. Results and Discussions
3.1. Characterization of MIP Particles
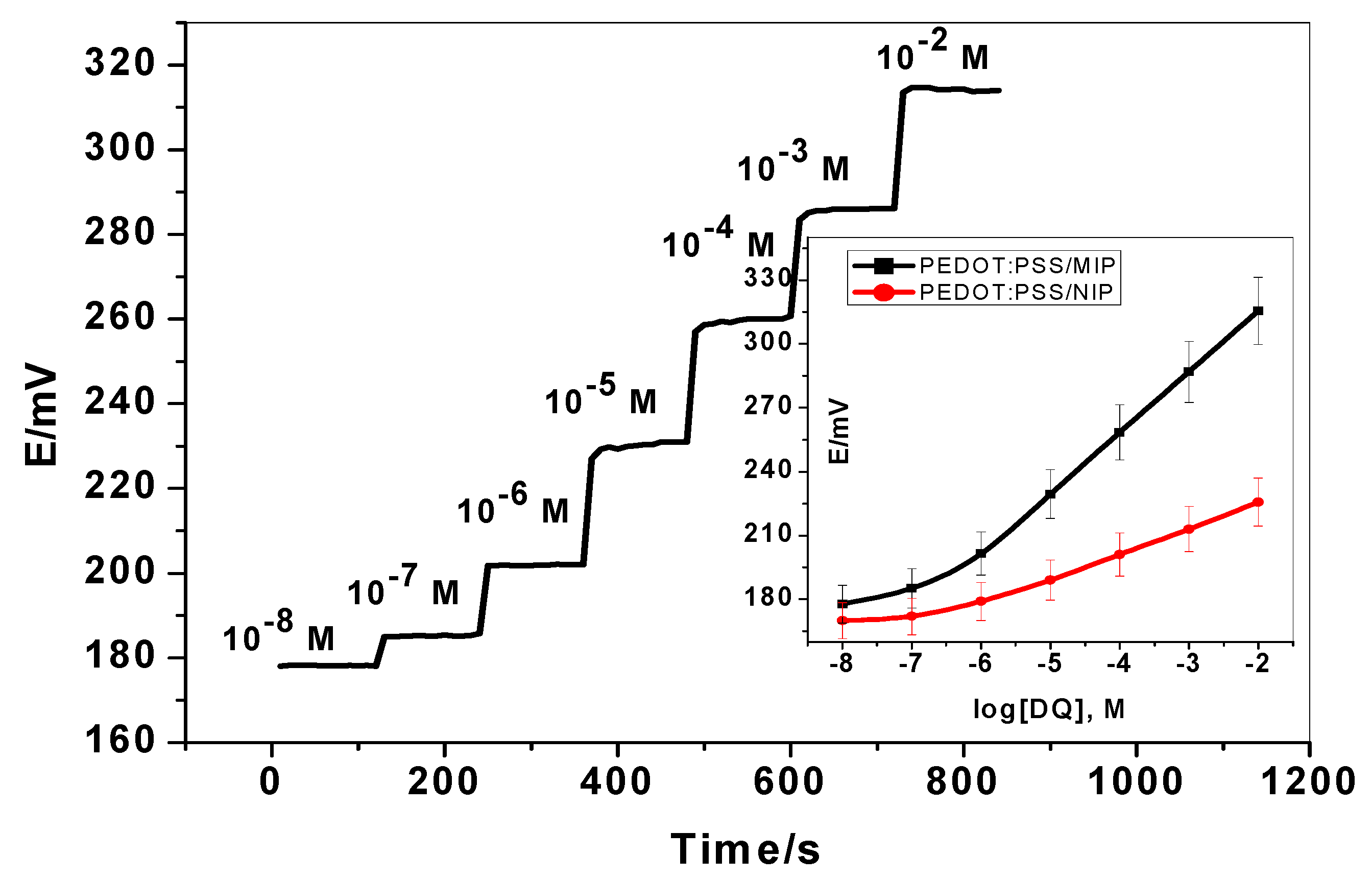
3.2. Potentiometric Characteristics of the Proposed Sensors
3.3. Long-Term Potential Stability
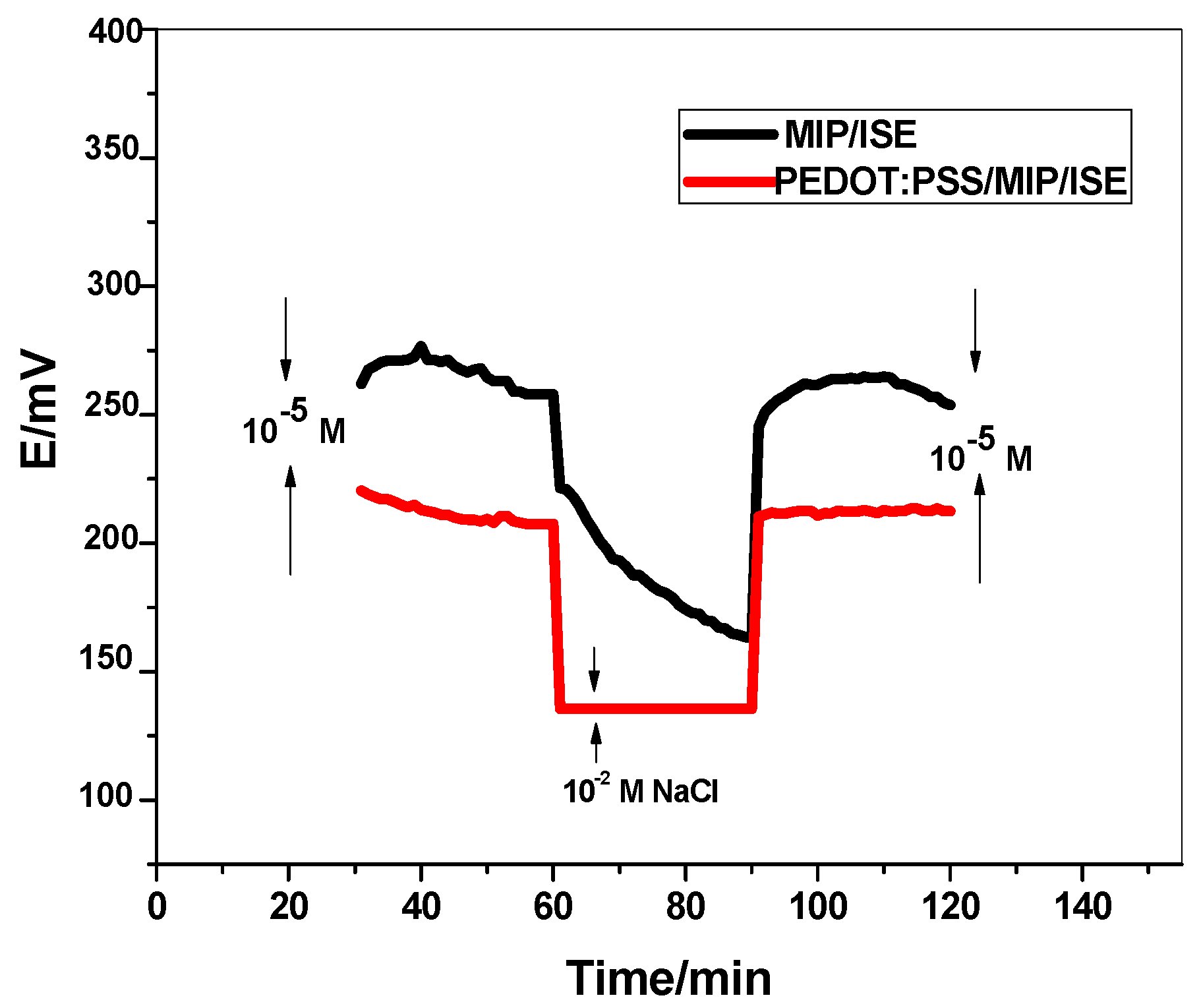
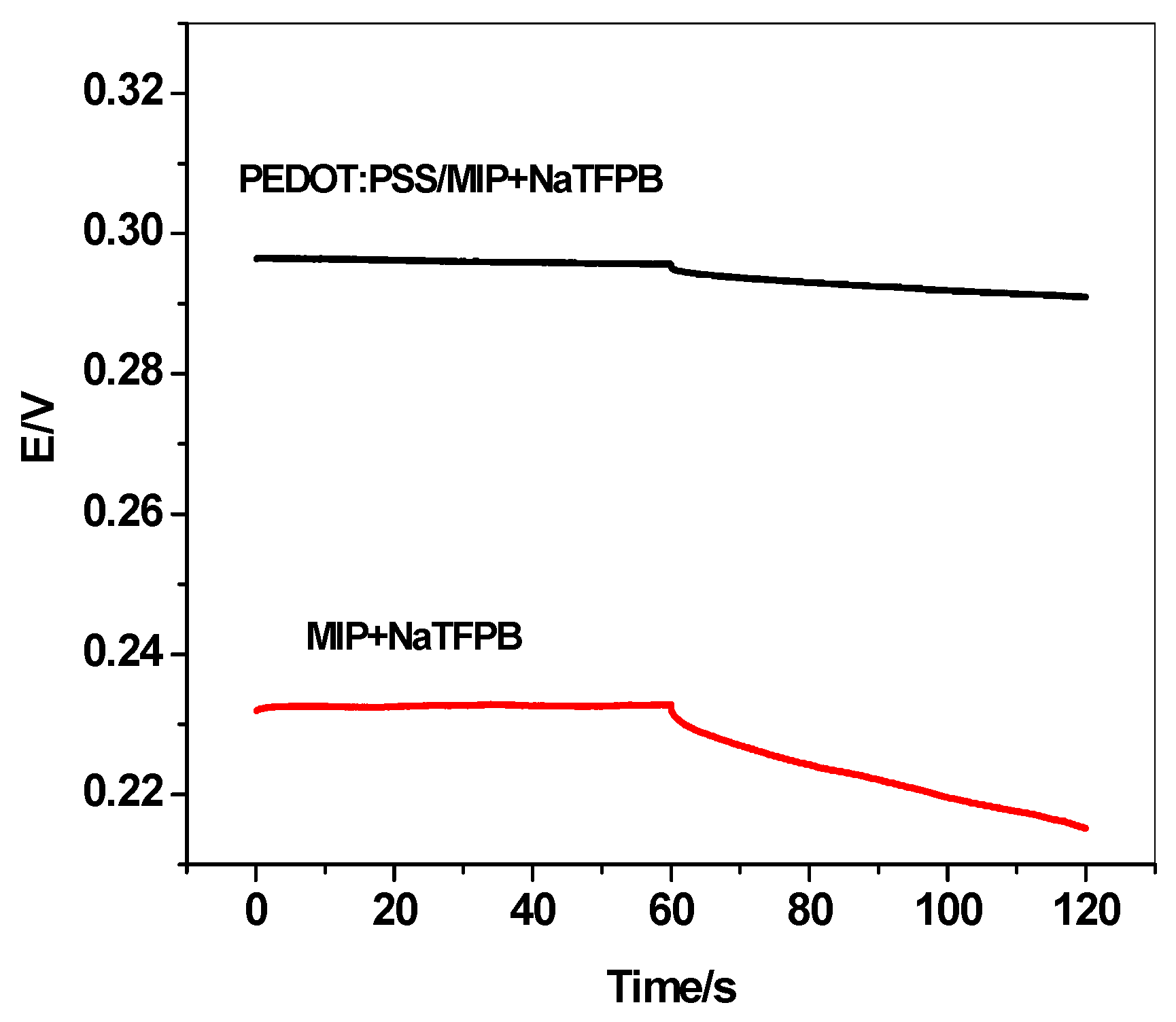
3.4. Short-Term Potential Stability
3.5. Diquat Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gonzalez-Pradas, E.; Villafranca-Sainchez, M.; Rey-Bueno, F.D.; Urena-Amate, M.D.; Socias-Viciana, M.; Femfindez-Perez, M. Removal of diquat and deisopropylatrazine from water by ontmorillonite-(Ce or Zr) phosphate crosslinked compound. Chemosphere 1999, 39, 455–466. [Google Scholar] [CrossRef]
- Pateiro-Moure, M.; Arias-Estévez, M.; Simal-Gándara, J. Critical Review on the Environmental Fate of Quaternary Ammonium Herbicides in Soils Devoted to Vineyards. Environ. Sci. Technol. 2013, 47, 4984–4998. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Peer Review. Available online: https://ec.europa.eu/social/main.jsp?catId=1024&langId=en (accessed on 16 January 2020).
- Hazardous Substances Databank, Toxicology Data Network, U.S. National Library of Medicine, Bethesda, MD. 1988. Available online: https://www.nih.gov/about-nih/what-we-do/nih-almanac/national-library-medicine-nlm (accessed on 16 January 2020).
- Chichila, T.; Walters, S. Liquid Chromatographic Determination of Paraquat and Diquat in Crops Using a Silica Column with Aqueous Ionic Mobile Phase. JAOAC 1991, 74, 961–967. [Google Scholar] [CrossRef]
- Abu Ghalwa, N.; Abu-Shawish, H.M.; Hamada, M.; Hartani, K.; Basheer, A.A. Studies on Degradation of Diquat Pesticide in Aqueous Solutions Using Electrochemical Method. Am. J. Anal. Chem. 2012, 3, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.; Colardyn, F.; De Reuck, J.; Praet, M.; Lameire, N.; Ringoir, S. Diquat intoxication: Report of two cases and review of the literature. Am. J. Med. 1981, 70, 1267–1271. [Google Scholar] [CrossRef]
- Sax, N.I. Dangerous Properties of Industrial Materials, 6th ed.; Van Nostrand Reinhold Company: New York, NY, USA, 1984. [Google Scholar]
- Material safety data sheet: Ortho Diquat Herbicide. h/a No. 1 1978. Richmond, CA: Chevron Environmental Health Center, Inc. June 1986. Available online: http://pmep.cce.cornell.edu/profiles/extoxnet/dienochlor-glyphosate/diquat-ext.html (accessed on 16 January 2020).
- Harmoudi, H.E.; Achak, M.; Lahrich, S.; Farahi, A.; Gaini, L.E.; Bakasse, M.; Mhammedi, M.A.E. Square wave voltammetric determination of diquat using natural phosphate modified platinum electrode. Arab. J. Chem. 2017, 10, S671–S676. [Google Scholar] [CrossRef] [Green Version]
- El Mhammedi, A.M.; Bakasse, M.; Chtaini, A. Investigation of square wave voltammetric detection of diquat at carbon paste electrode impregnated with Ca10(PO4)6F2: Application in natural water samples. Mater. Chem. Phys. 2008, 109, 519–525. [Google Scholar] [CrossRef]
- El Mhammedi, M.A.; Bakasse, M.; Najih, R.; Chtaini, A. A carbon paste electrode modified with kaolin for the detection of diquat. Appl. Clay Sci. 2009, 43, 130–134. [Google Scholar] [CrossRef]
- Walcarius, A.; Lamberts, L. Square wave voltammetric determination of paraquat and diquat in aqueous solution. J. Electroanal. Chem. 1996, 406, 59–68. [Google Scholar] [CrossRef]
- Galeano Díaz, T.; Cabanillas, A.G.; Salinas, F. Square-wave and differential pulse oxidative voltammetric determination of diquat and paraquat in alkaline medium. Electroanalysis 2000, 12, 616–621. [Google Scholar] [CrossRef]
- de Figueiredo-Filho, L.C.S.; Baccarin, M.; Janegitz, B.C.; Fatibello-Filho, O. A disposable and inexpensive bismuth film minisensor for a voltammetric determination of diquat and paraquat pesticides in natural watersamples. Sens. Actuators B 2017, 240, 749–756. [Google Scholar] [CrossRef]
- Lu, T.; Sun, W. Determination of diquat at a nafion film modified glassy carbon electrode using electrocatalytic voltammetry. Electroanalysis 2000, 12, 605–609. [Google Scholar] [CrossRef]
- Yuen, S.H.; Bagness, J.E.; Myles, D. Spectrophotometric determination of diquat and paraquat in aqueous herbicide formulations. Analyst 1967, 92, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, T.; Martínez-Lozano, C.; Tomás, V.; Fenoll, J. Sensitive determination of diquat by a kinetic method using the stopped-flow mixing technique. Analyst 2000, 125, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, T.; Martinez-Lozano, C.; Tomas, V. Spectrofluorimetric determination of diquat by manual and flow-injection methods. Anal. Chim. Acta 1991, 244, 99–104. [Google Scholar] [CrossRef]
- Carrillo-Carrión, C.; Simonet, B.M.; Valcárcel, M. Rapid fluorescence determination of diquat herbicide in food grains using quantum dots as new reducing agent. Anal. Chim. Acta 2011, 692, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Tang, Q.; Huang, Y.; Wang, R.; Xi, Y.; Ni, X.; Tao, Z.; Xue, S.; Zhang, J. A host–guest complexation based fluorescent probe for the detection of paraquat and diquat herbicides in aqueous solutions. RSC Adv. 2015, 5, 100316–100321. [Google Scholar] [CrossRef]
- Núñez, O.; Moyano, E.; Puignou, L.; Galceran, M.T. Sample stacking with matrix removal for the determination of paraquat, diquat and difenzoquat in water by capillary electrophoresis. J. Chromatogr. A 2001, 912, 353–361. [Google Scholar] [CrossRef]
- Ishiwata, T.; Onuki, S.; Okada, H.; Ohashi, K. Determination of paraquat and diquat in human blood by capillary electrophoresis after solid-phase extraction using an Oasis® MCX cartridge. Jpn. J. Forensic Toxicol. 2002, 20, 284–294. [Google Scholar]
- Taguchi, V.Y.; Jenkins, S.W.; Crozier, P.W.; Wang, D.T. Determination of diquat and paraquat in water by liquid chromatography-(electrospray ionization) mass spectrometry. J. Am. Soc. Mass Spectrom. 1998, 9, 830–839. [Google Scholar] [CrossRef] [Green Version]
- Castro, R.; Moyano, E.; Galceran, M.T. Ion-trap versus quadrupole for analysis of quaternary ammonium Herbicides by LC-MS. Chromatographia 2001, 53, 273–278. [Google Scholar] [CrossRef]
- Grey, L.; Nguyen, B.; Yang, P. Liquid chromatography-electrospray ionization isotope dilution mass spectrometry analysis of paraquat and diquat using conventional and multilayer solid-phase extraction cartridges. J. Chromatogr. A 2002, 958, 25–33. [Google Scholar] [CrossRef]
- Hao, C.; Zhao, X.; Morse, D.; Yang, P.; Taguchi, V.; Morra, F. Optimized liquid chromatography tandem mass spectrometry approach for the determination of diquat and paraquat herbicides. J. Chromatogr. A 2013, 1304, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Qiu, J.; Wu, C.; Huang, T.; Meng, R.; Lai, Y. Magnetic single-walled carbon nanotubes-dispersive solid-phase extraction method combined with liquid chromatography-tandem mass spectrometry for the determination of paraquat in urine. J. Chromatogr. B 2014, 965, 85–90. [Google Scholar] [CrossRef]
- Whitehead, R.D., Jr.; Montesano, M.A.; Jayatilaka, N.K.; Buckley, B.; Winnik, B.; Needham, L.L.; Barr, D.B. Method for measurement of the quaternary amine compounds paraquat and diquat in human urine using high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2010, 878, 2548–2553. [Google Scholar] [CrossRef]
- de Almeida, R.M.; Yonamine, M. Gas chromatographic–mass spectrometric method for the determination of the herbicides paraquat and diquat in plasma and urine samples. J. Chromatogr. B 2007, 853, 260–264. [Google Scholar] [CrossRef]
- Shawish, H.M.A.; Ghalwa, N.A.; Hamada, M.; Basheer, A. Modified carbon paste electrode for potentiometric determination of diquat dibromide pesticide in water and urine samples. Mater. Sci. Eng. C 2012, 32, 140–145. [Google Scholar] [CrossRef]
- Moody, G.J.; Owusu, R.K.; Thomas, J.D.R. Analysis of diquat by ion selective electrodes. Anal. Let. 1988, 21, 1653–1664. [Google Scholar] [CrossRef]
- Thomas, J.D.R. Ion-selective electrode studies on novel organic molecule sensors. Analyst 1991, 116, 1211–1215. [Google Scholar] [CrossRef]
- Moody, G.J.; Owusu, R.K.; Thomas, J.D.R. Studies on crown ether based potentiometric sensors for 4,4’-dipyridinium and related dications. Analyst 1988, 113, 65–69. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Galal Eldin, A.; Amr, A.E.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Improved Solid-Contact Nitrate Ion Selective Electrodes Based on Multi-Walled Carbon Nanotubes (MWCNTs) as an Ion-to-Electron Transducer. Sensors 2019, 19, 3891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.S.M.; Amr, A.E.; Elbehery, N.H.A.; Al-Omar, M.A.; Kamel, A.H. Non-equilibrium potential responses towards neutral orcinol using all-solid-state potentiometric sensors integrated with molecularly imprinted polymers. Polymers 2019, 11, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashmawy, N.H.; Almehizia, A.A.; Youssef, T.A.; El-Galil, E.A.A.; Al-Omar, M.A.; Kamel, A.H. Novel Carbon/PEDOT/PSS-Based screen-printed biosensors for acetylcholine neurotransmitter and acetylcholinesterase detection in human serum. Molecules 2019, 24, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Velde, L.; d’Angremont, E.; Olthuis, W. Solid contact potassium selective electrodes for biomedical applications—A review. Talanta 2016, 160, 56–65. [Google Scholar] [CrossRef]
- Mahony, J.O.; Nolan, K.; Smyth, M.R.; Mizaiko, B. Molecularly imprinted polymers—Potential and challenges in analytical chemistry. Anal. Chim. Acta 2005, 36, 31–39. [Google Scholar] [CrossRef]
- Kamel, A.H.; Jiang, X.; Li, P.; Liang, R. A paper-based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal. Methods 2018, 10, 3890–3895. [Google Scholar] [CrossRef]
- El-Kosasy, A.; Kamel, A.H.; Hussin, L.; Ayad, M.F.; Fares, N. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2,4-dichlorophenol as a food taint. Food Chem. 2018, 250, 188–196. [Google Scholar] [CrossRef]
- Kamel, A.H.; Hassan, A.M.E. Solid Contact Potentiometric Sensors Based on Host-Tailored Molecularly Imprinted Polymers for Creatine Assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- Bakker, E. Determination of Unbiased Selectivity Coefficients of Neutral Carrier-Based Cation-Selective Electrodes. Anal. Chem. 1997, 69, 1061–1069. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. The Pesticide Manual—World Compendium, 10th ed.; The British Crop Protection Council: Surrey, UK, 1994; p. 370. [Google Scholar]
- Lagman, L.H.; Hale, J.R. Analytical Method for the Determination of Diquat in Aquatic Weed Infested Lakes and Rivers in South Carolina. In Proceedings of the Water Quality Technology Conference, WQTC-15, Baltimore, MD, USA, 15–20 November 1987. [Google Scholar]
- Moody, G.J.; Owusu, R.K.; Thomas, J.D.R. Liquid Membrane Ion-selective Electrodes for Diquat and Paraquat. Analyst 1987, 112, 121–127. [Google Scholar] [CrossRef]
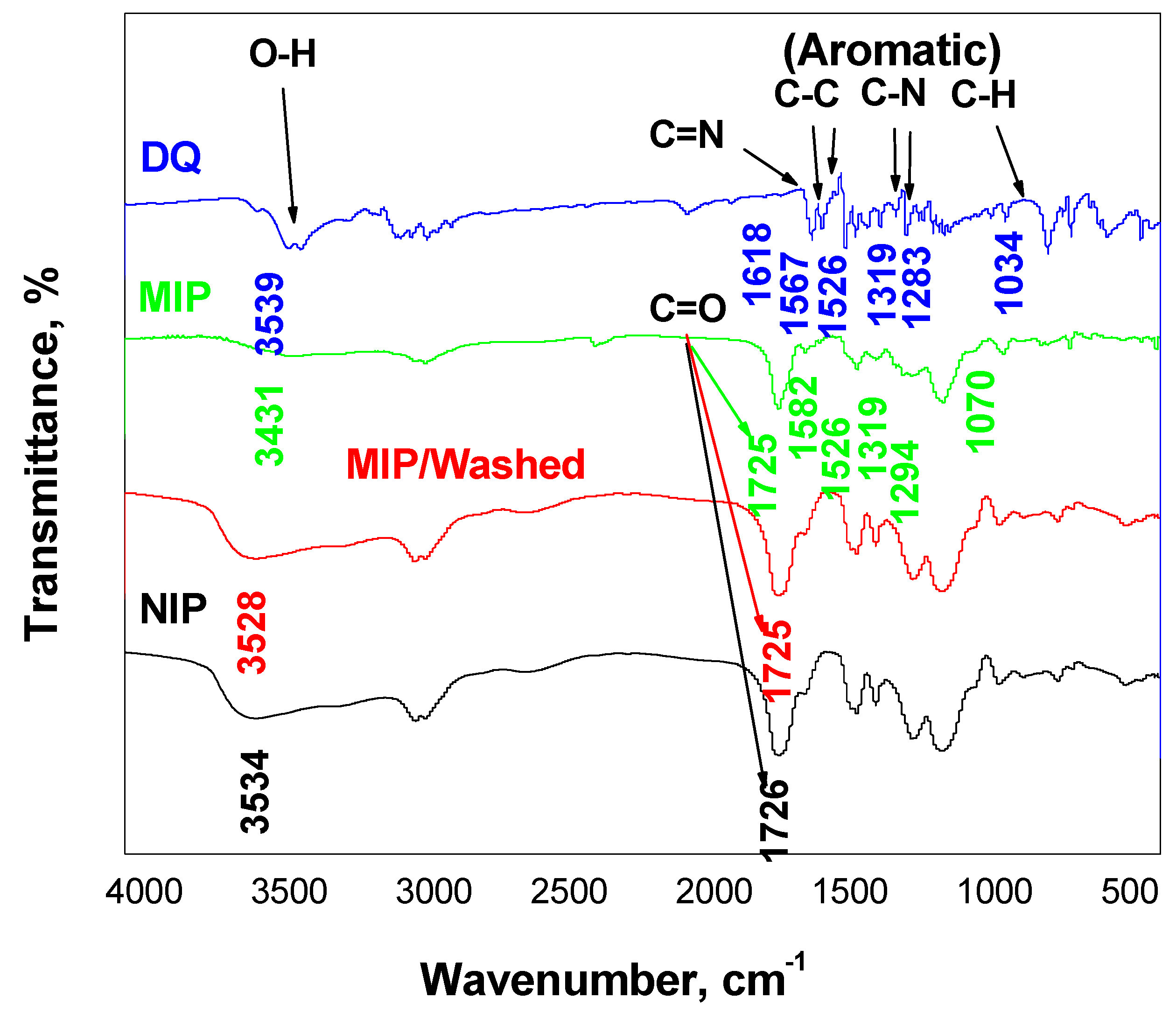
| Peaks | Diquat Dibromide Hydrate | Non-Washed MIP | Washed-MIP | NIP |
|---|---|---|---|---|
| υOH | 3390, 3432 | 3431 | 3528 | 3543 |
| υC–H stretch (aromatic and aliphatic) | 3049–3006, 2949 | 2988–2955 | 2988–2956 | 2990–2955 |
| υ-C=X stretching (X=N or O) | 1618 C=N | 1725 C=O | 1726 C=O | 1725 C=O |
| υC-C | 1572, 1526 | 1582, 1526 | - | - |
| C–N stretch aromatic amines | 1319 | 1319 | - | - |
| C–N stretch aromatic amines | 1283 | 1294 | - | - |
| C-H in-plane Bending (Aromatic) | 1034 | 1070 | - | - |
| =C-H in-plane bending | 939 | 944 | 959 | 960 |
| C-H bending (out-of-plane) | 793 | 791 | 755 | 756 |
| C-H bend Alkene | 711 | 714 | 718 | 705 |
| C-H bending (out-of-plane) Aromatic ring | 643 | 645 | - | - |
| Parameter | PEDOT:PSS/MIP+NaTFPB | PEDOT:PSS/NIP+NaTFPB |
|---|---|---|
| Slope (mV/decade) | 28.2 ± 0.7 | 12.1 ± 0.5 |
| Correlation coefficient (r2) | 0.999 | 0.997 |
| Detection limit (µg/mL) | 0.026 | 0.05 |
| Linear range (M) | 1.0 × 10−6–1.0 × 10−2 | 5.0 × 10−6–1.0 × 10−2 |
| Working pH range (pH) | 4.2–9.0 | 4.5–9.0 |
| Response time (s) | <15 s | ~15 |
| Repeatability (% mV) | 0.9 | 1.2 |
| Reproducibility (% mV) | 1.1 | 0.9 |
| Accuracy (%) | 99.2 | 98.7 |
| Sensor | * Log KPot I,J | ||||||
|---|---|---|---|---|---|---|---|
| Paraquat | Cyromazine | Dinotefuran | Acetamipride | K+ | Na+ | Ca2+ | |
| PEDOT:PSS/MIP+NaTFPB | −3.5 ± 0.2 | −3.6 ± 0.4 | −5.1 ± 0.7 | −5.01 ± 0.2 | −6.1 ± 0.4 | −6.2 ± 0.7 | −6.7 ± 0.2 |
| Commercial Product | Label (w/v%) | * Found | |||
|---|---|---|---|---|---|
| Potentiometry | RSD, % | HPLC [44,45] | RSD, % | ||
| Reglone 200 SL, Syngenta Company (Cairo, Egypt) | 31.8 | 29.6 ± 1.1 | 93.1 | 30.3 ± 0.5 | 95.3 |
| Sample | Amount Spiked, (µg/g) | * Amount, (µg/g) | F-test a | |
|---|---|---|---|---|
| Potentiometry | HPLC [44,45] | |||
| 1 | 0.1 | 0.12 ± 0.03 | 0.11 ± 0.03 | 1.6 |
| 2 | 0.5 | 0.45 ± 0.01 | 0.52 ± 0.04 | 3.5 |
| 3 | 0.7 | 0.65 ± 0.04 | 0.68 ± 0.03 | 4.8 |
| 4 | 1.0 | 0.92 ± 0.07 | 1.01 ± 0.06 | 2.7 |
| Ionophore | Electrode Type | Slope, mV/decade | Linear Range, M | Detection Limit, M | Working pH Range | Ref. |
|---|---|---|---|---|---|---|
| Diquat-phosphotungstate | Carbon paste | 30.8 | 3.8 × 10−6–1.0 × 10−3 | 9.0 × 10−7 | 4.5–9.5 | [31] |
| Diquat bis (tetra-4-chloro phenyl borate) | Polymeric PVC | 30.0 | 4 × 10−9–3 × 10−6 | - | 2.0–11 | [32] |
| Different crown ethers | Polymeric PVC | 33–41 | - | 1.7 × 10−6–6 × 10−6 | - | [34] |
| Dibenzo 30-crown 10 | Liquid | 32.6 | - | - | 1.2–8.5 | [46] |
| diquat ion pair with tetraphenyl borate | membrane | 28.8 | - | - | 1.2–8.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamel, A.H.; Amr, A.E.-G.E.; Abdalla, N.S.; El-Naggar, M.; Al-Omar, M.A.; Almehizia, A.A. Modified Screen-Printed Potentiometric Sensors based on Man-Tailored Biomimetics for Diquat Herbicide Determination. Int. J. Environ. Res. Public Health 2020, 17, 1138. https://doi.org/10.3390/ijerph17041138
Kamel AH, Amr AE-GE, Abdalla NS, El-Naggar M, Al-Omar MA, Almehizia AA. Modified Screen-Printed Potentiometric Sensors based on Man-Tailored Biomimetics for Diquat Herbicide Determination. International Journal of Environmental Research and Public Health. 2020; 17(4):1138. https://doi.org/10.3390/ijerph17041138
Chicago/Turabian StyleKamel, Ayman H., Abd El-Galil E. Amr, Nashwa S. Abdalla, Mohamed El-Naggar, Mohamed A. Al-Omar, and Abdulrahman A. Almehizia. 2020. "Modified Screen-Printed Potentiometric Sensors based on Man-Tailored Biomimetics for Diquat Herbicide Determination" International Journal of Environmental Research and Public Health 17, no. 4: 1138. https://doi.org/10.3390/ijerph17041138
APA StyleKamel, A. H., Amr, A. E.-G. E., Abdalla, N. S., El-Naggar, M., Al-Omar, M. A., & Almehizia, A. A. (2020). Modified Screen-Printed Potentiometric Sensors based on Man-Tailored Biomimetics for Diquat Herbicide Determination. International Journal of Environmental Research and Public Health, 17(4), 1138. https://doi.org/10.3390/ijerph17041138








