Effectiveness and Safety in Remote Monitoring of Patients with Pacemakers Five Years after an Implant: The Poniente Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
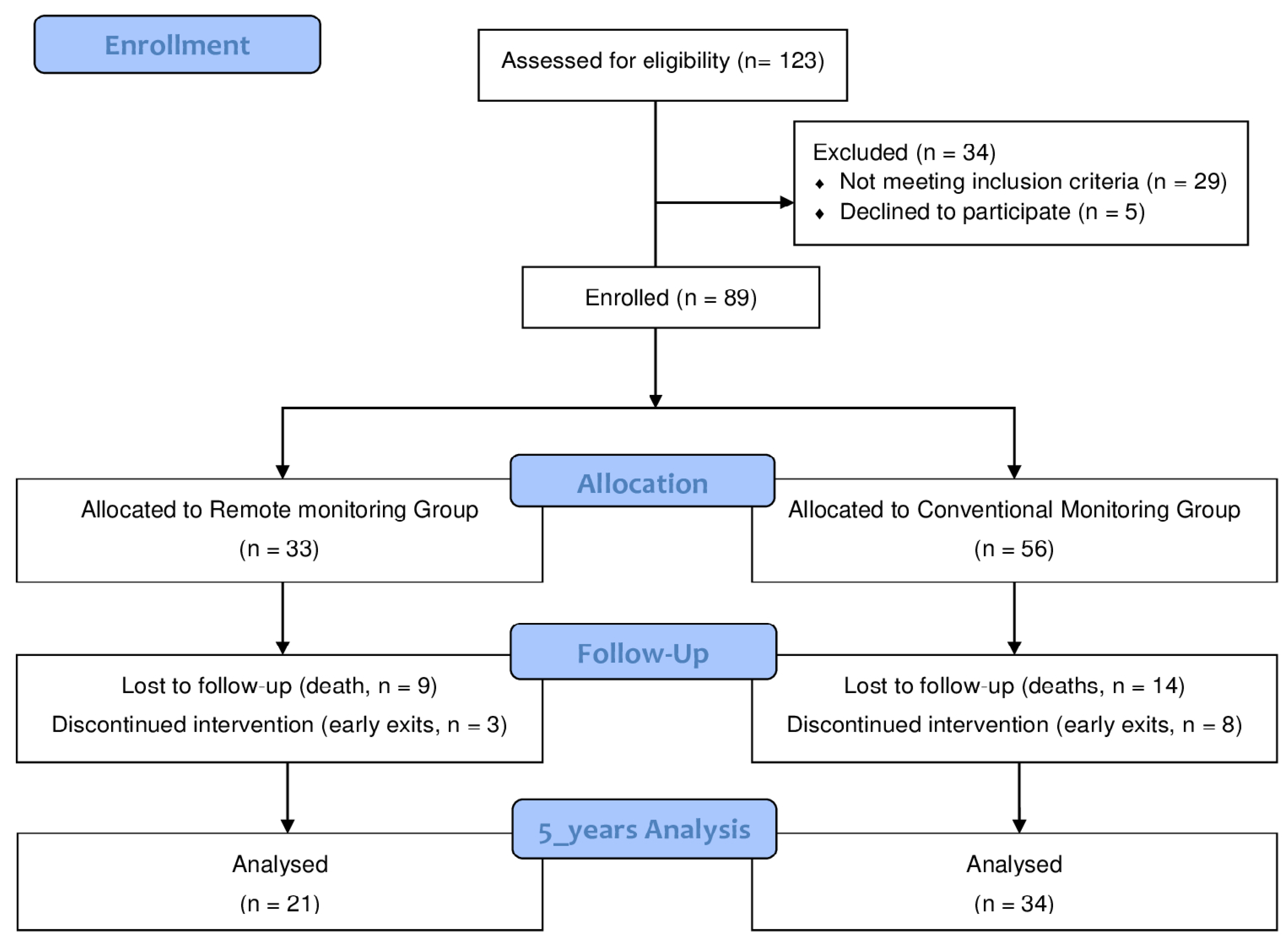
2.2. Setting and Patients
2.3. Telecardiology System
2.4. Procedure
2.5. Measures and Instruments
2.6. Statistical Analysis
3. Results
Health-Related Quality of Life and Functional Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Udo, E.O.; van Hemel, N.M.; Zuithoff, N.P.; Barrett, M.J.; Ruiter, J.H.; Doevendans, P.A.; Moons, K.G. Incidence and predictors of pacemaker reprogramming: Potential consequences for remote follow-up. Europace 2013, 15, 978–983. [Google Scholar] [CrossRef]
- Andreozzi, E.; Gargiulo, G.D.; Fratini, A.; Esposito, D.; Bifulco, P. A Contactless Sensor for Pacemaker Pulse Detection: Design Hints and Performance Assessment. Sensors 2018, 18, 2715. [Google Scholar] [CrossRef]
- Deering, T.F.; Clair, W.K.; Delaughter, M.C.; Fisher, W.G.; Garlitski, A.C.; Wilkoff, B.L.; Gillis, A.M. A Heart Rhythm Society Electrophysiology Workforce study: Current survey analysis of physician workforce trends. Heart Rhythm 2010, 7, 1346–1355. [Google Scholar] [CrossRef]
- García-Fernández, F.J.; Osca Asensi, J.; Romero, R.; Fernández Lozano, I.; Larrazabal, J.M.; Martínez Ferrer, J.; Ortiz, R.; Pombo, M.; Tornés, F.J.; Moradi Kolbolandi, M. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: A long-term randomized trial (RM-ALONE). Eur. Heart J. 2019, 40, 1837–1846. [Google Scholar] [CrossRef]
- Andrès, E.; Talha, S.; Zulfiqar, A.A.; Hajjam, M.; Ervé, S.; Hajjam, J.; Gény, B.; El Hassani, A.H. Current research and new perspectives of telemedicine in chronic heart failure: Narrative review and points of interest for the clinician. Clin. Med. 2018, 7, 544. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Task Force Members; Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O. Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013, 15, 1070–1118. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cruzado y Barba, M.; López-Villegas, A.; Catalán-Matamoros, D. Conclusiones y recomendaciones del I Congreso Internacional de Telemedicina e Investigación Sanitaria (Conclusions and recommendations of the 1st International Congress of Telemedicine and Health Research). Rev. Esp. Comun. Salud. 2016, 7, 164–166. [Google Scholar] [CrossRef][Green Version]
- Freeman, J.V.; Saxon, L. Remote Monitoring and Outcomes in Pacemaker and Defibrillator Patients. Big Data Saving Lives? J. Am. Coll. Cardiol. 2015, 65, 2611–2613. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.; Bayer, S.; Curry, R. Implementing complex innovations in fluid multi-stakeholder environments: Experiences of “telecare”. Technovation 2006, 26, 396–406. [Google Scholar] [CrossRef]
- Catalán Matamoros, D.; López Villegas, A. La Telesalud y la sociedad actual: Retos y oportunidades (Telehealth and the current society: Challenges and opportunities). Rev. Esp. Comun. Salud. 2016, 7, 336–345. [Google Scholar]
- Christensen, J. The emergence and unfolding of telemonitoring practices in different healthcare organizations. Int. J. Environ. Res. Public Health 2018, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario. Remote Monitoring of Implantable Cardioverter-Defibrillators, Cardiac Resynchronization Therapy, and Permanent Pacemakers: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2018, 18, 1–199. Available online: http://www.hqontario.ca/evidenceto-improve-care/journal-ontario-health-technology assessment-series (accessed on 22 February 2020).
- Raatikainen, M.J.P.; Uusimaa, P.; van Ginneken, M.M.E.; Janssen, J.P.G.; Linnaluoto, M. Remote monitoring of implantable cardioverter defibrillator patients: A safe, timesaving, and cost-effective means for follow-up. EP Europace 2008, 10, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Exley, J.; Parks, S.; Ball, S.; Bienkowska-Gibbs, T.; MacLure, C.; Harte, E.; Stewart, K.; Larkin, J.; Bottomley, A.; et al. The use and impact of quality of life assessment tools in clinical care settings for cancer patients, with a particular emphasis on brain cancer: Insights from a systematic review and stakeholder consultations. Qual. Life Res. 2016, 25, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Mlynarski, R.; Wlodyka, A.; Kargul, W. Changes in the mental and physical components of the quality of life for patients six months after pacemaker implantation. Cardiol. J. 2009, 16, 250–253. [Google Scholar]
- Oliveira, B.G.; Velasquez-Melendez, G.; Rincon, L.G.; Ciconelli, R.M.; Sousa, L.A.; Ribeiro, A.L. Health-related quality of life in Brazilian pacemaker patients. Pacing Clin. Electrophysiol. 2008, 31, 1178–1183. [Google Scholar] [CrossRef]
- Udo, E.O.; van Hemel, N.M.; Zuithoff, N.P.; Hijboer, H.; Taks, W.; Doevendans, P.A.; Moons, K.G. Long term quality-of-life in patients with bradycardia pacemaker implantation. Int. J. Cardiol. 2013, 168, 2159–2163. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Potpara, T.S.; Boveda, S.; Deharo, J.C.; Chen, J.; Dobreanu, D.; Sciarrafia, E. Patients’ knowledge and attitudes regarding living with implantable electronic devices: Results of a multicentre, multinational patient survey conducted by the European Heart Rhythm Association. Ep Europace 2017, 20, 386–391. [Google Scholar] [CrossRef]
- Mabo, P.; Victor, F.; Bazin, P.; Ahres, S.; Babuty, D.; Da Costa, A.; Binet, D.; Daubert, J.C.; CONNECT Investigators. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial). Eur. Heart J. 2012, 33, 1105–1111. [Google Scholar] [CrossRef]
- Guedon-Moreau, L.; Lacroix, D.; Sadoul, N.; Clementy, J.; Kouakam, C.; Hermida, J.S.; Aliot, E.; Boursier, M.; Bizeau, O.; Kacet, S.; et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: Safety and efficacy report of the ECOST trial. Eur. Heart J. 2013, 34, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Slotwiner, D.; Varma, N.; Akar, J.G.; Annas, G.; Beardsall, M.; Fogel, R.I.; Galizio, N.O.; Glotzer, T.V.; Leahy, R.A.; Love, C.J. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015, 12, e69–e100. [Google Scholar] [CrossRef] [PubMed]
- López-Villegas, A.; Catalán-Matamoros, D.; Robles-Musso, E.; Peiró, S. Comparative efectiveness of remote monitoring of people with cardiac Pacemaker versus conventional: Quality of life at the six months. Rev. Esp. Salud Pública 2015, 89, 149–158. [Google Scholar]
- López-Villegas, A.; Catalán-Matamoros, D.; Robles-Musso, E.; Peiró, S. Effectiveness of pacemaker tele-monitoring on quality of life, functional capacity, event detection and workload: The PONIENTE Trial. Geriatr. Gerontol. Int. 2016, 16, 1188–1195. [Google Scholar]
- Medtronic. How the CareLink Network Works. Available online: http://www.medtronic.com/uk-en/patients/treatments-therapies/pacemaker/important-safety-information.html#Carelink (accessed on 9 November 2018).
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.A., 3rd; Freedman, R.A.; Gettes, L.S.; Gillinov, A.M.; Gregoratos, G.; Hammill, S.C.; Hayes, D.L.; et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2008, 27, e1–e62. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Auricchio, A.; Brugada, J.; Cowie, M.; Ellenbogen, K.A.; Gillis, A.M.; Hayes, D.L.; Howlett, J.G.; Kautzner, J.; Love, C.J.; et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm 2008, 5, 907–925. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Boineau, R.E.; Higginbotham, M.B.; Lee, K.L.; Mark, D.B.; Califf, R.M.; Cobb, F.R.; Pryor, D.B. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am. J. Cardiol. 1989, 64, 651–654. [Google Scholar] [CrossRef]
- Alonso, J.; Permanyer-Miralda, G.; Cascant, P.; Brotons, C.; Prieto, L.; Soler-Soler, J. Measuring functional status of chronic coronary patients. Reliability, validity and responsiveness to clinical change of the reduced version of the Duke Activity Status Index (DASI). Eur. Heart J. 1997, 18, 414–419. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Badia, X.; Roset, M.; Montserrat, S.; Herdman, M.; Segura, A. The Spanish version of EuroQol: A description and its applications. European Quality of Life scale. Med. Clin. (Barc) 1999, 112, 79–85. [Google Scholar]
- Perl, S.; Stiegler, P.; Rotman, B.; Prenner, G.; Lercher, P.; Anelli-Monti, M.; Sereinigg, M.; Riegelnik, V.; Kvas, E.; Kos, C.; et al. Socioeconomic effects and cost saving potential of remote patient monitoring (SAVE-HM trial). Int. J. Cardiol. 2013, 169, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Amara, W.; Montagnier, C.; Cheggour, S.; Boursier, M.; Gully, C.; Barnay, C.; Georger, F.; Deplagne, A.; Fromentin, S.; Mlotek, M.; et al. Early detection and treatment of atrial arrhythmias alleviates the arrhythmic burden in paced patients: The SETAM study. Pacing Clin. Electrophysiol. 2017, 40, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Martinelli, M.; Peixoto, G.L.; Siquiera, S.F.; Wajngarten, M.; Silva, R.T.; Costa, R.; Filho, R.; Ramires, J.A. Silent atrial fibrillation in elderly pacemaker users: A randomized trial using home monitoring. Ann. Noninvasive Electrocardiol. 2016, 21, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Crossley, G.H.; Chen, J.; Choucair, W.; Cohen, T.J.; Gohn, D.C.; Johnson, W.B.; Kennedy, E.E.; Mongeon, L.R.; Serwer, G.A.; Qiao, H.; et al. Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J. Am. Coll. Cardiol. 2009, 54, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Halimi, F.; Clementy, J.; Attuel, P.; Dessenne, X.; Amara, W. Optimized post-operative surveillance of permanent pacemakers by home monitoring: The OEDIPE trial. Europace 2008, 10, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- López-Liria, R.; López-Villegas, A.; Enebakk, T.; Thunhaug, H.; Lappegård, K.T.; Catalán-Matamoros, D. Telemonitoring and Quality of Life in Patients after 12 Months Following a Pacemaker Implant: The Nordland Study, a Randomised Trial. Int. J. Environ. Res. Public Health 2019, 16, 2001. [Google Scholar] [CrossRef]
- Crossley, G.H.; Boyle, A.; Vitense, H.; Chang, Y.; Mead, R.H.; COMPAS Trial Investigators. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: The value of wireless remote monitoring with automatic clinician alerts. J. Am. Coll. Cardiol. 2011, 57, 1181–1189. [Google Scholar] [CrossRef]
- Lim, P.C.Y.; Lee, A.S.Y.; Chua, K.C.M.; Lim, E.T.S.; Chong, D.T.T.; Tan, B.Y.; Ho, K.L.; Teo, W.S.; Ching, C.K. Remote monitoring of patients with cardiac implantable electronic devices: A Southeast Asian, single-centre pilot study. Singap. Med. J. 2016, 57, 372–377. [Google Scholar] [CrossRef]
- Ricci, R.P.; Vicentini, A.; D’Onofrio, A.; Sagone, A.; Rovaris, G.; Padeletti, L.; Morichelli, L.; Fusco, A.; De Vivo, S.; Lombardi, L.; et al. Economic analysis of remote monitoring of cardiac implantable electronic devices: Results of the Health Economics Evaluation Registry for Remote Follow up (TARIFF) study. Heart Rhythm 2017, 14, 50–57. [Google Scholar] [CrossRef]
- Hindricks, G.; Taborsky, M.; Glikson, M.; Heinrich, U.; Schumacher, B.; Katz, A.; Brachmann, J.; Lewalter, T.; Goette, A.; Block, M.; et al. Implant based multiparameter telemonitoring of patients with heart failure (IN-TIME): A randomised controlled trial. Lancet 2014, 384, 583–590. [Google Scholar] [CrossRef]
- Hindricks, G.; Elsner, C.; Piorkowski, C.; Taborsky, M.; Geller, J.C.; Schumacher, B.; Bytesnik, J.; Kottkamp, H. Quarterly vs. yearly clinical follow-up of remotely monitored recipients of prophylactic implantable cardioverter-defibrillators: Results of the REFORM trial. Eur. Heart J. 2014, 35, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F. The Trans-European Network—Home-Care Management System (TEN-HMS) Study: An Investigation of the Effect of Telemedicine on Outcomes in Europe. Dis. Manag. Health Outcomes 2006, 14, 23–28. [Google Scholar] [CrossRef]
- Chaudhry, S.I.; Mattera, J.A.; Curtis, J.P.; Spertus, J.A.; Herrin, J.; Lin, Z.; Phillips, C.O.; Hodshon, B.V.; Cooper, L.S.; Krumholz, H.M. Telemonitoring in patients with heart failure. N. Engl. J. Med. 2010, 363, 2301–2309. [Google Scholar] [CrossRef]
- Koehler, F.; Winkler, S.; Schieber, M.; Sechtem, U.; Stangl, K.; Böhm, M.; Boll, H.; Baumann, G.; Honold, M.; Koehler, K.; et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The telemedical interventional monitoring in heart failure study. Circulation 2011, 123, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.K.; Romano, P.S.; Edgington, S.; Aronow, H.U.; Auerbach, A.D.; Black, J.T.; De Marco, T.; Escarce, J.J.; Evangelista, L.S.; Hanna, B.; et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients with Heart Failure—The Better Effectiveness After Transition-Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Chang, S.; Davidson, P.M.; Newton, P.J.; Horowitz, J.D.; Krum, H.; Reid, C.M.; Chan, Y.K.; et al. Composite outcome measures in a pragmatic clinical trial of chronic heart failure management: A comparative assessment. Int. J. Cardiol. 2015, 185, 62–68. [Google Scholar] [CrossRef]
- Pocock, S.J.; Gersh, B.J. Do current clinical trials meet society’s needs: A critical review of recent evidence. J. Am. Coll. Cardiol. 2014, 64, 1615–1628. [Google Scholar] [CrossRef]
- Cronin, E.; Varma, N. Remote monitoring of cardiovascular implanted electronic devices: A paradigm shift for the 21st century. Expert Rev. Med. Devices 2012, 9, 367–376. [Google Scholar] [CrossRef]
- Folino, A.F.; Breda, R.; Calzavara, P.; Migliore, F.; Iliceto, S.; Buja, G. In-home controls of pacemakers in debilitated elderly patients. Geriatr. Gerontol. Int. 2012, 12, 30–35. [Google Scholar] [CrossRef]
- Ricci, R.P.; Morichelli, L.; Santini, M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: Impact on medical management and health-care resource utilization. Europace 2008, 10, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Halimi, F.; Cantu, F. Remote monitoring for active cardiovascular implantable electronic devices: A European survey. Europace 2010, 12, 1778–1780. [Google Scholar] [CrossRef]
- Zanaboni, P.; Landolina, M.; Marzegalli, M.; Lunati, M.; Perego, G.B.; Guenzati, G.; Curnis, A.; Valsecchi, S.; Borghetti, F.; Borghi, G.; et al. Cost-utility analysis of the EVOLVO study on remote monitoring for heart failure patients with implantable defibrillators: Randomized controlled trial. J. Med. Internet Res. 2013, 15, e106. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, R.; Völler, H.; Nagels, K.; Bindl, D.; Vettorazzi, E.; Dittmar, R.; Wohlgemuth, W.; Neumann, T.; Störk, S.; Bruder, O.; et al. First outline and baseline data of a randomized, controlled multicenter trial to evaluate the health economic impact of home telemonitoring in chronic heart failure—CardioBBEAT. Trials 2015, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Cano Pérez, O.; Pombo Jiménez, M.; Lorente Carreño, D.; Chimeno García, J. Spanish Pacemaker Registry. 16th Official Report of the Spanish Society of Cardiology Working Group on Cardiac Pacing (2018). Rev. Esp. Cardiol. 2019, 72, 944–953. [Google Scholar]
| All | Groups | p-Value | ||
|---|---|---|---|---|
| Remote Monitoring | Conventional Monitoring | |||
| Age (mean) ± SD | 81.00 ± 6.47 | 81.14 ± 7.30 | 80.91 ± 6.01 | 0.690 |
| Women (%) | 17 (30.91) | 8 (38.10) | 9 (26.47) | 0.365 |
| DASI (mean) [95 CI] | 19.46 [19.22; 21.76] | 19.05 [16.42; 21.67] | 19.72 [17.39; 22.05] | 0.842 |
| EQ5D utilities (mean) [95 CI] | 0.73 [0.64; 0.83] | 0.68 [0.50; 0.85] | 0.77 [0.65; 0.90] | 0.232 |
| EQ5D VAS (mean) | 73.27 [68.95; 77.60] | 73.81 [67.46; 80.16] | 72.9 [66.90; 78.99] | 0.879 |
| Pacing indication (%) | ||||
| Sinus node disease | 11 (20.00) | 3 (14.29) | 8 (23.53) | 0.493 |
| Atrioventricular block | 39 (70.91) | 15 (71.43) | 24 (70.59) | |
| Others | 5 (9.09) | 3 (14.29) | 2 (5.88) | |
| Disease manifestations (%) | ||||
| Syncope | 33 (60.00) | 13 (61.90) | 20 (58.82) | 0.681 |
| Dizziness | 16 (29.09) | 7 (33.33) | 9 (26.47) | |
| Dyspnoea | 3 (5.45) | 0 (0) | 3 (8.82) | |
| Angina | 3 (5.45) | 1 (4.76) | 2 (5.88) | |
| Service derived (%) | ||||
| Emergencies | 11 (20.00) | 4 (19.05) | 7 (20.59) | 0.516 |
| Cardiology | 32 (58.18) | 14 (66.67) | 18 (52.94) | |
| Other service | 12 (21.82) | 3 (14.29) | 9 (26.47) | |
| Stimulation (%) | ||||
| VDD | 14 (25.45) | 5 (23.81) | 9 (26.47) | 0.595 |
| DDD | 30 (54.55) | 12 (57.14) | 18 (52.94) | |
| VVI | 8 (14.55) | 4 (19.05) | 4 (11.76) | |
| VVIR | 3 (5.45) | 0 (0) | 3 (8.82) | |
| AF Paroxismal episodes (%) | ||||
| Yes | 26 (47.27) | 14 (66.67) | 12 (35.29) | 0.024 |
| No | 29 (52.73) | 7 (33.33) | 22 (64.71) | |
| AF episodes duration (mean) ± SD | ||||
| 2.62 ± 1.55 | 2.57 ± 1.65 | 2.67 ± 1.50 | 0.829 | |
| Ischemic cerebrovascular event (%) | ||||
| Yes | 2 (3.64) | 1 (4.76) | 1 (2.94) | 0.622 |
| No | 53 (96.36) | 20 (95.24) | 33 (97.06) | |
| Anticoagulation (%) | ||||
| Yes | 20 (36.36) | 11 (52.38) | 9 (26.47) | 0.052 |
| No | 35 (63.64) | 10 (47.62) | 25 (73.53) | |
| Hospitalisation causes (%) | ||||
| No hospitalisation | 52 (94.55) | 19 (90.48) | 33 (97.06) | |
| Friedrich | 1 (1.82) | 1 (4.76) | 0 (0.00) | 0.323 |
| PM electrode dislocation | 1 (1.82) | 1 (4.76) | 0 (0.00) | |
| PM Fracture electrode | 1 (1.82) | 0 (0.00) | 1 (2.94) | |
| Hospitalisation days after PM implantation (mean) ± SD | ||||
| 0.13 ± 0.70 | 0.95 ± 0.30 | 0.15 ± 0.86 | 0.322 | |
| Hospital visits (mean) ± SD | ||||
| 6.58 ± 2.74 | 4.38 ± 2.62 | 7.94 ± 1.79 | <0.001 | |
| Home transmissions (mean) ± SD | ||||
| 2.53 ± 3.41 | 6.62 ± 1.72 | --- | ||
| Total transmissions (mean) ± SD | ||||
| 9.11 ± 2.72 | 11 ± 2.93 | 7.94 ± 1.79 | <0.001 | |
| All | Remote Monitoring | Conventional Monitoring | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Functional Capacity | ||||||||||||
| Baseline | Year 5 | Differences | p-value | Baseline | Year 5 | Differences | p-value | Baseline | Year 5 | Differences | p-value | |
| DASI [95CI] | 20.49 [19.22; 21.76] | 20.64 [18.84; 22.43] | 0.15 [–1.33; 2.47] | 0.548 | 21.42 [19.32; 23.52] | 20.48 [17.63; 23.32] | −0.94 [−2,06; 4.01] | 0.510 | 19.95 [18.32; 21.58] | 20.74 [18.31; 23.16] | 0.79 [−1.40; 4.79] | 0.799 |
| Health-Related Quality of Life | ||||||||||||
| EQ5D VAS [95CI] | 58.41 [53.27; 63.56] | 73.27 [68.95; 77.60] | 14.86 [5.79; 20.21] | <0.001 | 59.00 [51.09; 66.91] | 73.81 [67.46; 80.16] | 14.81 [6.28; 29.91] | <0.01 | 58.08 [51.18; 75.33] | 72.94 [66.90; 78.99] | 14.86 [7.93; 29.13] | <0.001 |
| EQ5D utilities [95CI] | 0.70 [0.62; 0.78] | 0.73 [0.64; 0.83] | 0.03 [−0.11; 0.15] | 0.745 | 0.74 [0.62; 0.86] | 0.68 [0.50; 0.85] | −0.06 [−0.13; 0.27] | 0.484 | 0.67 [0.56; 0.78] | 0.77 [0.65; 0.90] | 0.10 [−0.36; 0.06] | 0.143 |
| Remote Monitoring | Conventional Monitoring | p-Value | |
|---|---|---|---|
| Functional Capacity | |||
| DASI [95 CI] | 20.48 [17.63; 23.32] | 20.74 [18.31; 23.16] | 0.84 |
| Health-related Quality of Life | |||
| EQ5D VAS [95 CI] | 73.81 [67.46; 80.16] | 72.94 [66.90; 78.99] | 0.88 |
| EQ5D utilities [95 CI] | 0.68 [0.50; 0.85] | 0.77 [0.65; 0.90] | 0.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Liria, R.; López-Villegas, A.; Leal-Costa, C.; Peiró, S.; Robles-Musso, E.; Bautista-Mesa, R.; Rocamora-Pérez, P.; Lappegård, K.T.; Catalán-Matamoros, D. Effectiveness and Safety in Remote Monitoring of Patients with Pacemakers Five Years after an Implant: The Poniente Study. Int. J. Environ. Res. Public Health 2020, 17, 1431. https://doi.org/10.3390/ijerph17041431
López-Liria R, López-Villegas A, Leal-Costa C, Peiró S, Robles-Musso E, Bautista-Mesa R, Rocamora-Pérez P, Lappegård KT, Catalán-Matamoros D. Effectiveness and Safety in Remote Monitoring of Patients with Pacemakers Five Years after an Implant: The Poniente Study. International Journal of Environmental Research and Public Health. 2020; 17(4):1431. https://doi.org/10.3390/ijerph17041431
Chicago/Turabian StyleLópez-Liria, Remedios, Antonio López-Villegas, César Leal-Costa, Salvador Peiró, Emilio Robles-Musso, Rafael Bautista-Mesa, Patricia Rocamora-Pérez, Knut Tore Lappegård, and Daniel Catalán-Matamoros. 2020. "Effectiveness and Safety in Remote Monitoring of Patients with Pacemakers Five Years after an Implant: The Poniente Study" International Journal of Environmental Research and Public Health 17, no. 4: 1431. https://doi.org/10.3390/ijerph17041431
APA StyleLópez-Liria, R., López-Villegas, A., Leal-Costa, C., Peiró, S., Robles-Musso, E., Bautista-Mesa, R., Rocamora-Pérez, P., Lappegård, K. T., & Catalán-Matamoros, D. (2020). Effectiveness and Safety in Remote Monitoring of Patients with Pacemakers Five Years after an Implant: The Poniente Study. International Journal of Environmental Research and Public Health, 17(4), 1431. https://doi.org/10.3390/ijerph17041431







