Shallow Groundwater Quality and Its Controlling Factors in the Su-Xi-Chang Region, Eastern China
Abstract
:1. Introduction
2. Outline of Study Area and Methodology
2.1. Study Area
2.2. Hydrogeological Conditions
2.3. Sampling and Analytical Methods
2.4. Data Treatment and Analytical Methods
3. Results and Discussion
3.1. Physicochemical Parameters, Hydrochemical Characteristics and Groundwater Quality
3.2. Hydrochemical Origin and Correlation Analysis
3.3. Principal Component Analysis
3.4. Anthropogenic Activities and Impacts
3.4.1. The Status of Groundwater Exploitation and Utilization
3.4.2. Geological Environmental Problem: Land Subsidence
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harguinteguy, C.A.; Cofré, M.N.; Fernández-Cirelli, A.; Pignata, M.L. The macrophytes Potamogeton pusillus L. and Myriophyllum aquaticum (Vell.) Verdc. as potential bioindicators of a river contaminated by heavy metals. Microchem. J. 2015, 124, 228–234. [Google Scholar] [CrossRef]
- Hillel, N.; Geyer, S.; Licha, T.; Khayat, S.; Laronne, J.B.; Siebert, C. Water quality and discharge of the Lower Jordan River. J. Hydrol. 2015, 527, 1096–1105. [Google Scholar] [CrossRef]
- Palácio, S.M.; Pauli, A.R.D.; Piana, P.A.; Queiroz, C.B.; Fabris, S.C.; Fagundes-Klen, M.R.; Veit, M.T. Assessment of anthropogenic impacts on the water quality of Marreco River, Brazil, based on principal component analysis and toxicological assays. Water Air Soil Pollut. 2016, 227, 307. [Google Scholar] [CrossRef]
- Hanrahan, M.; Jnr, B.D. The rocky path to source water protection: A cross-case analysis of drinking water crises in small communities in Canada. Water 2017, 9, 388. [Google Scholar] [CrossRef] [Green Version]
- Kiedrzyńska, E.; Kiedrzyński, M.; Urbaniak, M.; Magnuszewski, A.; Skłodowski, M.; Wyrwicka, A.; Zalewski, M. Point sources of nutrient pollution in the lowland river catchment in the context of the Baltic Sea eutrophication. Ecol. Eng. 2014, 70, 337–348. [Google Scholar] [CrossRef]
- Qiang, F.; Tian, X.L.; Dong, L.; He, D. Simulation study of the sustainable utilization of urban water resources based on system dynamics: A case study of Jiamusi. Water Sci. Technol. Water Supply 2016, 16, 980–991. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Xue, C.; Wu, J. Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ. Sci. Pollut. Res. Int. 2017, 24, 13224. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Sarver, E.; Krometis, L.H. Interactive effects of water quality, physical habitat, and watershed anthropogenic activities on stream ecosystem health. Water Res. 2017, 130, 69. [Google Scholar] [CrossRef]
- Hoang, H.; Duong, T.T.; Nguyen, K.T.; Le, Q.; Luu, M.; Trinh, D.A.; Le, A.H.; Ho, C.T.; Dang, K.D.; Némery, J. Impact of anthropogenic activities on water quality and plankton communities in the Day River (Red River Delta, Vietnam). Environ. Monit. Assess. 2018, 190, 67. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Non-point pollution of surface waters eith phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Huang, J.; Du, P. Assessment of temporal and spatial variation of coastal water quality and source identification along Macau peninsula. Stoch. Environ. Res. Risk Assess. 2011, 25, 353–361. [Google Scholar] [CrossRef]
- Mouri, G.; Takizawa, S.; Oki, T. Spatial and temporal variation in nutrient parameters in stream water in a rural-urban catchment, Shikoku, Japan: Effects of land cover and human impact. J. Environ. Manag. 2011, 92, 1837. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A. A GIS based DRASTIC model for assessing groundwater vulnerability in shallow aquifer in Aligarh, India. Appl. Geogr. 2008, 28, 32–53. [Google Scholar] [CrossRef]
- Gao, X.; Bai, Y.; Huo, Z.; Xu, X.; Huang, G.; Xia, Y.; Steenhuis, T.S. Deficit irrigation enhances contribution of shallow groundwater to crop water consumption in arid area. Agric. Water Manag. 2017, 185, 116–125. [Google Scholar] [CrossRef]
- Leite, N.K.; Stolberg, J.; Cruz, S.P.D.; Tavela, A.D.O.; Safanelli, J.L.; Marchini, H.R.; Exterkoetter, R.; Leite, G.M.C.; Krusche, A.V.; Johnson, M.S. Hydrochemistry of shallow groundwater and springs used for potable supply in Southern Brazil. Environ. Earth Sci. 2018, 77, 80. [Google Scholar] [CrossRef]
- Chen, A.; Lei, B.; Hu, W.; Wang, H.; Zhai, L.; Mao, Y.; Fu, B.; Zhang, D. Temporal-spatial variations and influencing factors of nitrogen in the shallow groundwater of the nearshore vegetable field of Erhai Lake, China. Environ. Sci. Pollut. Res. 2018, 25, 4858–4870. [Google Scholar] [CrossRef]
- Wang, Z.R.; Tian, X.; Wu, X. Hydrochemical characteristics and quality assessment of shallow groundwater and CBM co-produced water in the Shizhuangnan block, Qinshui Basin, China. Environ. Earth Sci. 2018, 77, 57. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Z. Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, Mid-west China. Expo. Health 2016, 8, 311–329. [Google Scholar] [CrossRef]
- Baker, A. Land use and water quality. Hydrol. Process. 2003, 17, 2499–2501. [Google Scholar] [CrossRef]
- He, C.; Malcolm, S.B.; Dahlberg, K.A.; Fu, B. A conceptual framework for integrating hydrological and biological indicators into watershed management. Landsc. Urban Plan. 2000, 49, 25–34. [Google Scholar] [CrossRef]
- Sun, R.H.; Wang, Z.M.; Chen, L.D. Assessment of surface water quality at large watershed scale: Land-use, anthropogenic, and administrative impacts. J. Am. Water Resour. Assoc. 2013, 49, 741–752. [Google Scholar] [CrossRef]
- Shi, X.Q.; Xue, Y.Q.; Ye, S.J.; Wu, J.C.; Zhang, Y.; Yu, J. Characterization of land subsidence induced by groundwater withdrawals in Su-Xi-Chang area, China. Environ. Geol. 2007, 52, 27–40. [Google Scholar] [CrossRef]
- Shi, X.; Wu, J.; Ye, S.; Zhang, Y.; Xue, Y.; Wei, Z.; Li, Q.; Yu, J. Regional land subsidence simulation in Su-Xi-Chang area and Shanghai City, China. Eng. Geol. 2008, 100, 27–42. [Google Scholar] [CrossRef]
- Wu, J.C.; Shi, X.Q.; Ye, S.J.; Xue, Y.Q.; Zhang, Y.; Yu, J. Numerical simulation of land subsidence induced by groundwater overexploitation in Su-Xi-Chang area, China. Environ. Geol. 2009, 57, 1409–1421. [Google Scholar] [CrossRef]
- Yun, Z.; Xue, Y.Q.; Wu, J.C.; Shi, X.Q.; Yu, J. Excessive groundwater withdrawal and resultant land subsidence in the Su-Xi-Chang area, China. Environ. Earth Sci. 2010, 61, 1135–1143. [Google Scholar]
- Fohrer, N. Assessment of anthropogenic impacts on water quality. Phys. Chem. Earth 2005, 30, 471. [Google Scholar] [CrossRef]
- Simeonov, V.; Stratis, J.A.; Samara, C.; Zachariadis, G.; Voutsa, D.; Anthemidis, A.; Sofoniou, M.; Kouimtzis, T. Assessment of the surface water quality in Northern Greece. Water Res. 2003, 37, 4119. [Google Scholar] [CrossRef]
- Singh, K.P.; Malik, A.; Mohan, D.; Sinha, S. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—A case study. Water Res. 2004, 38, 3980. [Google Scholar] [CrossRef]
- Vega, M.; Pardo, R.; Barrado, E.; Debán, L. Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res. 1998, 32, 3581–3592. [Google Scholar] [CrossRef]
- Tong, S.T.; Chen, W. Modeling the relationship between land use and surface water quality. J. Environ. Manag. 2002, 66, 377. [Google Scholar] [CrossRef]
- Preston, S.D.; Alexander, R.B.; Schwarz, G.E.; Crawford, C.G. Factors affecting stream nutrient loads: A synthesis of regional SPARROW model results for the continental United States. J. Am. Water Resour. Assoc. 2011, 47, 891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.A.; Schwarz, G.E.; Alexander, R.B. Regional interpretation of water-quality monitoring data. Water Resour. Res. 1997, 33, 2781–2798. [Google Scholar] [CrossRef] [Green Version]
- Gémesi, Z.; Downing, J.A.; Cruse, R.M.; Anderson, P.F. Effects of watershed configuration and composition on downstream lake water quality. J. Environ. Qual. 2011, 40, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Mei, K.; Zhang, Y.; Liao, L.; Long, B.; Dahlgren, R.A.; Zhang, M. Spatial and temporal variations of nitrogen pollution in Wen-Rui Tang River watershed, Zhejiang, China. Environ. Monit. Assess. 2011, 180, 501–520. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Kazama, F. Assessment of surface water quality using multivariate statistical techniques: A case study of the Fuji river basin, Japan. Environ. Model. Softw. 2007, 22, 464–475. [Google Scholar] [CrossRef]
- Wise, D.R.; Johnson, H.M. Surface-water nutrient conditions and sources in the United States Pacific Northwest. J. Am. Water Resour. Assoc. 2011, 47, 1110. [Google Scholar] [CrossRef]
- Long, H.; Liu, Y.; Wu, X.; Dong, G. Spatio-temporal dynamic patterns of farmland and rural settlements in Su–Xi–Chang region: Implications for building a new countryside in coastal China. Land Use Policy 2009, 26, 322–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Xue, Y.; Wu, J.; Yu, J. Mechanisms for earth fissure formation due to groundwater extraction in the Su-Xi-Chang area, China. Bull. Eng. Geol. Environ. 2016, 75, 745–760. [Google Scholar] [CrossRef]
- Cuoco, E.; Darrah, T.H.; Buono, G.; Eymold, W.K.; Tedesco, D. Differentiating natural and anthropogenic impacts on water quality in a hydrothermal coastal aquifer (Mondragone Plain, Southern Italy). Environ. Earth Sci. 2015, 73, 7115–7134. [Google Scholar] [CrossRef]
- Mapoma, H.W.T.; Xie, X.; Liu, Y.; Zhu, Y.; Kawaye, F.P.; Kayira, T.M. Hydrochemistry and quality of groundwater in alluvial aquifer of Karonga, Malawi. Environ. Earth Sci. 2017, 76, 335. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Bu, J.W.; Sun, Z.Y.; Zhou, A.G.; Xu, Y.N.; Ma, R.; Wei, W.H.; Liu, M. Heavy metals in surface soils in the upper reaches of the Heihe River, northeastern Tibetan Plateau, China. Int. J. Environ. Res. Public Health 2016, 13, 247. [Google Scholar] [CrossRef] [Green Version]
- Loska, K.; Wiechuła, D. Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere 2003, 51, 723–733. [Google Scholar] [CrossRef]
- Ma, H.; Liu, L.; Chen, T. Water security assessment in Haihe River Basin using principal component analysis based on Kendall tau. Environ. Monit. Assess. 2010, 163, 539. [Google Scholar] [CrossRef]
- Han, Y.M.; Du, P.X.; Cao, J.J.; Posmentier, E.S. Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci. Total Environ. 2006, 355, 176–186. [Google Scholar]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- CSBTS (China State Bureau of Technical Supervision). Quality standard for ground water (GB/T 14848-93). 1993. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT14848-1993 (accessed on 17 December 2019).
- MH (Ministry of Health of China). Standards for Drinking Water Qual. (GB 5749-2006). 2006. Available online: http://tradechina.dairyaustralia.com.au/wp-content/uploads/2018/08/GB-5749-2006-Standards-for-Drinking-Water-Quality.pdf (accessed on 17 December 2019).
- WHO (World Health Organization). Guidelines for Drinking-Water Quality, 4rd ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- SEPA (State Environmental Protection Administration of China). Environmental Quality Standards for Surface Water (GB 3838-2002). 2002. Available online: https://www.codeofchina.com/standard/GB3838-2002.html (accessed on 17 December 2019).
- Dong, Z.H.; Ji, L.I.; Sun, L.M. Nitrate contamination in the groundwater of intensive vegetable cultivation areas in Shouguang City, Shandong Province, China. J. Agro-Environ. Sci. 2005, 24, 1139–1144. [Google Scholar]
- Jin, Z.; Pan, Z.; Jin, M.; Li, F.; Wan, Y.; Gu, B. Determination of nitrate contamination sources using isotopic and chemical indicators in an agricultural region in China. Agric. Ecosyst. Environ. 2012, 155, 78–86. [Google Scholar] [CrossRef]
- Ju, X.T.; Kou, C.L.; Zhang, F.S.; Christie, P. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain. Environ. Pollut. 2006, 143, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Su, X.S.; Wang, H.; Zhang, Y.L. Health risk assessment of nitrate contamination in groundwater: A case study of an agricultural area in Northeast China. Water Resour. Manag. 2013, 27, 3025–3034. [Google Scholar] [CrossRef]
- Yang, R.; Liu, W.J. Nitrate contamination of groundwater in an agroecosystem in Zhangye Oasis, Northwest China. Environ. Earth Sci. 2010, 61, 123–129. [Google Scholar] [CrossRef]
- Marandi, A.; Shand, P. Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Banks, D.; Frengstad, B. Evolution of groundwater chemical composition by plagioclase hydrolysis in Norwegian anorthosites. Geochim. Cosmochim. Acta 2006, 70, 1337–1355. [Google Scholar] [CrossRef]
- Pablo, H.T.F.; Chacón–Moscoso, S.; Barbero–García, I.; Vila–Abad, E. Polychoric versus Pearson correlations in exploratory and confirmatory factor analysis of ordinal variables. Qual. Quant. 2010, 44, 153–166. [Google Scholar]
- Adams, S.; Titus, R.; Pietersen, K.; Tredoux, G.; Harris, C. Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J. Hydrol. 2001, 241, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Ly, A.; Marsman, M.; Wagenmakers, E.J. Analytic posteriors for Pearson’s correlation coefficient. Stat. Neerl. 2018, 72, 4–13. [Google Scholar] [CrossRef]
- An, Y.; Lu, W. Hydrogeochemical processes identification and groundwater pollution causes analysis in the northern Ordos Cretaceous Basin, China. Environ. Geochem. Health 2017, 40, 1209–1219. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Wang, X.; Wan, L.; Shi, Z.; Wanke, H.; Uugulu, S.; Uahengo, C.-I. Groundwater quality and associated hydrogeochemical processes in Northwest Namibia. J. Geochem. Explor. 2018, 186, 202–214. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Ma, Y. Characterization of controlling hydrogeochemical processes using factor analysis in Puyang Yellow River irrigation district (China). Hydrol. Res. 2017, 48, 1438–1454. [Google Scholar] [CrossRef]
- Keita, S.; Tang, Z. The assessment of processes controlling the spatial distribution of hydrogeochemical groundwater types in Mali using multivariate statistics. J. Afr. Earth Sci. 2017, 134, 573–589. [Google Scholar] [CrossRef]
- El Alfy, M.; Lashin, A.; Abdalla, F.; Al-Bassam, A. Assessing the hydrogeochemical processes affecting groundwater pollution in arid areas using an integration of geochemical equilibrium and multivariate statistical techniques. Environ. Poll. 2017, 229, 760–770. [Google Scholar] [CrossRef]
- Sánchez, E.R.S.; Hoyos, S.E.G.; Esteller, M.V.; Morales, M.M.; Astudillo, A.O. Hydrogeochemistry and water-rock interactions in the urban area of Puebla Valley aquifer (Mexico). J. Geochem. Explor. 2017, 181, 219–235. [Google Scholar] [CrossRef]
- Subramani, T.; Rajmohan, N.; Elango, L. Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ. Monit. Assess. 2010, 162, 123. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Samie, S.G.A.; Badawy, H.A. Factors controlling mechanisms of groundwater salinization and hydrogeochemical processes in the Quaternary aquifer of the Eastern Nile Delta, Egypt. Environ. Earth Sci. 2013, 68, 369–394. [Google Scholar] [CrossRef]
- Larssen, T.; Lydersen, E.; Tang, D.; He, Y.; Gao, J.; Liu, H.; Duan, L.; Seip, H.M.; Vogt, R.D.; Mulder, J. Acid rain in China. Environ. Sci. Technol. 2006, 40, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, D.A. Statistics for the Social and Behavioral Sciences; Little, Brown and Company: Boston, MA, USA, 1986. [Google Scholar]



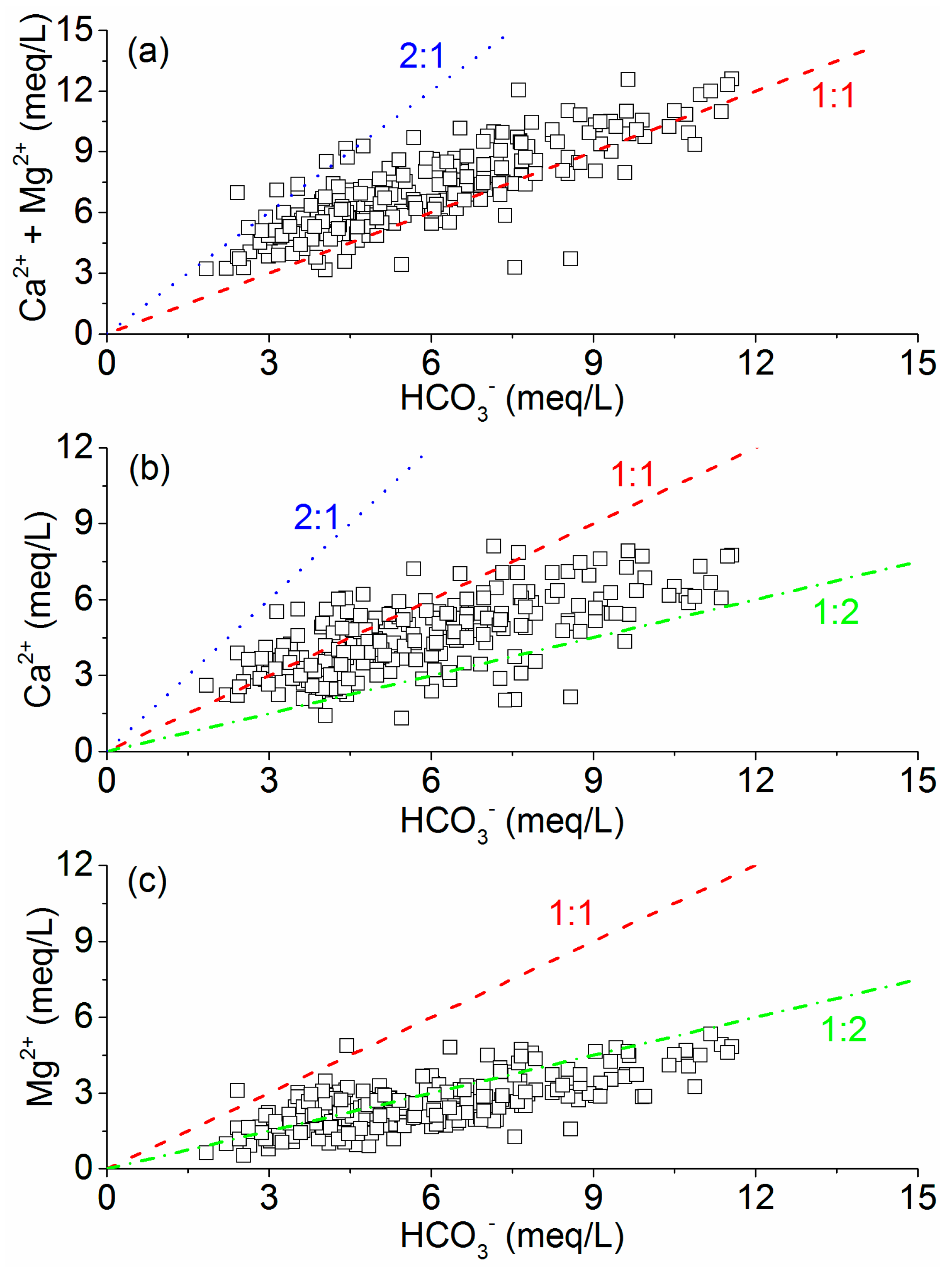

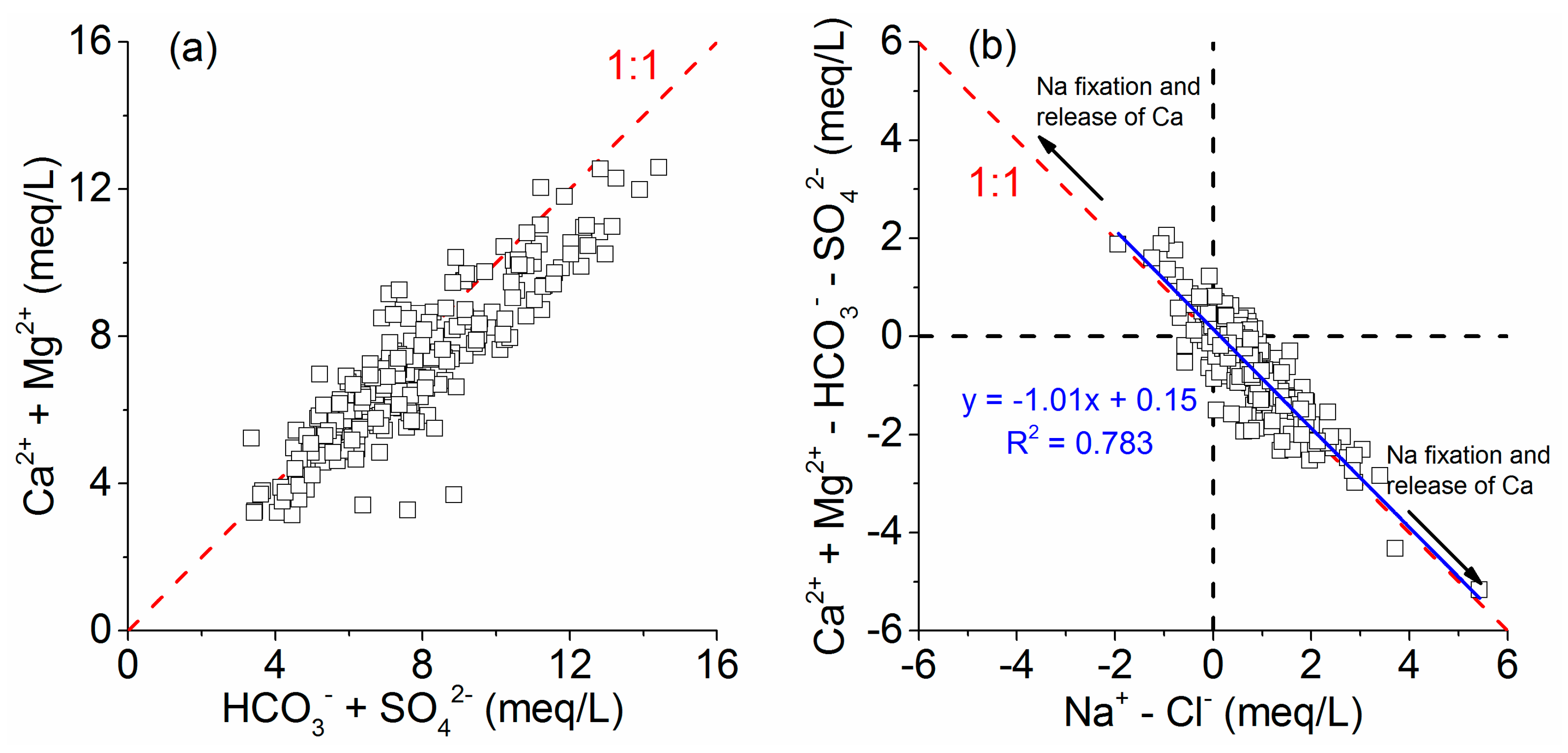



| Parameter | Min. | Max. | Mean | QSGW a | EQSSW b | SDWQ c | GDWQ d |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 11.00 | 22.50 | 16.89 | n.s. | n.s. | n.s. | n.s. |
| EC (μS cm−1) | 120.00 | 2850.00 | 1031.26 | n.s. | n.s. | n.s. | n.s. |
| pH | 5.59 | 7.82 | 7.00 | 6.5–8.5 | 6–9 | 6.5–8.5 | 6.5–8.5 |
| TDS (mg L−1) | 224.89 | 1086.70 | 616.69 | ≤500 | n.s. | ≤1000 | ≤1000 |
| Turbidity (NTU) | 0.1 | 18.60 | 3.85 | ≤3 | n.s. | ≤1 | ≤1 |
| DO (mg O2 L−1) | 0.03 | 5.51 | 0.97 | n.s. | ≥6 | n.s. | n.s. |
| Ammonia (mg N L−1) | 0.01 | 32.90 | 0.72 | ≤0.02 | ≤0.5 | ≤0.5 | n.s. |
| TP (mg P L−1) | 0.01 | 2.02 | 0.15 | n.s. | ≤0.1 | n.s. | n.s. |
| Parameter | Min. | Max. | Mean | SD | Skewness | Kurtosis |
|---|---|---|---|---|---|---|
| Ca2+ | 26.20 | 162.00 | 90.09 | 28.93 | 0.26 | −0.50 |
| Mg2+ | 6.07 | 64.00 | 30.23 | 11.86 | 0.58 | −0.07 |
| Na+ | 8.32 | 159.00 | 72.63 | 29.23 | 0.63 | 0.05 |
| Cl− | 7.96 | 191.00 | 84.39 | 36.18 | 0.79 | 0.28 |
| SO42− | 2.45 | 187.00 | 89.40 | 39.04 | 0.34 | −0.17 |
| HCO3− | 113.00 | 706.00 | 353.70 | 132.67 | 0.64 | −0.25 |
| NO3− | 0.01 | 154.00 | 22.07 | 26.85 | 2.04 | 4.73 |
| H2SiO3 | 11.10 | 55.40 | 25.14 | 6.87 | 1.31 | 3.01 |
| Parameter | pH | TDS | Ca2+ | Mg2+ | Na+ | Cl− | SO42− | HCO3− | NO3− | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | 0.136 * | 0.021 | 0.129 | 0.170 ** | 0.211 ** | 0.108 | 0.048 | 0.044 | 0.080 |
| TDS | 0.136 * | 1 | 0.745 ** | 0.651 ** | 0.705 ** | 0.695 ** | 0.538 ** | 0.701 ** | 0.311 ** | 0.163 * |
| Ca2+ | 0.021 | 0.745 ** | 1 | 0.424 ** | 0.262 ** | 0.395 ** | 0.421 ** | 0.666 ** | 0.247 ** | 0.064 |
| Mg2+ | 0.129 | 0.651 ** | 0.424 ** | 1 | 0.442 ** | 0.466 ** | 0.246 ** | 0.710 ** | −0.019 | −0.037 |
| Na+ | 0.170 ** | 0.705 ** | 0.262 ** | 0.442 ** | 1 | 0.706 ** | 0.329 ** | 0.526 ** | 0.010 | 0.112 |
| Cl− | 0.211 ** | 0.695 ** | 0.395 ** | 0.466 ** | 0.706 ** | 1 | 0.345 ** | 0.311 ** | 0.109 | 0.109 |
| SO42− | 0.108 | 0.538 ** | 0.421 ** | 0.246 ** | 0.329 ** | 0.345 ** | 1 | 0.008 | 0.343 ** | −0.141 * |
| HCO3‑ | 0.048 | 0.701 ** | 0.666 ** | 0.710 ** | 0.526 ** | 0.311 ** | 0.008 | 1 | −0.093 | 0.121 |
| NO3− | 0.044 | 0.311 ** | 0.247 ** | −0.019 | 0.010 | 0.109 | 0.343 ** | −0.093 | 1 | 0.005 |
| H2SiO3 | 0.080 | 0.163 * | 0.064 | −0.037 | 0.112 | 0.109 | −0.141 * | 0.121 | 0.005 | 1 |
| Parameter | Factors | Communality | ||
|---|---|---|---|---|
| FA1 | FA2 | FA3 | ||
| TDS | 0.880 | 0.409 | 0.120 | 0.956 |
| Ca2+ | 0.682 | 0.340 | −0.041 | 0.582 |
| Mg2+ | 0.823 | −0.076 | −0.197 | 0.722 |
| Na+ | 0.740 | 0.130 | 0.190 | 0.600 |
| Cl− | 0.676 | 0.297 | 0.198 | 0.584 |
| SO42− | 0.305 | 0.756 | −0.226 | 0.717 |
| HCO3− | 0.879 | −0.234 | 0.008 | 0.828 |
| NO3− | −0.052 | 0.820 | 0.089 | 0.683 |
| H2SiO3 | 0.083 | −0.057 | 0.948 | 0.908 |
| Eigenvalues | 3.796 | 1.697 | 1.087 | - |
| % of Variance | 42.175 | 18.853 | 12.078 | - |
| Cumulative % | 42.175 | 61.028 | 73.106 | - |
| Factors | TDS | Ca2+ | Mg2+ | Na+ | Cl− | SO42− | HCO3− | NO3− | H2SiO3 |
|---|---|---|---|---|---|---|---|---|---|
| FA1 | 0.81 | 0.80 | 0.94 | 0.91 | 0.78 | 0.13 | 0.93 | 0.00 | 0.01 |
| FA2 | 0.18 | 0.20 | 0.01 | 0.03 | 0.15 | 0.80 | 0.07 | 0.98 | 0.00 |
| FA3 | 0.02 | 0.00 | 0.05 | 0.06 | 0.07 | 0.07 | 0.00 | 0.01 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, J.; Sun, Z.; Ma, R.; Liu, Y.; Gong, X.; Pan, Z.; Wei, W. Shallow Groundwater Quality and Its Controlling Factors in the Su-Xi-Chang Region, Eastern China. Int. J. Environ. Res. Public Health 2020, 17, 1267. https://doi.org/10.3390/ijerph17041267
Bu J, Sun Z, Ma R, Liu Y, Gong X, Pan Z, Wei W. Shallow Groundwater Quality and Its Controlling Factors in the Su-Xi-Chang Region, Eastern China. International Journal of Environmental Research and Public Health. 2020; 17(4):1267. https://doi.org/10.3390/ijerph17041267
Chicago/Turabian StyleBu, Jianwei, Ziyong Sun, Rui Ma, Yunde Liu, Xulong Gong, Zhao Pan, and Wenhao Wei. 2020. "Shallow Groundwater Quality and Its Controlling Factors in the Su-Xi-Chang Region, Eastern China" International Journal of Environmental Research and Public Health 17, no. 4: 1267. https://doi.org/10.3390/ijerph17041267







