Factors Associated with the Leisure-Time Physical Activity (LTPA) during the First Trimester of the Pregnancy: The Cross-Sectional Study among Pregnant Women in Serbia
Abstract
:1. Introduction
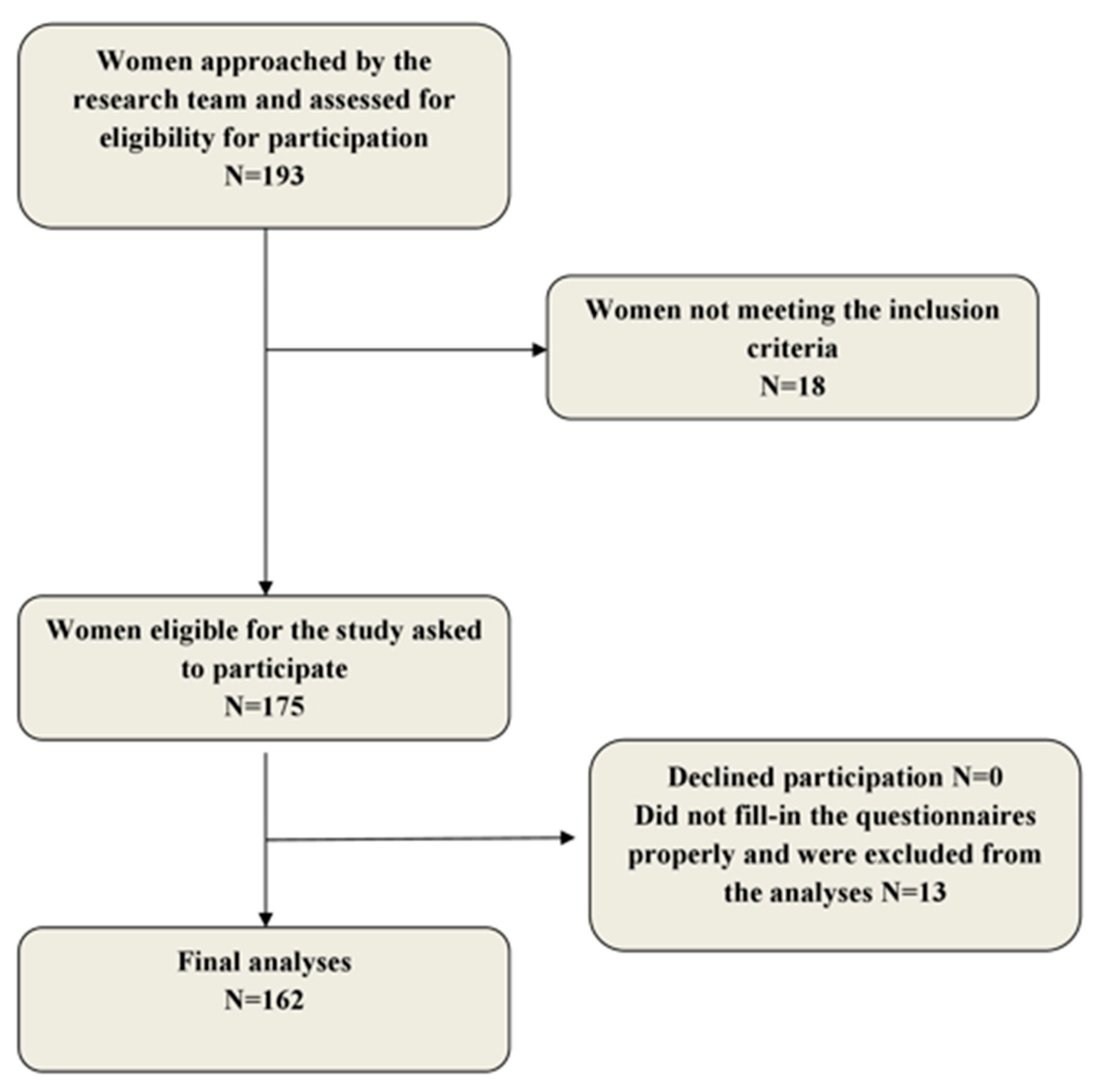
2. Methods
2.1. Population
2.2. Ethical Approval
2.3. Research Instrument
2.4. Variables
2.5. Analyzed Variables
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 1–16. [Google Scholar] [CrossRef]
- Shin, C.; Lee, Y.; Belyea, M. Physical activity, benefits, and barriers across the aging continuum. Appl. Nurs. Res. 2018, 44, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Han, L.H.; Zhang, J.H.; Luo, S.; Hu, J.W.; Sun, K. The influence of physical activity, sedentary behavior on health-related quality of life among the general population of children and adolescents: A systematic review. PLoS ONE 2017, 12, e0187668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puciato, D.; Rozpara, M.; Borysiuk, Z. Physical Activity as a Determinant of Quality of Life in Working-Age People in Wrocław, Poland. Int. J. Environ. Res. Phys. Act. 2018, 15, 623–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puciato, D.; Borysiuk, Z.; Rozpara, M. Quality of life and physical activity in an older working-age population. Clin. Interv. Aging 2017, 12, 1627–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schramm, W.; Stockbauer, J.; Hoffman, H. Exercise, Employment, Other Daily Activities, and Adverse Pregnancy Outcomes. Am. J. Epidemiol. 1996, 143, 211–218. [Google Scholar] [CrossRef]
- ACOG Technical Bulletin Number 189 February 1994. Exercise during pregnancy and the postpartum period. Int. J. Gynecol. Obstet. 1994, 45, 65–70. [Google Scholar] [CrossRef]
- Sanabria-Martínez, G.; García-Hermoso, A.; Poyatos-Leõn, R.; Álvarez-Bueno, C.; Sánchez-Lõpez, M.; Martínez-Vizcaíno, V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: A meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1167–1174. [Google Scholar] [CrossRef]
- Domenjoz, I.; Kayser, B.; Boulvain, M. Effect of physical activity during pregnancy on mode of delivery. Am. J. Obstet. Gynecol. 2014, 211, 401.el–401.e11. [Google Scholar] [CrossRef]
- Mottola, M.F. Physical activity and maternal obesity: Cardiovascular adaptations, exercise recommendations, and pregnancy outcomes. Nutr. Rev. 2013, 71 (Suppl. 1), 31–36. [Google Scholar] [CrossRef]
- The American College of Obstetricians and Gynecologists. Committee opinion summary No.650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet. Gynecol. 2015, 126, 1326–1327. [Google Scholar] [CrossRef]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sport Med. 2018, 40, 1339–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Information Sheet: Global Recommendations on Physical Activity for Health 18-64 Years Old; World Health Organization: Geneva, Switzerland, 2011; p. 1. [Google Scholar]
- Streuling, I.; Beyerlein, A.; Rosenfeld, E.; Hofmann, H.; Schulz, T.; Von Kries, R. Physical activity and gestational weight gain: A meta-analysis of intervention trials. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.; Zhang, C.; van Dam, R.M.; Bower, K.; Hu, F. Physical Activity Before and During Pregnancy and Risk of Gestational. Diabetes Care 2011, 34, 223–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badon, S.; Wartko, P.; Qiu, C.; Sorensen, T.; Williams, M.; Enquobahrie, D. Leisure-time physical activity and gestational diabetes mellitus in the Omega study. Med. Sci. Sports Exerc. 2016, 48, 1044–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harizopoulou, V.C.; Kritikos, A.; Papanikolaou, Z.; Saranti, E.; Vavilis, D.; Klonos, E.; Papadimas, I.; Goulis, D.G. Maternal physical activity before and during early pregnancy as a risk factor for gestational diabetes mellitus. Acta Diabetol. 2010, 47 (Suppl. 1), 83–89. [Google Scholar] [CrossRef]
- Momeni Javid, F.; Simbar, M.; Dolatian, M.; Alavi Majd, H. Comparison of Lifestyles of Women With Gestational Diabetes and Healthy Pregnant Women. Glob. J. Health Sci. 2014, 7, 162–169. Available online: http://www.ccsenet.org/journal/index.php/gjhs/article/view/39368 (accessed on 5 March 2019). [CrossRef] [Green Version]
- Aune, D.; Saugstad, O.D.; Henriksen, T.; Tonstad, S. Physical Activity and the Risk of Preeclampsia. Epidemiology 2014, 25, 331–343. Available online: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00001648-201405000-00003 (accessed on 3 February 2020). [CrossRef]
- Catov, J.M.; Parker, C.B.; Gibbs, B.B.; Bann, C.M.; Carper, B.; Silver, R.M.; Simhan, H.N.; Parry, S.; Chung, J.H.; Haas, D.M.; et al. Patterns of leisure-time physical activity across pregnancy and adverse pregnancy outcomes. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.H.; Rothenberger, S.D.; Kline, C.E.; Okun, M.L. Exercise during Early Pregnancy is Associated with Greater Sleep Continuity. Behav. Sleep Med. 2018, 16, 482–493. [Google Scholar] [CrossRef]
- Wang, S.-M.; Dezinno, P.; Maranets, I.; Berman, M.R.; Caldwell-Andrews, A.A.; Kain, Z.N. Low Back Pain During Pregnancy. Obstet. Gynecol. 2004, 104, 65–70. Available online: http://insights.ovid.com/crossref?an=00006250-200407000-00011 (accessed on 5 March 2019). [CrossRef]
- Patrícia, V.; De Sousa, S.; Cury, A.; Eufrásio, L.S. The influence of gestational trimester, physical activity practice and weight gain on the low back and pelvic pain intensity in low risk pregnant women. J. Back Musculoskelet. Rehabil. 2019, 32, 671–676. [Google Scholar]
- Thorell, E.; Kristiansson, P. Pregnancy related back pain, is it related to aerobic fitness? A longitudinal cohort study. BMC Pregnancy Childbirth 2012, 12, 30. Available online: http://www.biomedcentral.com/1471-2393/12/30 (accessed on 5 March 2019). [CrossRef] [PubMed]
- Taylor, P.; Ko, Y.; Chen, C.; Lin, P. Physical activities during pregnancy and type of delivery in nulliparae. Eur. J. Sport Sci. 2016, 16, 374–380. [Google Scholar]
- Takami, M.; Tsuchida, A.; Takamori, A.; Aoki, S.; Ito, M. Effects of physical activity during pregnancy on preterm delivery and mode of delivery: The Japan Environment and Children’s Study, birth cohort study. PLoS ONE 2018, 13, e02066160. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, A.; Maharlouei, N.; Rezaianzadeh, A. Physical activities ( exercises or choreses ) during pregnancy and mode of delivery in nulliparous women: A prospective cohort study. Taiwan J. Obstet. Gynecol. 2018, 57, 18–22. [Google Scholar] [CrossRef]
- Cid, M.; González, M. Potential benefits of physical activity during pregnancy for the reduction of gestational diabetes prevalence and oxidative stress. Early Hum. Dev. 2016, 94, 57–62. [Google Scholar] [CrossRef]
- Barakat, R.; Perales, M.; Garatachea, N.; Ruiz, J.R.; Lucia, A. Exercise during pregnancy. A narrative review asking: What do we know? Br. J. Sports Med. 2015, 49, 1377–1381. [Google Scholar] [CrossRef] [Green Version]
- Vargas-terrones, M.; Barakat, R.; Santacruz, B.; Fernandez-buhigas, I.; Mottola, M.F. Physical exercise programme during pregnancy decreases perinatal depression risk: A randomised controlled trial. Br. J. Sports Med. 2018, 53, 348–353. [Google Scholar] [CrossRef]
- Szegda, K.; Bertone-johnson, E.R.; Pekow, P.; Powers, S.; Markenson, G.; Dole, N.; Chasan-Taber, L. Physical activity and depressive symptoms during pregnancy among Latina women: A prospective cohort study. BMC Pregnancy Childbirth 2018, 18, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Padmapriya, N.; Bernard, J.Y.; Liang, S.; Loy, S.L.; Shen, Z.; Kwek, K.; Godfrey, K.M.; Gluckman, P.D.; Chong, S.M. Association of physical activity and sedentary behavior with depression and anxiety symptoms during pregnancy in a multiethnic cohort of Asian women. Arch Womens Ment. Health 2016, 19, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Moyer, C.; Roldán Reoyo, O. The Influence of Prenatal Exercise on Offspring Health: A Review. Clin. Med. Insights Women’s Health 2016, 9, 85. Available online: http://www.la-press.com/the-influence-of-prenatal-exercise-on-offspring-health-a-reviewsupplem-article-a5967 (accessed on 5 March 2019).
- Schou Andersen, C.; Juhl, M.; Gamborg, M.; Sørensen, T.I.A.; Nohr, E.A. Maternal Recreational Exercise during Pregnancy in relation to Children’s BMI at 7 Years of Age. Int. J. Pediatr. 2012, 2012, 1–8. Available online: http://www.hindawi.com/journals/ijpedi/2012/920583/ (accessed on 5 March 2019). [CrossRef] [PubMed]
- May, L.E.; Scholtz, S.A.; Suminski, R.; Gustafson, K.M. Aerobic exercise during pregnancy influences infant heart rate variability at one month of age. Early Hum Dev. 2014, 90, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.L.; Surita, F.G.; Godoy, A.C.; Kasawara, K.T.; Morais, S.S. Physical activity patterns and factors related to exercise during pregnancy: A cross sectional study. PLoS ONE 2015, 10, 1–14. [Google Scholar]
- Marshall, E.S.; Bland, H.; Melton, B. Perceived barriers to physical activity among pregnant women living in a rural community. Public Health Nurs. 2012, 30, 361–369. [Google Scholar] [CrossRef]
- Currie, S.; Sinclair, M.; Murphy, M.H.; Madden, E.; Dunwoody, L.; Liddle, D. Reducing the Decline in Physical Activity during Pregnancy: A Systematic Review of Behaviour Change Interventions. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi-Noguchi, H.; Shiraishi, M.; Matsuzaki, M. Physical activity levels in the second trimester of pregnancy and related demographic factors. Cogent. Med. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Savvaki, D.; Taousani, E.; Goulis, D.G.; Tsirou, E.; Voziki, E.; Douda, H.; Nikolettos, N.; Tokmakidis, S.P. Guidelines for exercise during normal pregnancy and gestational diabetes: A review of international recommendations American College of Sport Medicine Canadian Society for Exercise Physiology. Hormones 2018, 17, 521–529. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Schmidt, M.D.; Roberts, D.E.; Hosmer, D.; Markenson, G.; Freedson, P.S. Development and validation of a pregnancy physical activity questionnaire. Med. Sci. Sports Exerc. 2004, 36, 1750–1760. [Google Scholar] [CrossRef]
- Centers For Disease Control and Prevention. PRAMS Phase 7 Questionnaire Topic Reference 1. 2012. Available online: https://www.cdc.gov/prams/pdf/questionnaire/Phase-7-Topics-Reference_508tagged.pdf (accessed on 11 December 2017).
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. Available online: http://www.journals.cambridge.org/abstract_S1368980006001261 (accessed on 10 September 2016). [CrossRef] [PubMed]
- World Health Organization. Process of Translation and Adaptation of Instruments. 2008. Available online: http://www.who.int/substance_abuse/research_tools/translation/en/index.html (accessed on 25 December 2017).
- Todorovic, J.; Terzic-Supic, Z.; Djikanovic, B.; Nesic, D.; Piperac, P.; Stamenkovic, Z. Can social media intervention improve physical activity of medical students? Public Health 2019, 174, 69–73. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Activity and Adults: Recommended Levels of Physical Activity for Adults Aged 18–64 Years; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Nikitovic, V. Population of Serbia at the Beggining of the 21st Century; Populacija Srbije Pocetkom 21 Veka; Nikitovic, V., Ed.; Republic Institute of Statistics: Belgrade, Serbia, 2015. [Google Scholar]
- Evenson, K.R.; Wen, F. National trends in self-reported physical activity and sedentary behaviors among pregnant women: NHANES 1999–2006. Prev. Med. (Baltim.) 2010, 50, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Mannalithara, A.; Myer, P.A.; Singh, G. Obesity, Abdominal Obesity, Physical Activity, and Caloric Intake in US Adults: 1988 to 2010. Am. J. Med. 2010, 127, 717–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loyen, A.; Verloigne, M.; Van Hecke, L.; Hendriksen, I.; Lakerveld, J.; Steene-Jochannessen, J.; Koster, A.; Donnelly, A.; Ekelund, U.; Deforche, B.; et al. Variation in population levels of sedentary time in European adults according to cross-European studies: A systematic literature review within DEDIPAC. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, M.; Emmons, M.; Lipkus, M. Understanding the potential of teachable moments: The case of smoking cessation. Health Educ. Res. 2003, 18, 156–170. [Google Scholar] [CrossRef]
- Bacchi, E.; Bonin, C.; Zanolin, M.E.; Zambotti, F.; Livornese, D.; Donic, S.; Bonora, E.; Baldisser, G.; Ihnatava, T.; Di Sarra, D.; et al. Physical activity patterns in normal-weight and overweight/obese pregnant women. PLoS ONE 2016, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Amezcua-Prieto, C.; Olmedo-Requena, R.; Jiménez-Mejías, E.; Mozas-Moreno, J.; Lardelli-Claret, P.; Jiménez-Moleón, J.J. Factors associated with changes in leisure time physical activity during early pregnancy. Int. J. Gynecol. Obstet. 2013, 121, 127–131. [Google Scholar] [CrossRef]
- Cioffi, J.; Schmied, V.; Dahlen, H.; Mills, A.; Thornton, C.; Duff, M.; Cummings, J.; Kolt, G.S. Physical activity in pregnancy: Women’s perceptions, practices, and influencing factors. J. Midwifery Women’s Health 2010, 55, 455–461. [Google Scholar] [CrossRef]
- Renault, K.; Nørgaard, K.; Andreasen, K.R.; Secher, N.J.; Nilas, L. Physical activity during pregnancy in obese and normal-weight women as assessed by pedometer. Acta Obstet. Gynecol. Scand. 2010, 89, 956–961. [Google Scholar] [CrossRef]
- Domingues, M.; Santos, I.; Matijasevich, A.; Horta, B.L.; Hallal, P.C.; Coll, C. Changes in Leisure-Time Physical Activity from the Prepregnancy to the Postpartum Period: 2004 Pelotas (Brazil) Birth Cohort Study. J. Phys. Act. Health 2016, 13, 361–365. [Google Scholar] [CrossRef]
- Sui, Z.; Moran, L.J.; Dodd, J.M. Physical activity levels during pregnancy and gestational weight gain among women who ara overweight or obese. Physicl. Act. Diet. 2013, 24, 206–213. [Google Scholar]
- Lof, M.; Forsum, E. Activity pattern and energy expenditure due to physical activity before and during pregnancy in healthy Swedish women. Br. J. Nutr. 2006, 95, 296. Available online: http://www.who.int/substance_abuse/research_tools/translation/en/index.html (accessed on 25 December 2017). [CrossRef] [PubMed] [Green Version]
- Hegaard, H.K.; Pedersen, B.K.; Nielsen, B.B.; Damm, P. Leisure time physical activity during pregnancy and impact on gestational diabetes mellitus, pre-eclampsia, preterm delivery and birth weight: A review. Acta Obstet. Gynecol. Scand. 2007, 86, 1290–1296. [Google Scholar] [CrossRef]
- Padmapriya, N.; Shen, L.; Soh, S.; Shen, Z.; Kwek, K.; Godfrey, K.; Gluckman, P.D.; Chong, Y.S.; Saw, S.M.; Müller-Riemenschneider, F. Physical Activity and Sedentary Behavior Patterns Before and During Pregnancy in a Multi-ethnic Sample of Asian Women in Singapore. Matern. Child Health J. 2015, 19, 2523–2535. [Google Scholar] [CrossRef]
| Sufficient LTPA | Insufficient LTPA | p-Value | |
|---|---|---|---|
| Age (mean ± SD) | 31.42 ± 5.29 | 32.16 ± 4.99 | 0.426 |
| Nationality N (%) | |||
| Serbian | 117 (99.2) | 44 (100) | |
| Other | 1 (0.8) | 0 (0) | 0.540 |
| Place of residence N (%) | |||
| Urban | 110 (94.8) | 38 (86.4) | |
| Rural | 6 (5.2) | 6 (13.6) | 0.07 |
| Marital status N (%) | |||
| Married/permanent relationship | 118 (100) | 44 (100) | |
| Single | 0 | 0 | / |
| Working status N (%) | |||
| Employed | 98 (83.1) | 39 (88.6) | |
| Unemployed | 20 (16.9) | 5 (11.4) | 0.381 |
| Years of education N (%) | |||
| ≤12 years of education | 38 (32.2) | 23 (52.3) | |
| >12 years of education | 80 (67.8) | 21 (47.7) | 0.019 |
| Self-rated financial status N (%) | |||
| Poor | 14 (12.2) | 7 (16.3) | |
| Average | 66 (57.4) | 14 (32.6) | |
| Good | 35 (30.4) | 22 (51.2) | 0.019 |
| Planned pregnancy N (%) | 85 (72) | 37 (84.1) | 0.113 |
| Nausea N (%) | 80 (67.8) | 33 (75.0) | 0.375 |
| Has children N (%) | 55 (46.6) | 25 (56.8) | 0.248 |
| Daily fruit intake N (%) | 79 (66.9) | 31 (70.5) | 0.671 |
| Daily vegetables intake N (%) | 67 (56.8) | 22 (50.0) | 0.440 |
| Chronic disease N (%) | 34 (28.8) | 13 (29.5) | 0.927 |
| Height in cm (mean ± SD) | 167.65 ± 6.43 | 168.14 ± 5.73 | 0.660 |
| Weight in kg (mean ± SD) | 68.53 ± 15.96 | 66.81 ± 14.35 | 0.533 |
| Waist circumference in cm (mean ± SD) | 78.88 ± 11.90 | 77.25 ± 12.42 | 0.569 |
| BMI in kg/m2 (mean ± SD) | 24.29 ± 4.95 | 23.64 ± 5.05 | 0.457 |
| PA level before pregnancy N (%) | |||
| Low | 21 (18.9) | 17 (39.5) | |
| Moderate | 63 (56.8) | 17 (39.5) | |
| High | 27 (24.3) | 9 (20.0) | 0.027 |
| Sufficient LTPA (Mean ± SD) | Insufficient LTPA (Mean ± SD) | p-Value | |
|---|---|---|---|
| Sedentary before pregnancy (Hours/week) | 25.88 ± 2.62 | 22.97 ± 4.06 | 0.942 |
| Walking before pregnancy (Hours/week) | 4.73 ± 0.44 | 3.17 ± 0.48 | 0.006 |
| Moderate recreational physical activity before pregnancy (Hours/week) | 5.37 ± 0.5 | 4.83 ± 0.74 | 0.326 |
| Vigorous physical activity before pregnancy (Hours/week) | 4.80 ± 0.44 | 3.57 ± 0.54 | 0.379 |
| Characteristic | OR (95% CI) |
|---|---|
| Years of education | |
| ≤12 years of education | 2.30 (1.05–5.04) |
| >12 years of education | 1.0 (reference category) |
| Self-rated financial status | |
| Poor | 0.34 (0.14–0.79) |
| Average | 0.85 (0.27-2.69) |
| Good | 1.0 (reference category) |
| PA level before pregnancy | |
| Low | 1.01 (0.26-3.91) |
| Moderate | 0.54 (0.18-1.57) |
| High | 1.0 (reference category) |
| Walking before pregnancy (Hours/week) | 0.87 (0.77–0.99) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorovic, J.; Terzic-Supic, Z.; Bjegovic-Mikanovic, V.; Piperac, P.; Dugalic, S.; Gojnic-Dugalic, M. Factors Associated with the Leisure-Time Physical Activity (LTPA) during the First Trimester of the Pregnancy: The Cross-Sectional Study among Pregnant Women in Serbia. Int. J. Environ. Res. Public Health 2020, 17, 1366. https://doi.org/10.3390/ijerph17041366
Todorovic J, Terzic-Supic Z, Bjegovic-Mikanovic V, Piperac P, Dugalic S, Gojnic-Dugalic M. Factors Associated with the Leisure-Time Physical Activity (LTPA) during the First Trimester of the Pregnancy: The Cross-Sectional Study among Pregnant Women in Serbia. International Journal of Environmental Research and Public Health. 2020; 17(4):1366. https://doi.org/10.3390/ijerph17041366
Chicago/Turabian StyleTodorovic, Jovana, Zorica Terzic-Supic, Vesna Bjegovic-Mikanovic, Pavle Piperac, Stefan Dugalic, and Miroslava Gojnic-Dugalic. 2020. "Factors Associated with the Leisure-Time Physical Activity (LTPA) during the First Trimester of the Pregnancy: The Cross-Sectional Study among Pregnant Women in Serbia" International Journal of Environmental Research and Public Health 17, no. 4: 1366. https://doi.org/10.3390/ijerph17041366





