Lesson from Ecotoxicity: Revisiting the Microbial Lipopeptides for the Management of Emerging Diseases for Crop Protection
Abstract
:1. Introduction
2. Isolation and Characterization of Lipopeptides
2.1. Isolation and Purification of Lipopeptides
2.2. Molecular Characterization of Antifungal Lipopeptides
3. Different Classes of Lipopeptides
3.1. Iturins
3.2. Surfactins
3.3. Fengycins
3.4. Pseudofactins
3.5. Viscosins
3.6. Daptomycins
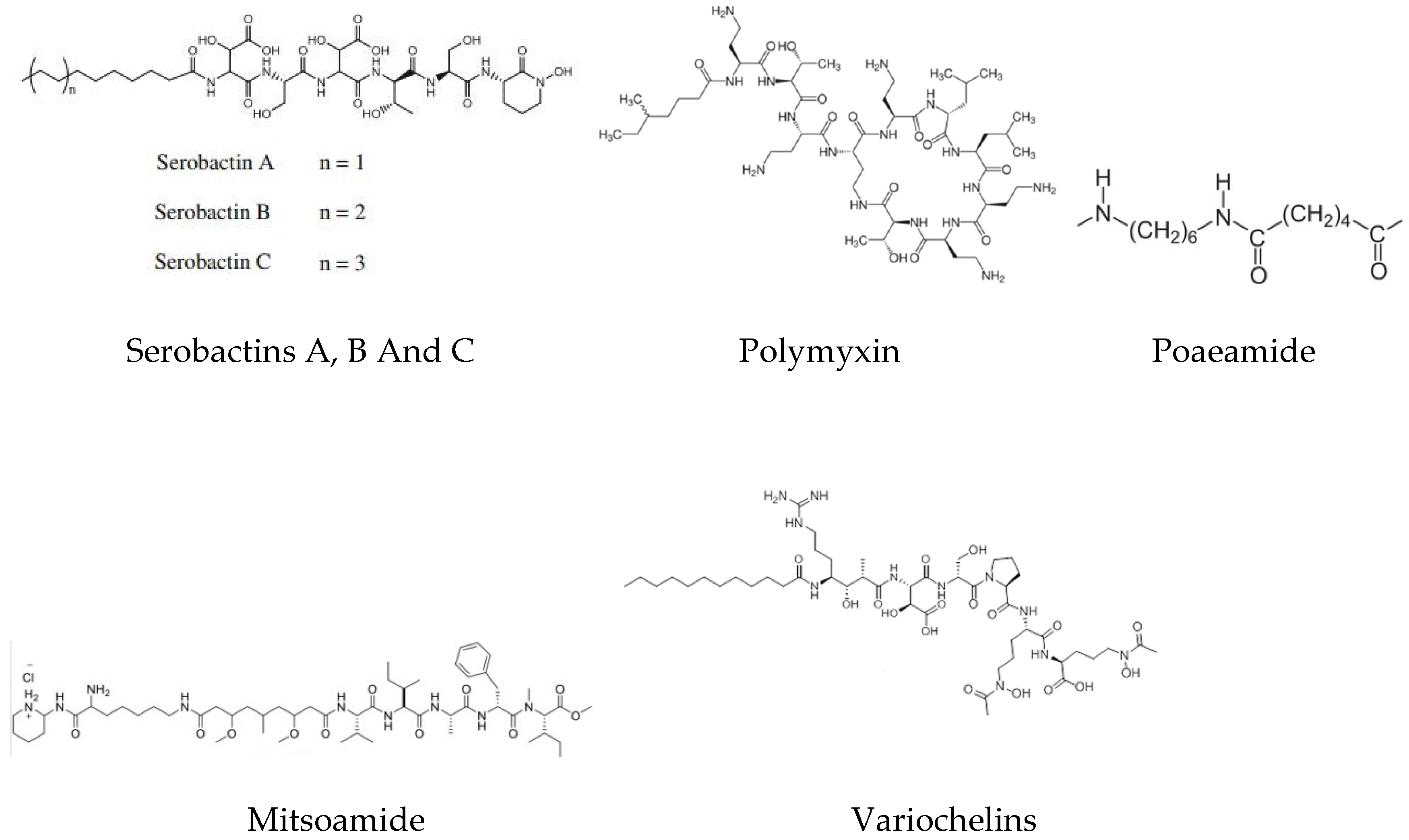
3.7. Poaeamides
4. Biocontrol Potential of Lipopeptides
4.1. Lipopeptides as Biosurfactants Distressing Membrane Integrity and Permeability
4.2. Lipopeptides as Siderophores
4.3. Lipopeptides ISR-Inducer in Plants
5. Future prospects of Microbial Lipopeptides in Plant Disease Management
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brahmaprakash, G.P.; Sahu, P.K. Biofertilizers for sustainability. J. Indian Inst. Sci. 2012, 92, 37–62. [Google Scholar]
- Singh, U.B.; Malviya, D.; Singh, S.; Imran, M.; Pathak, N.; Alam, M.; Rai, J.P.; Singh, R.K.; Sarma, B.K.; Sharma, P.K.; et al. Compatible salt-tolerant rhizosphere microbe-mediated induction of phenylpropanoid cascade and induced systemic responses against Bipolaris sorokiniana (Sacc.) Shoemaker causing spot blotch disease in wheat (Triticum aestivum L.). Appl. Soil Ecol. 2016, 108, 300–306. [Google Scholar] [CrossRef]
- Singh, U.B.; Malviya, D.; Singh, S.; Pradhan, J.K.; Singh, B.P.; Roy, M.; Imram, M.; Pathak, N.; Baisyal, B.M.; Rai, J.P.; et al. Bio-protective microbial agents from rhizosphere eco-systems trigger plant defense responses provide protection against sheath blight disease in rice (Oryza sativa L.). Microbiol. Res. 2016, 192, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Ratna, P.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Sahu, P.K.; Brahmaprakash, G.P. Microbial inoculants for agriculture in changing climates. Mysore J. Agril. Sci. 2017, 51, 27–44. [Google Scholar]
- Sahu, P.K.; Brahmaprakash, G.P. Modified liquid dual culture methodology for screening bacterial endophytes against fungal pathogens. Mysore J. Agril. Sci. 2018, 52, 234–240. [Google Scholar]
- Sahu, P.K.; Singh, D.P.; Prabha, R.; Meena, K.K.; Abhilash, P.C. Connecting microbial capabilities with the soil and plant health: Options for agricultural sustainability. Ecol. Indic. 2019, 105, 601–612. [Google Scholar] [CrossRef]
- Sahu, P.K.; Singh, S.; Gupta, A.; Singh, U.B.; Brahmaprakash, G.P.; Saxena, A.K. Antagonistic potential of bacterial endophytes and induction of systemic resistance against collar rot pathogen Sclerotium Rolfsii in tomato. Biol. Control 2019, 137, 104014. [Google Scholar] [CrossRef]
- Singh, U.B.; Malviya, D.; Singh, S.; Kumar, M.; Sahu, P.K.; Singh, H.V.; Kumar, S.; Roy, M.; Imran, M.; Rai, J.P.; et al. Trichoderma harzianum-and methyl jasmonate-induced resistance to Bipolaris sorokiniana through enhanced phenylpropanoid activities in bread wheat (Triticum aestivum L.). Front. Microbiol. 2019, 10, 1697. [Google Scholar] [CrossRef] [Green Version]
- Singh, U.B.; Singh, S.; Malviya, D.; Karthikeyan, N.; Imran, M.; Chaurasia, R.; Alam, M.; Singh, P.; Sarma, B.K.; Rai, J.P.; et al. Integration of anti-penetrant tricyclazole, signaling molecule salicylic acid and root associated Pseudomonas fluorescens enhances suppression of Bipolaris sorokiniana in bread wheat (Triticum aestivum L.). J. Plant Pathol. 2019, 101, 943–954. [Google Scholar] [CrossRef]
- Gudina, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactants produced by marine microorganisms with therapeutic applications. Mar. Drugs 2016, 14, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitschke, M.; Costa, S.G.V.A.O. Biosurfactants in food industry. Trends Food Sci. Technol. 2007, 18, 252–259. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Gudina, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1544. [Google Scholar] [CrossRef]
- Peypoux, F.; Bonmatin, J.M.; Wallach, J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 1999, 51, 553–563. [Google Scholar] [CrossRef]
- Cai, X.C.; Li, H.; Xue, Y.R.; Liu, C.H. Study of endophytic Bacillus amyloliquefaciens CC09 and its antifungal CLPs. J. Appl. Biol. Biotechnol. 2013, 1, 1–5. [Google Scholar]
- Stein, T. Bacillus subtilis antibiotics: Structure, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. Aneurinifactin, a new lipopeptide biosurfactant produced by a marine Aneurinibacillus aneurinilyticus SBP-11 isolated from Gulf of Mannar: Purification, characterization and its biological evaluation. Microbiol. Res. 2017, 194, 1–9. [Google Scholar] [CrossRef]
- Arrebola, E.; Jacobs, R.; Korsten, L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against post harvest fungal pathogens. J. Appl. Microbiol. 2010, 108, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Wang, A.H.; Wang, C.L.; Mao, D.Z.; Lu, M.F.; Cui, Y.Q.; Jiao, R.Z. Surfactin induces apoptosis in human breast cancer MCF-7 cells through aROS/JNK-mediated mitochondrial/caspase pathway. Chem. Biol. Interact. 2010, 183, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, next term as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Sanchez, A.J.; Hernandez-Sanchez, H.; Jaramillo-Flores, M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013, 168, 22–32. [Google Scholar] [CrossRef]
- Christova, N.; Lang, S.; Wray, V.; Kaloyanov, K.; Konstantinov, S.; Stoineva, I. Production, structural elucidation, and in vitro antitumor activity of trehalose lipid biosurfactant from Nocardia farcinica strain. J. Microbiol. Biotechnol. 2015, 25, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arutchelvi, J.; Bhaduri, S.; Uppara, P.V.; Doble, M. Mannosylerythritol lipids: A review. J. Ind. Microbiol. Biotechnol. 2008, 35, 1559–1570. [Google Scholar] [CrossRef]
- Leclere, V.; Bechet, M.; Adam, A.; Guez, J.S.; Wathelet, B.; Ongena, M.; Thonart, P.; Gancel, F.; Chollet-Imbert, M.; Jacques, P. Myco-subtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl. Environ. Microbiol. 2005, 71, 4577–4584. [Google Scholar] [CrossRef] [Green Version]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Arshad, M.; Frankenberger, W.T. Plant growth promoting rhizobacteria: Applications and perspectives in agriculture. Adv. Agron. 2004, 81, 97–168. [Google Scholar]
- Ongena, M.; Jacques, P.; Touré, Y.; Destain, J.; Jabrane, A.; Thonart, P. Involvement of fengycin-type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. Appl. Microbiol. Biotechnol. 2005, 69, 29–38. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van Pelt, J.A.; Van Wees, S.C.M.; Ton, J.; Leon-Kloosterziel, K.M.; Keurentjes, J.J.B.; Verhagen, B.W.M.; Knoester, M.; Van der Sluis, I.; Bakker, P.A.H.M.; et al. Rhizobacteria-mediated induced systemic resistance: Triggering, signalling and expression. Eur. J. Plant. Pathol. 2001, 107, 51–61. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.A.H.M. Induced systemic resistance as a mechanism of disease suppression by rhizobacteria. In PGPR: Biocontrol and Biofertilization; Siddiqui, Z.A., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 39–66. [Google Scholar]
- Coventry, H.S.; Dubery, I.A. Lipopolysaccharides from Burkholderia cepacia contribute to an enhanced defensive capacity and the induction of pathogenesis-related proteins in Nicotianae tabacum. Physiol. Mol. Plant Pathol. 2001, 58, 149–158. [Google Scholar] [CrossRef]
- Reitz, M.; Oger, P.; Meyer, A.; Niehaus, K.; Farrand, S.K.; Hallmann, J.; Sikora, R.A. Importance of the O-antigen, core-region and lipid A of rhizobial lipopolysaccharides for the induction of systemic resistance in potato to Globodera pallida. Nematology 2002, 4, 73–79. [Google Scholar] [CrossRef]
- Meziane, H.; Van der Sluis, I.; Van Loon, L.C.; Höfte, M.; Bakker, P.A.H.M. Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol. Plant Pathol. 2005, 6, 177–185. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W.; Pare, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Audenaert, K.; Pattery, T.; Cornelis, P.; Höfte, M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic acid, pyochelin, and pyocyanin. Mol. Plant Microbe Interact. 2002, 15, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Ongena, M.; Jourdan, E.; Schafer, M.; Kech, C.; Budzikiewicz, H.; Luxen, A.; Thonart, P. Isolation of an n-alkylated benzylamine derivative from Pseudomonas putida BTP1 as elicitor of induced systemic resistance in bean. Mol. Plant Microbe Interact. 2005, 18, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Ran, L.X.; Li, Z.N.; Wu, G.J.; Van Loon, L.C.; Bakker, P.A.H.M. Induction of systemic resistance against bacterial wilt in Eucalyptus urophylla by fluorescent Pseudomonas spp. Eur. J. Plant. Pathol. 2005, 113, 59–70. [Google Scholar] [CrossRef]
- Iavicoli, A.; Boutet, E.; Buchala, A.; Métraux, J.P. Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Mol. Plant Microbe Interact. 2003, 16, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, I.A.; Shaukat, S.S. Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: Importance of bacterial secondary metabolite, 2,4-diacetylpholoroglucinol. Soil Biol. Biochem. 2003, 35, 1615–1623. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Cornelis, P.; Höfte, M. Redox-active pyocyanin secreted by Pseudomonas aeruginosa 7NSK2 triggers systemic resistance to Magnaporthe grisea but enhances Rhizoctonia solani susceptibility in rice. Mol. Plant Microbe Interact. 2006, 19, 1406–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raaijmakers, J.M.; de Bruijn, I.; de Kock, M.J.D. Cyclic lipopeptide production by plant-associated Pseudomonas spp.: Diversity, activity, biosynthesis, and regulation. Mol. Plant Microbe Int. 2006, 19, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; Van Breusegem, F.; Eberl, L.; et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006, 29, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Ficke, A.; Asiimwe, T.; Höfte, M.; Raaijmakers, J.M. Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol. 2007, 175, 731–742. [Google Scholar] [CrossRef] [Green Version]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.L.; Thonart, P. Surfactin and fengycinlipopeptides of Bacillus subtilis as elicitors of induced systemic resistance inplants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Gómez-Gómez, L. Plant perception systems for pathogen recognition and defense. Mol. Immunol. 2004, 41, 1055–1062. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signalling events induced by elicitors of plant defenses. Mol. Plant-Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Jourdan, E.; Henry, G.; Duby, F.; Dommes, J.; Barthelemy, J.P.; Thonart, P.; Ongena, M.A.R.C. Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol. Plant-Microbe Int. 2009, 22, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Ghribi, D.; Abdelkefi-Mesrati, L.; Mnif, I.; Kammoun, R.; Ayadi, I.; Saadaoui, I.; Maktouf, S.; Chaabouni-Ellouze, S. Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Malfanova, N.; Franzil, L.; Lugtenberg, B.; Chebotar, V.; Ongena, M. Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch. Microbiol. 2012, 194, 893–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Lu, Z.; Bie, X.; Lu, F.; Yang, S. Isolation and characterization of a co-producer of fengycins and surfactins, endophytic Bacillus amyloliquefaciens ES-2, from Scutellaria baicalensis Georgi. World J. Microbiol. Biotechnol. 2006, 22, 1259–1266. [Google Scholar] [CrossRef]
- Zhang, S.M.; Wang, Y.X.; Meng, L.Q.; Li, J.; Zhao, X.Y.; Cao, X.; Chen, X.L.; Wang, A.X.; Li, J.F. Isolation and characterization of antifungal lipopeptides produced by endophytic Bacillus amyloliquefaciens TF28. Afri. J. Microbiol. Res. 2012, 6, 1747–1755. [Google Scholar]
- Arguelles-Arias, A.; Ongena, M.; Halimi, B.; Lara, Y.; Brans, A.; Joris, B.; Fickers, P. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb. Cell Fact. 2009, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Li, Y.; Zhang, C.; Yao, Z.; Zhang, L.; Bie, X.; Lu, F.; Lu, Z. Genome shuffling of Bacillus amyloliquefaciens for improving antimicrobial lipopeptide production and an analysis of relative gene expression using FQ RT-PCR. J. Ind. Microbiol. Biot. 2012, 39, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Gond, S.K.; Bergen, M.S.; Torres, M.S.; White, J.F., Jr. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015, 172, 79–87. [Google Scholar] [CrossRef]
- Chung, S.; Kong, H.; Buyer, J.S.; Lakshman, D.K.; Lydon, J.; Kim, S.D.; Roberts, D.P. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soil borne pathogens of cucumber and pepper. Appl. Microbiol. Biotechnol. 2008, 80, 115–123. [Google Scholar] [CrossRef]
- Alvarez, F.; Castro, M.; Príncipe, A.; Borioli, G.; Fischer, S.; Mori, G.; Jofré, E. The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of Sclerotinia stem rot disease. J. Appl. Microbiol. 2012, 112, 159–174. [Google Scholar] [CrossRef]
- Mora, I.; Cabrefiga, J.; Montesinos, E. Cyclic lipopeptide biosynthetic genes and products, and inhibitory activity of plant-associated Bacillus against phytopathogenic bacteria. PLoS ONE 2015, 10, e0127738. [Google Scholar] [CrossRef] [Green Version]
- Ramarathnam, R.; Bo, S.; Chen, Y.; Fernando, W.D.; Xuewen, G.; De Kievit, T. Molecular and biochemical detection of fengycin-and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can. J. Microbiol. 2007, 53, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Athukorala, S.N.P.; Fernando, W.G.D.; Rashid, K.Y. Identification of antifungal antibiotics of Bacillus species isolated from different microhabitats using polymerase chain reaction and MALDI-TOF mass spectrometry. Can. J. Microbiol. 2009, 55, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Gracia, E.; Macedo-Raygoza, G.; Villafaña-Rojas, J.; Martinez-Rodriguez, A.; Chavez-Castrillon, Y.Y.; Espinosa-Escalante, F.M.; Di Mascio, P.; Ogura, T.; Beltran-Garcia, M.J. Production of lipopeptides by fermentation processes: Endophytic bacteria, fermentation strategies and easy methods for bacterial selection. In Fermentation Processes; InTech: Rijeka, Croatia; p. 12.
- Moyne, A.L.; Cleveland, T.E.; Tuzun, S. Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiol. Lett. 2004, 234, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, G.; Hajirezaei, M.R.; Hofemeister, J. bac genes for recombinant bacilysin and anticapsin production in Bacillus host strains. Arch. Microbiol. 2005, 183, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.; Borchert, S.; Conrad, B.; Feesche, J.; Hofemeister, B.; Hofemeister, J.; Entian, K.D. Two different lantibiotic-like peptides originate from the ericin gene cluster of Bacillus subtilis A1/3. J. Bacterial. 2002, 184, 1703–1711. [Google Scholar] [CrossRef] [Green Version]
- Herzner, A.M.; Dischinger, J.; Szekat, C.; Josten, M.; Schmitz, S.; Yakéléba, A.; Reinartz, R.; Jansen, A.; Sahl, H.G.; Piel, J.; et al. Expression of the lantibiotic mersacidin in Bacillus amyloliquefaciens FZB42. PLoS ONE 2011, 6, e22389. [Google Scholar] [CrossRef] [Green Version]
- Duitman, E.H.; Hamoen, L.W.; Rembold, M.; Venema, G.; Seitz, H.; Saenger, W.; Bernhard, F.; Reinhardt, R.; Schmidt, M.; Ullrich, C. The mycosubtilin synthetase of Bacillus subtilis ATCC6633: A multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc. Natl. Acad. Sci. USA 1999, 96, 13294–13299. [Google Scholar] [CrossRef] [Green Version]
- Abderrahmani, A.; Tapi, A.; Nateche, F.; Chollet, M.; Leclère, V.; Wathelet, B.; Hacene, H.; Jacques, P. Bioinformatics and molecular approaches to detect NRPS genes involved in the biosynthesis of kurstakin from Bacillus thuringiensis. Appl. Microbiol. Biotechnol. 2011, 92, 571–581. [Google Scholar] [CrossRef]
- Das, P.; Mukherjee, S.; Sen, R. Genetic regulations of the biosynthesis of microbial surfactants: An overview. Biotechnol. Genet. Eng. Rev. 2008, 25, 165–186. [Google Scholar] [CrossRef]
- Yu, G.Y.; Sinclair, J.B.; Hartman, G.L.; Bertagnolli, B.L. Production of Iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol. Biochem. 2002, 34, 955–963. [Google Scholar] [CrossRef]
- Vater, J.; Kablitz, B.; Wilde, C.; Franke, P.; Mehta, N.; Cameotra, S.S. Matrix-assisted laser desorption ionization-time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl. Environ. Microbiol. 2002, 68, 6210–6219. [Google Scholar] [CrossRef] [Green Version]
- Pueyo, M.T.; Bloch, C., Jr.; Carmon-Ribeiro, A.M.; di Mascio, P. Lipopeptides produced by a soil Bacillus megaterium Strain. Microb. Ecol. 2005, 57, 367–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nihorimbere, V.; Cawoy, H.; Seyer, A.; Brunelle, A.; Thonart, P.; Ongena, M. Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol. Ecol. 2012, 79, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Ahmed, S.; Eswari, J.S. Therapeutic cyclic lipopeptides mining from microbes: Latest strides and hurdles. World J. Microbiol. Biotechnol. 2015, 31, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hameed, S.; Imran, A.; Iqbal, M.; Lazarovits, G. Genetic, physiological and biochemical characterization of Bacillus sp. strain RMB7 exhibiting plant growth promoting and broad spectrum antifungal activities. Microbial. Cell Factories 2014, 13, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Wang, N.; Hu, J.; Wang, S. Isolation and characterization of a new iturinic lipopeptide, mojavensin A produced by a marine-derived bacterium Bacillus mojavensis B0621A. J. Antibiot. 2012, 65, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koumoutsi, A.; Chen, X.H.; Henne, A.; Liesegang, H.; Hitzeroth, G.; Franke, P.; Vater, J.; Borriss, R. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 2004, 186, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Seydlov’a, G.; ˇCabala, R.; Svobodov´a, J. Biomedical engineering, trends, research and technologies. In Surfactin—Novel Solutions for Global Issues; InTech: Rijeka, Croatia, 2011; Volume 13, pp. 306–330. [Google Scholar]
- Korenblum, E.; de Araujo, L.V.; Guimarães, C.R.; De Souza, L.M.; Sassaki, G.; Abreu, F.; Nitschke, M.; Lins, U.; Freire, D.M.G.; Barreto-Bergter, E. Purification and characterization of a surfactin-like molecule produced by Bacillus sp. H2O-1 and its antagonistic effect against sulfate reducing bacteria. BMC Microbiol. 2012, 12, 252. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Jung, S.Y.; Lee, D.K.; Jung, J.K.; Park, J.K.; Kim, D.K.; Lee, C.H. Suppression of inflammatory responses by surfactin, 1 a selective inhibitor of platelet cytosolic phospholipase A2. Biochem. Pharmacol. 1998, 55, 975–985. [Google Scholar] [CrossRef]
- Hamley, I.W. Lipopeptides: From self-assembly to bioactivity. Chem. Commun. 2015, 51, 8574–8583. [Google Scholar] [CrossRef] [Green Version]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the antifungal and antibacterial agents: Applications in food safety and therapeutics. Biomed. Res. Int. 2015, 2015, 473050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawoy, H.; Mariutto, M.; Henry, G.; Fisher, C.; Vasilyeva, N.; Thonart, P.; Dommes, J.; Ongena, M. Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol. Plant–Microbe Interact. 2014, 27, 87–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deleu, M.; Paquot, M.; Nylander, T. Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes. Biophys. J. 2008, 94, 2667–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.Y.; Chen, C.L.; Lee, Y.H.; Cheng, Y.C.; Wu, Y.C.; Shu, H.Y. Non ribosomal synthesis of fengycin on an enzyme complex formed by fengycin synthetases. J. Biol. Chem. 2007, 282, 5608–5616. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Wang, Y.H.; He, Y.Q. Diversity and active mechanism of fengycin-type cyclopeptides from Bacillus subtilis XF-1 against Plasmodiophora brassicae. J. Microbiol. Biotechnol. 2013, 23, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Janek, T.; Łukaszewicz, M.; Krasowska, A. Antiadhesive activity of the biosurfactant pseudofactin II secreted by the Arctic bacterium Pseudomonas fluorescens BD5. BMC Microbiol. 2012, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- Alsohim, A.S.; Taylor, T.B.; Barrett, G.A.; Gallie, J.; Zhang, X.X.; Altamirano-Junqueira, A.E.; Johnson, L.J.; Rainey, P.B.; Jackson, R.W. The biosurfactant viscosin produced by Pseudomonas fluorescens SBW 25 aids spreading motility and plant growth promotion. Environ Microbiol. 2014, 16, 2267–2281. [Google Scholar] [CrossRef]
- Enoch, D.A.; Bygott, J.M.; Daly, M.L.; Karas, J.A. Daptomycin. J. Infect. 2007, 55, 205–213. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef] [Green Version]
- Zachow, C.; Jahanshah, G.; de Bruijn, I.; Song, C.; Ianni, F.; Pataj, Z.; Gerhardt, H.; Pianet, I.; Lämmerhofer, M.; Berg, G.; et al. The novel lipopeptide poaeamide of the endophyte Pseudomonas poae RE* 1-1-14 is involved in pathogen suppression and root colonization. Mol. Plant–Microbe Interact. 2015, 28, 800–810. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Wang, M.; Zhao, P.; Quan, C.; Li, X.; Wang, L.; Gao, W.; Li, J.; Zu, X.; Fu, D.; et al. Gram-negative bacilli-derived peptide antibiotics developed since 2000. Biotechnol. Lett. 2018, 40, 1271–1287. [Google Scholar] [CrossRef]
- Gong, M.; Wang, J.D.; Zhang, J.; Yang, H.; Lu, X.F.; Pei, Y.; Cheng, J.Q. Study of the antifungal ability of Bacillus subtilis strain PY-1 in vitro and identification of its antifungal substance (iturin A). Acta Biochem. Biophysiol. Sin. 2006, 38, 233–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, C.D.; Li, B.Q.; Zhao, T.; Guo, Q.G.; Lu, X.Y.; Li, S.Z.; Ma, P. Isolation and stability analysis of lipopeptides produced by Bacillus subtilis strain BAB21. China J. Agric. Sci. Technol. 2009, 11, 69–74. [Google Scholar]
- Romero, D.; de Vicente, A.; Rakotoaly, R.H.; Dufour, S.E.; Veening, J.W.; Arrebola, E.; Pérez-García, A. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol. Plant–Microbe Interact. 2007, 20, 430–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debois, D.; Jourdan, E.; Smargiasso, N.; Thonart, P.; De Pauw, E.; Ongena, M. Spatiotemporal monitoring of the antibiome secreted by Bacillus biofilms on plant roots using MALDI mass spectrometry imaging. Anal. Chem. 2014, 86, 4431–4438. [Google Scholar] [CrossRef]
- Makovitzki, A.; Viterbo, A.; Brotman, Y.; Chet, I.; Shai, Y. Inhibition of fungal and bacterial plant pathogens in vitro and in planta with ultrashort cationic lipopeptides. Appl. Environ. Microbiol. 2007, 73, 6629–6636. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.M.; Math, R.K.; Hong, S.Y.; Islam, S.M.A.; Mandanna, D.K.; Cho, J.J.; Yun, M.G.; Kim, J.M.; Yun, H.D. Iturin produced by Bacillus pumilus HY1 from Korean soybean sauce (kanjang) inhibits growth of aflatoxin producing fungi. Food Control 2009, 20, 402–406. [Google Scholar] [CrossRef]
- Aretz, W.; Meiwes, J.; Seibert, G.; Vobis, G.; Wink, J. Friulimicins: Novel lipopeptide antibiotics with peptidoglycan synthesis inhibiting activity from Actinoplanes friuliensis sp. nov. J. Antibiot. 2000, 53, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, M.; Daido, H.; Takao, T.; Murata, S.; Shimonishi, Y.; Imanaka, T. A new lipopeptide biosurfactant produced by Arthrobacter sp. strain MIS38. J. bacterial. 1993, 175, 6459–6466. [Google Scholar] [CrossRef] [Green Version]
- Pathak, K.V.; Keharia, H.; Gupta, K.; Thakur, S.S.; Balaram, P. Lipopeptides from the banyan endophyte, Bacillus subtilis K1: Mass spectrometric characterization of a library of fengycins. J. Am. Soc. Mass Spectrom. 2012, 23, 1716–1728. [Google Scholar] [CrossRef] [Green Version]
- Toure, Y.; Ongena, M.A.R.C.; Jacques, P.; Guiro, A.; Thonart, P. Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J. Appl. Microbiol. 2004, 96, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.B.; Shi, Z.Q.; Zhang, T.; Yang, Z.M. Fengycin antibiotics isolated from B-FS01 culture inhibit the growth of Fusarium moniliforme Sheldon ATCC 38932. FEMS Microbiol. Lett. 2007, 272, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mnif, I.; Grau-Campistany, A.; Coronel-León, J.; Hammami, I.; Triki, M.A.; Manresa, A.; Ghribi, D. Purification and identification of Bacillus subtilis SPB1 lipopeptide biosurfactant exhibiting antifungal activity against Rhizoctonia bataticola and Rhizoctonia solani. Environ. Sci. Poll. Res. 2016, 23, 6690–6699. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.Y.; Yin, Z.G.; Wang, K.X.; Chen, J.G.; Shen, S.S. Purification and structural analysis of surfactin produced by endophytic Bacillus subtilis EBS05 and its antagonistic activity against Rhizoctonia cerealis. Plant Pathol. J. 2011, 27, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.I.; Ryu, J.; Kim, Y.H.; Chl, Y.T. Production of biosurfactant lipopeptides iturin A, fengycin, and surfactin A from Bacillus subtilis CMB32 for control of colletotrichum gloeosporioides. J. Microbiol. Biotechnol. 2010, 20, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Nair, D.; Vanuopadath, M.; Nair, B.G.; Pai, J.G.; Nair, S.S. Identification and characterization of a library of surfactins and fengycins from a marine endophytic Bacillus sp. J. Basic Microbial. 2016, 56, 1159–1172. [Google Scholar] [CrossRef]
- Sajitha, K.L.; Dev, S.A.; Florence, E.M. Identification and characterization of lipopeptides from Bacillus subtilis B1 against sapstain fungus of rubberwood through MALDI-TOF-MS and RT-PCR. Curr. Microbiol. 2016, 73, 46–53. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Su, C.X.; Gong, G.H.; Wang, P.; Yu, Z.L. Isolation and characterization of lipopeptide antibiotics produced by Bacillus subtilis. Lett. Appl. Microbiol. 2008, 47, 180–186. [Google Scholar] [CrossRef]
- Fan, H.; Ru, J.; Zhang, Y.; Wang, Q.; Li, Y. Fengycin produced by Bacillus subtilis 9407 plays a major role in the biocontrol of apple ring rot disease. Microbiol. Res. 2017, 199, 89–97. [Google Scholar] [CrossRef]
- Bie, X.; Lu, Z.; Lu, F. Identification of fengycin homologues from Bacillus subtilis with ESI–MS/CID. J. Microbiol. Methods 2009, 79, 272–278. [Google Scholar] [CrossRef]
- Prabhukarthikeyan, S.R.; Karthikeyan, G.; Jeyarani, S.; Raguchander, T. PCR based identification and characterization of lipopeptides producing Bacillus against Fusarium oxysporum f. sp. lycopersici. Biochem. Cell. Arch. 2014, 14, 133–140. [Google Scholar]
- Liu, J.; Liu, M.; Wang, J.; Yao, J.M.; Pan, R.R.; Yu, Z.L. Enhancement of the Gibberella zeae growth inhibitory lipopeptides from a Bacillus subtilis mutant by ion beam implantation. Applied Microbiol. Biotechnol. 2005, 69, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Farace, G.; Fernandez, O.; Jacquens, L.; Coutte, F.; Krier, F.; Jacques, P.; Dorey, S. Cyclic lipopeptides from Bacillussubtilis activate distinct patterns of defense responses in grapevine. Mol. Plant Pathol. 2015, 16, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Desoignies, N.; Schramme, F.; Ongena, M.; Legrève, A. Systemic resistance induced by Bacillus lipopeptides in Beta vulgaris reduces infection by the rhizomania disease vector Polymyxa betae. Mol. Plant Pathol. 2013, 14, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Ben, A.D.; Frikha-Gargouri, O.; Tounsi, S. Bacillus amyloliquefaciens strain 32a as a source of lipopeptides for biocontrol of Agrobacterium tumefaciens strains. J. Appl. Microbiol. 2015, 119, 196–207. [Google Scholar]
- Vitullo, D.; Di Pietro, A.; Romano, A.; Lanzotti, V.; Lima, G. Role of new bacterial surfactins in the antifungal interaction between Bacillus amyloliquefaciens and Fusarium oxysporum. Plant Pathol. 2012, 61, 689–699. [Google Scholar] [CrossRef]
- Abdallah, R.A.B.; Stedel, C.; Garagounis, C.; Nefzi, A.; Jabnoun-Khiareddine, H.; Papadopoulou, K.K.; Daami-Remadi, M. Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato. Crop Prot. 2017, 99, 45–58. [Google Scholar] [CrossRef]
- Medeot, D.B.; Bertorello-Cuenca, M.; Liaudat, J.P.; Alvarez, F.; Flores-Cáceres, M.L.; Jofré, E. Improvement of biomass and cyclic lipopeptides production in Bacillus amyloliquefaciens MEP218 by modifying carbon and nitrogen sources and ratios of the culture media. Biol. Cont. 2017, 115, 119–128. [Google Scholar] [CrossRef]
- Preecha, C.; Sadowsky, M.J.; Prathuangwong, S. Lipopeptide surfactin produced by Bacillus amyloliquefaciens KPS46 is required for biocontrol efficacy against Xanthomonasaxonopodis pv. glycines. Kasetsart J. (Nat. Sci.) 2010, 44, 84–99. [Google Scholar]
- Snook, M.E.; Mitchell, T.; Hinton, D.M.; Bacon, C.W. Isolation and characterization of Leu7-surfactin from the endophytic bacterium Bacillus mojavensis RRC 101, a biocontrol agent for Fusarium verticillioides. J. Agric. Food Chem. 2009, 57, 4287–4292. [Google Scholar] [CrossRef]
- Ayed, H.B.; Hmidet, N.; Béchet, M.; Chollet, M.; Chataigné, G.; Leclère, V.; Jacques, P.; Nasri, M. Identification and biochemical characteristics of lipopeptides from Bacillus mojavensis A21. Process Biochem. 2014, 49, 1699–1707. [Google Scholar] [CrossRef]
- Blacutt, A.A.; Mitchell, T.R.; Bacon, C.W.; Gold, S.E. Bacillus mojavensis RRC101 lipopeptides provoke physiological and metabolic changes during antagonism against Fusarium verticillioides. Mol. Plant Microbe Interact. 2016, 29, 713–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favaro, G.; Bogialli, S.; Di Gangi, I.M.; Nigris, S.; Baldan, E.; Squartini, A. Characterization of lipopeptides produced by Bacillus licheniformis using liquid chromatography with accurate tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 2237–2252. [Google Scholar] [CrossRef] [PubMed]
- Saggese, A.; Culurciello, R.; Casillo, A.; Corsaro, M.M.; Ricca, E.; Baccigalupi, L. A Marine isolate of Bacillus pumilus secretes a pumilacidin active against Staphylococcus aureus. Mar. Drugs 2008, 16, 180. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.I.; Bai, H.; Bai, D.; Chae, H.; Chung, S.; Kim, Y.; Park, R.; Chi, Y.T. Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J. Appl. Microbiol. 2004, 97, 942–949. [Google Scholar] [CrossRef]
- Hathout, Y.; Ho, Y.P.; Ryzhov, V.; Demirev, P.; Fenselau, C. Kurstakins: A new class of lipopeptides isolated from Bacillus thuringiensis. J. Nat. Prod. 2000, 63, 1492–1496. [Google Scholar] [CrossRef]
- Béchet, M.; Caradec, T.; Hussein, W.; Abderrahmani, A.; Chollet, M.; Leclère, V.; Dubois, T.; Lereclus, D.; Pupin, M.; Jacques, P. Structure, biosynthesis, and properties of kurstakins, non-ribosomal lipopeptides from Bacillus spp. Appl. Microbiol. Biotechnol. 2012, 95, 593–600. [Google Scholar]
- Kaur, P.K.; Joshi, N.; Singh, I.P.; Saini, H.S. Identification of cyclic lipopeptides produced by Bacillus vallismortis R2 and their antifungal activity against Alternaria alternata. J. Appl. Microbiol. 2017, 122, 139–152. [Google Scholar] [CrossRef]
- Ramarathnam, R.; Fernando, W.D. Molecular and biochemical detection of lipopeptide antibiotics producing Bacillus spp., antagonistic to common fungal pathogens of canola (Brassica napus L.). In Proceedings of the 12th International Rapeseed Congress, Wuhan, China, 26–30 March 2007; p. 180. [Google Scholar]
- Kalai-Grami, L.; Karkouch, I.; Naili, O.; Slimene, I.B.; Elkahoui, S.; Zekri, R.B.; Touati, I.; Mnari-Hattab, M.; Hajlaoui, M.R.; Limam, F. Production and identification of iturin A lipopeptide from Bacillus methyltrophicus TEB1 for control of Phoma tracheiphila. J. Basic Microbiol. 2016, 56, 864–871. [Google Scholar] [CrossRef]
- Pramudito, T.E.; Agustina, D.; Nguyen, T.K.N.; Suwanto, A. A Novel variant of narrow-spectrum antifungal bacterial lipopeptides that strongly inhibit Ganoderma boninense. Probiotics Antimicrob. Proteins 2017, 10, 110–117. [Google Scholar] [CrossRef]
- Perez, K.J.; Viana, J.D.S.; Lopes, F.C.; Pereira, J.Q.; dos Santos, D.M.; Oliveira, J.S.; Velho, R.V.; Crispim, S.M.; Nicoli, J.R.; Brandelli, A. Bacillus spp. isolated from puba as a source of biosurfactants and antimicrobial lipopeptides. Front. Microbiol. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasim, B.; Sreelakshmi, K.S.; Mathew, J.; Radhakrishnan, E.K. Surfactin, iturin, and fengycin biosynthesis by Endophytic Bacillus sp. from Bacopa monnieri. Microb. Ecol. 2016, 72, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, B.; Wang, J.; Che, J.; Liu, G.; Guan, X. Antifungal lipopeptides produced by Bacillus sp. FJAT-14262 isolated from rhizosphere soil of the medicinal plant Anoectochilus roxburghii. Appl. Biochem. Biotechnol. 2017, 182, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J. Identification and molecular characterization of three isoforms of iturin produced by endophytic Bacillus sp. CY22. J. Life Sci. 2005, 15, 1005–1012. [Google Scholar]
- Sarwar, A.; Hassan, M.N.; Imran, M.; Iqbal, M.; Majeed, S.; Brader, G.; Sessitsch, A.; Hafeez, F.Y. Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol. Res. 2018, 209, 1–13. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, H.S.; Yang, S.Y.; Kim, I.S.; Yamaguchi, T.; Sohng, J.K.; Park, S.K.; Kim, J.C.; Lee, C.H.; Gardener, B.M. Both extracellular chitinase and a new cyclic lipopeptide, chromobactomycin, contribute to the biocontrol activity of Chromobacterium sp. C61. Mol. Plant Pathol. 2014, 15, 122–132. [Google Scholar] [CrossRef]
- Shiono, Y.; Tsuchinari, M.; Shimanuki, K.; Miyajima, T.; Murayama, T.; Koseki, T.; Laatsch, H.; Funakoshi, T.; Takanami, K.; Suzuki, K. A and B, two new cyclic lipopeptides from an endophytic Fusarium sp. J. Antibiot. 2007, 60, 309. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Kusari, S.; Golz, C.; Strohmann, C.; Spiteller, M. Three cyclic pentapeptides and a cyclic lipopeptide produced by endophytic Fusarium decemcellulare LG53. RSC Adv. 2016, 6, 54092–54098. [Google Scholar] [CrossRef] [Green Version]
- Rosconi, F.; Davyt, D.; Martínez, V.; Martínez, M.; Abin-Carriquiry, J.A.; Zane, H.; Butler, A.; de Souza, E.M.; Fabiano, E. Identification and structural characterization of serobactins, a suite of lipopeptide siderophores produced by the grass endophyte Herbaspirillum seropedicae. Environ. Microbiol. 2013, 15, 916–927. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Thrane, C.; Christophersen, C.; Anthoni, U.; Sørensen, J. Structure, production characteristics and fungal antagonism of tensin–a new antifungal cyclic lipopeptide from Pseudomonas fluorescens strain 96.578. J. Appl. Microbiol. 2000, 89, 992–1001. [Google Scholar] [CrossRef]
- Schlusselhuber, M.; Godard, J.; Sebban, M.; Bernay, B.; Garon, D.; Seguin, V.; Oulyadi, H.; Desmasures, N. Characterization of milkisin, a novel lipopeptide with antimicrobial properties produced by Pseudomonas sp. UCMA 17988 isolated from bovine raw milk. Front. Microbiol. 2018, 9, 1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, B.; Rueckert, C.; Blom, J.; Wang, Q.; Borriss, R. The genome of the plant growth-promoting rhizobacterium Paenibacillus polymyxa M-1 contains nine sites dedicated to nonribosomal synthesis of lipopeptides and polyketides. J. Bacteriol. 2011, 193, 5862–5863. [Google Scholar] [CrossRef] [PubMed]
- Canova, S.P.; Petta, T.; Reyes, L.F.; Zucchi, T.D.; Moraes, L.A.; Melo, I.S. Characterization of lipopeptides from Paenibacillus sp.(IIRAC30) suppressing Rhizoctonia solani. World J. Microbiol. Biotechnol. 2010, 26, 2241–2247. [Google Scholar] [CrossRef]
- Heinemann, B.; Kaplan, M.; Muir, R.; Hooper, I. Amphomycin, a new antibiotic. Antibiot. Chemother. 1953, 3, 1239–1242. [Google Scholar]
- Tanaka, H.; Oiwa, R.; Matsukura, S.; Omura, S. Amphomycin inhibits phospho-N-acetylmuramyl-pentapeptide translocase in peptidoglycan synthesis of Bacillus. Biochem. Biophys. Res. Commun. 1979, 86, 902–908. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Shen, Q.; Yin, X. Molecular cloning and identification of the laspartomycin biosynthetic gene cluster from Streptomyces viridochromogenes. Gene 2011, 483, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurth, C.; Schieferdecker, S.; Athanasopoulou, K.; Seccareccia, I.; Nett, M. Variochelins, lipopeptide siderophores from Variovorax boronicumulans discovered by genome mining. J. Nat. Prod. 2016, 79, 865–872. [Google Scholar] [CrossRef]
- Inès, M.; Dhouha, G. Lipopeptide surfactants: Production, recovery and pore forming capacity. Peptides 2015, 71, 100–112. [Google Scholar] [CrossRef]
- Fracchia, L.; Cavallo, M.; Martinotti, M.G.; Banat, I.M. Biosurfactants and bioemulsifiers biomedical and related applications-present status and future potentials. Biomed. Sci. Eng. Technol. 2012, 14, 326–335. [Google Scholar]
- Bernheimer, A.W.; Avigad, L.S. Nature and properties of a cytolytic agent produced by Bacillus subtilis. J. Gen. Microbiol. 1970, 6, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, C.; Teruel, J.A.; Aranda, F.A.; Ortiz, A. Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochem. Biophys. Acta 2003, 1611, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, M.L.; Shai, Y. Short native antimicrobial peptides and engineered ultrashort lipopeptides: Similarities and differences in cell specificities and modes of action. Cell. Mol. Life Sci. 2011, 68, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Moyne, A.L.; Shelby, R.; Cleveland, T.E.; Tuzun, S. Bacillomycin D: An iturin with antifungal activity against Aspergillus flavus. J. Appl. Microbiol. 2001, 90, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Hiradate, S.; Yoshida, S.; Sugie, H.; Yada, H.; Fujii, Y. Mulberry anthracnose antagonists (iturins) produced by Bacillus amyloliquefaciens RC-2. Phytochemistry 2002, 61, 693–698. [Google Scholar] [CrossRef]
- Phae, C.G.; Phae, C.G.; Shoda, M.l.; Kubota, H. Suppressive effect of Bacillus subtilis and its products on phytopathogenic microorganisms. J. Ferment. Bioeng. 1990, 69, 1–7. [Google Scholar] [CrossRef]
- Aranda, F.J.; Teruel, J.A.; Ortiz, A. Further aspects on the hemolytic activity of the antibiotic lipopeptide iturin A. Biochim. Biophys. Acta Biomembr. 2005, 1713, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Vanittanakom, N.; Loeffler, W.; Koch, U.; Jung, G. Fengycin—A novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J. Antibiot. 1986, 39, 888–901. [Google Scholar] [CrossRef] [Green Version]
- Hofemeister, J.; Conrad, B.; Adler, B.; Hofemeister, B.; Feesche, J.; Kucheryava, N.; Steinborn, G.; Franke, P.; Grammel, N.; Zwintscher, A.; et al. Genetic analysis of the biosynthesis of nonribosomal peptide- and polyketide-like antibiotics, iron uptake and biofilm formation by Bacillus subtilis A1/3. Mol. Genet. Genom. 2004, 272, 363–378. [Google Scholar] [CrossRef]
- Sur, S.; Romo, T.D.; Grossfield, A. Selectivity and mechanism of Fengycin, an antimicrobial lipopeptide, from molecular dynamics. J. Phys. Chem. B 2018, 122, 2219–2226. [Google Scholar] [CrossRef]
- Deleu, M.; Paquot, M.; Nylander, T. Fengycin interaction with lipid monolayers at the air–aqueous interface-implications for the effect of fengycin on biological membranes. J. Colloid Interface Sci. 2005, 283, 358–365. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Gross, D.C. Lipopeptide phytotoxins produced by Pseudomonas syringae pv. syringae: Comparison of the biosurfactant and ion channel-forming activities of syringopeptin and syringomycin. Mol. Plant Microbe Interact. 1997, 10, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agner, G.; Kaulin, Y.A.; Gurnev, P.A.; Szabo, Z.; Schagina, L.V.; Takemoto, J.Y.; Blasko, K. Membrane-permeabilizing activities of cyclic lipodepsipeptides, syringopeptin 22A and syringomycin E from Pseudomonas syringae pv. syringae in human red blood cells and in bilayer lipid membranes. Bioelectrochemistry 2000, 52, 161–167. [Google Scholar] [CrossRef]
- Carpaneto, A.; Dalla Serra, M.; Menestrina, G.; Fogliano, V.; Gambale, F. The phytotoxic lipodepsipeptide syringopeptin 25A from Pseudomonas syringae pv. syringae forms ion channels in sugar beet vacuoles. J. Membr. Biol. 2002, 188, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Sy-Cordero, A.A.; Pearce, C.J.; Oberlies, N.H. Revisiting the enniatins: A review of their isolation, biosynthesis, structure determination, and biological activities. J. Antibiot. 2012, 65, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moryl, M.; Spętana, M.; Dziubek, K.; Paraszkiewicz, K.; Różalska, S.; Płaza, G.A.; Różalski, A. Antimicrobial, antiadhesive and antibiofilm potential of lipopeptides synthesised by Bacillus subtilis on uropathogenic bacteria. Acta Biochim. Polonica 2015, 62, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, I.; Lagendijk, E.L.; Pickford, R.; Derrick, J.P.; Lamers, G.E.; Thomas-Oates, J.E.; Lugtenberg, B.J.; Bloemberg, G.V. Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Mol. Microbiol. 2004, 51, 97–113. [Google Scholar] [CrossRef]
- Abdallah, D.B.; Tounsi, S.; Gharsallah, H.; Hammami, A.; Frikha-Gargouri, O. Lipopeptides from Bacillus amyloliquefaciens strain 32a as promising biocontrol compounds against the plant pathogen Agrobacterium tumefaciens. Environ. Sci. Pollut. Res. 2018, 25, 36518–36529. [Google Scholar] [CrossRef]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Sandy, M.; Butler, A. Microbial iron acquisition: Marine and terrestrial siderophores. Chem Rev. 2009, 109, 4580–4595. [Google Scholar] [CrossRef] [Green Version]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Gauglitz, J.M.; Zhou, H.; Butler, A. A suite of citrate-derived siderophores from a marine Vibrio species isolated following the Deepwater Horizon oil spill. J. Inorg. Biochem. 2012, 107, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, J.S.; Zhang, G.P.; Holt, P.D.; Jung, H.-T.; Carrano, C.J.; Haygood, M.G.; Butler, A. Self-assembling amphiphilic siderophores from marine bacteria. Science 2000, 287, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Butler, A. Structure of synechobactins, new siderophores of the marine cyanobacterium synechococcus sp. PCC 7002. Limnol. Oceanogr. 2005, 50, 1918–1923. [Google Scholar] [CrossRef]
- Homann, V.V.; Sandy, M.; Tincu, J.A.; Templeton, A.S.; Tebo, B.M.; Butler, A. Loihichelins A−F, a suite of amphiphilic siderophores produced by the marine bacterium Halomonas LOB-5. J. Nat. Prod. 2009, 72, 884–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traxler, M.F.; Seyedsayamdost, M.R.; Clardy, J.; Kolter, R. Interspecies modulation of bacterial development through iron competition and siderophore piracy. Mol. Microbiol. 2012, 86, 628–644. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, T.; Clardy, J. A chimeric siderophore halts swarming Vibrio. Angew. Chem. Int. Ed. 2014, 53, 3510–3513. [Google Scholar] [CrossRef] [Green Version]
- Barbeau, K.; Rue, E.L.; Bruland, K.W.; Butler, A. Photochemical cycling of iron in the surface ocean mediated by microbial iron (III)-binding ligands. Nature 2001, 413, 409. [Google Scholar] [CrossRef]
- Butler, A.; Theisen, R.M. Iron (III)–siderophore coordination chemistry: Reactivity of marine siderophores. Coord. Chem. Rev. 2010, 254, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Küpper, F.C.; Carrano, C.J.; Kuhn, J.U.; Butler, A. Photoreactivity of iron (III)-aerobactin: Photoproduct structure and iron (III) coordination. Inorg. Chem. 2006, 45, 6028–6033. [Google Scholar] [CrossRef]
- Yarimizu, K.; Polido, G.; Gardes, A.; Carter, M.L.; Hilbern, M.; Carrano, C.J. Evaluation of photo-reactive siderophore producing bacteria before, during and after a bloom of the dinoflagellate Lingulodinium polyedrum. Metallomics 2014, 6, 1156–1163. [Google Scholar] [CrossRef]
- Amin, S.A.; Green, D.H.; Hart, M.C.; Küpper, F.C.; Sunda, W.G.; Carrano, C.J. Photolysis of iron–siderophore chelates promotes bacterial–algal mutualism. Proc. Natl. Acad. Sci. USA 2009, 106, 17071–17076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldani, J.; Baldani, V.L.D.; Seldin, L.; Döbereiner, J. Characterization of Herbaspirillumseropedicae gen. Nov., a root-asociated nitrogen-fixing bacterium. Int. J. Syst. Bacteriol. 1986, 36, 86–93. [Google Scholar] [CrossRef] [Green Version]
- James, E.K.; Olivares, F.L.; Baldani, J.I.; Döbereiner, J. Herbaspirillum, an endophytic diazotroph colonizing vascular tissue Sorghum bicolor L. Moench. J. Exp. Bot. 1997, 48, 785–798. [Google Scholar] [CrossRef] [Green Version]
- Gyaneshwar, P.; James, E.K.; Reddy, P.M.; Ladha, J.K. Herbaspirillum colonization increases growth and nitrogen accumulation in aluminium-tolerant rice varieties. New Phytol. 2002, 154, 131–145. [Google Scholar] [CrossRef]
- Roncato-Maccari, L.D.; Ramos, H.J.; Pedrosa, F.O.; Alquini, Y.; Chubatsu, L.S.; Yates, M.G.; Rigo, L.U.; Steffens, M.B.R.; Souza, E.M. Endophytic Herbaspirillum seropedicae expresses nif genes in gramineous plants. FEMS Microbiol. Ecol. 2003, 45, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [Green Version]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S.A. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [Green Version]
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Henry, G.; Deleu, M.; Jourdan, E.; Thonart, P.; Ongena, M. The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell. Microbiol. 2011, 13, 1824–1837. [Google Scholar] [CrossRef]
- Rahman, A.; Uddin, W.; Wenner, N.G. Induced systemic resistance responses in perennial ryegrass against Magnaporthe oryzae elicited by semi-purified surfactin lipopeptides and live cells of Bacillus amyloliquefaciens. Mol. Plant Pathol. 2015, 16, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ongena, M.; Höfte, M. The cyclic lipopeptide orfamide induces systemic resistance in rice to Cochliobolus miyabeanus but not to Magnaporthe oryzae. Plant Cell Rep. 2017, 36, 1731–1746. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.A.X.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McManus, P.S.; Stockwell, V.O.; Sundin, V.O.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 46, 443–465. [Google Scholar] [CrossRef]
- Montesinos, E.; Badosa, E.; Cabrefiga, J.; Planas, M.; Feliu, L.; Bardají, E. Antimicrobial peptides for plant disease control. From discovery to application. In Small Wonders: Peptides for Disease Control; ACS Symposium Series; Rajasekaran, K., Cary, J.W., Jaynes, J.M., Montesinos, E., Eds.; American Chemical Society: Washington, DC, USA, 2012; pp. 235–262. [Google Scholar]
- Oliveras, À.; Baró, A.; Montesinos, L.; Badosa, E.; Montesinos, E.; Feliu, L.; Planas, M. Antimicrobial activity of linear lipopeptides derived from BP100 towards plant pathogens. PLoS ONE 2018, 13, e0201571. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Mingshan, J. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Hazarika, D.J.; Goswami, G.; Gautom, T.; Parveen, A.; Das, P.; Barooah, M.; Boro, R.C. Lipopeptide mediated biocontrol activity of endophytic Bacillus subtilis against fungal phytopathogens. BMC Microbiol. 2019, 19, 71. [Google Scholar] [CrossRef]
- Wang, X.; Gong, X.P.; Li, D.; Lai, I. Structural diversity and biological activities of cyclic depsipeptides from fungi. Molecules 2018, 23, 169. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Xue, Y.; Li, X.; Li, J.; Zhao, Z.; Quan, C.; Gao, W.; Zu, X.; Bai, X.; Feng, S. Fungi-derived lipopeptide antibiotics developed since 2000. Peptides 2019, 113, 52–65. [Google Scholar] [CrossRef]
- Deravel, J.; Lemiere, S.; Coutte, F.; Krier, F.; Van Hese, N.; Bechet, M.; Sourdeau, N.; Hofte, M.; Lepretre, A.; Jacques, P. Mycosubtilin and surfactin are efficient, low ecotoxicity molecules for the biocontrol of lettuce downy mildew. Appl. Environ. Microbiol. 2014, 98, 6255–6264. [Google Scholar] [CrossRef]
- Hu, F.; Liu, Y.; Li, S. Rational strain improvement for surfactin production: Enhancing the yield and generating novel structures. Microb. Cell factories 2004, 18, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vindya, N.G.; Sharma, N.; Yadav, M.; Ethiraj, K.R. Tubulins—The target for anticancer therapy. Curr. Top. Med. Chem. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Lone, M.Y.; Athar, M.; Manhas, A.; Jha, P.C.; Bhatt, S.; Shah, A. In Silico exploration of Vinca domain tubulin inhibitors: A combination of 3D-QSAR-Based pharmacophore modeling, docking and molecular dynamics simulations. Chem. Sel. 2017, 2, 10848–10853. [Google Scholar] [CrossRef]
- Shanmugam, G.; Lee, S.; Jeon, J. Identification of potential nematicidal compounds against the pine wood nematode, Bursaphelenchus xylophilus through an in Silico approach. Molecules 2018, 23, 1828. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Héloir, M.; Zhang, X.; Geissler, M.; Trouvelot, S.; Jacquens, L.; Henkel, M.; Su, X.; Fang, X.; Wang, Q.; et al. Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol. Plant Pathol. 2019, 20, 1037–1050. [Google Scholar]
- Cob-Calan, N.N.; Chi-Uluac, L.A.; Ortiz-Chi, F.; Cerqueda-García, D.; Navarrete-Vázquez, G.; Ruiz-Sánchez, E.; Hernández-Núñez, E. Molecular docking and dynamics simulation of protein β-tubulin and antifungal cyclic lipopeptides. Molecules 2019, 24, 3387. [Google Scholar] [CrossRef] [Green Version]




| S.No. | Lipopeptide Class | Gene | Primer Name | Primer Sequences (5′-3′) | Reference |
|---|---|---|---|---|---|
| 1. | Surfactins | ||||
| (a) | Surfactin | sfP | SFP-F1 SFP-R1 | ATGAAGATTTACGGAATTTA TTATAAAAGCTCTTCGTACG | [58] |
| (b) | Surfactin | Srfc | Sur3f Sur3r | ACAGTATGGAGGCATGGTC TTCCGCCACTTTTTCAGTTT | [57] |
| (c) | Surfactin | SrfA-A | srfAA-Fw srfAA-Rv | AAAGGATCCAGCCGAAGGGTG TCATGGT AAAAAGCTTGTTTTTCTCAAAGAACCAGCG | [59] |
| (d) | Surfactin | srfAA (Surfactin synthetase subunit 1) | SRFAF SRFAR | TCGGGACAGGAAGACATCAT CCACTCAAACGGATAATCCTGA | [60] |
| (e) | Surfactin | srfA | F3726 R3879 | GAAGTCTTCAGCGGCGAACTG GGGTGGCTCCGTTTTTCTCG | [56] |
| (f) | Surfactin | srfDB | SUR3F SUR3R | ACAGTATGGAGGCATGGTC TTCCGCCACTTTTTCAGTTT | [61] |
| 2. | Iturins | ||||
| (a) | Iturin A | ItuD | ItuD1f ItuD1r | GATGCGATCTCCTTGGATGT ATCGTCATGTGCTGCTTGAG | [57] |
| (b) | Iturin A | Itu-C | ituC-Fw ituC-Rv | AAAGGATCCAAGCGTGCCTTTTACGGGAAA AAAAAGCTT AATGACGCCAGCTTTCTCTT | [59] |
| (c) | Iturin | ituC (Iturin A synthetase C) | ITUCF ITUCR | GGCTGCTGCAGATGCTTTAT TCGCAGATAATCGCAGTGAG | [60] |
| 3. | Fengycins | ||||
| (a) | Fengycin | FenD | FenD1f FenD1d | TTTGGCAGCAGGAGAAGTTT GCTGTCCGTTCTGCTTTTTC | [62] |
| (b) | Fengycin | fenD (Fengycin synthetase) | FENDF FENDR | GGCCCGTTCTCTAAATCCAT GTCATGCTGACGAGAGCAAA | [60] |
| (c) | Fengycin | FenE | FenEF FenER | GTTTCATGGCGGCGAGCACG GATTCGCGGGAAGCGGATTGAGC | [62] |
| (d) | Fengycin | Fen | Af2-F Tf1-R | GAATAYMTCGGMCGTMTKGA GCTTTWADKGAATSBCCGCC | [63] |
| 4. | Bacillomycins | ||||
| (a) | Bacillomycin | bmyB (Bacillomycin L synthetase B) | BMYBF BMYBR | GAATCCCGTTGTTCTCCAAA GCGGGTATTGAATGCTTGTT | [60] |
| (b) | Bacillomycin D | BamC | Bacc1f Bacc1r | GAAGGACACGGAGAGAGTC CGCTGATGACTGTTCATGCT | [57] |
| (c) | Bacillomycin D | bam D | ITUD-F1 ITUD-R1 | TTGAAYGTCAGYGCSCCTTT TGCGMAAATAATGGSGTCGT | [64] |
| 5. | Bacilysin | ||||
| (a) | Bacilysin | bacAB | BACD-F1 BAMD-R1 | AAAAACAGTATTGGTYATCGCTGA CCATGATGCCTTCKATRCTGAT | [65] |
| (b) | Bacilysin | bacAB | BACAB-F1 BACAB-R1 | CTTCTCCAAGGGGTGAACAG TGTAGGTTTCACCGGCTTTC | |
| 6. | Ericin | ||||
| Ericin | eriB | eriBF eriBR | GAWKNACWCCWTWTGG CCRCCATATCSWTMTRYYTC | [66] | |
| 7. | Mersacidin | ||||
| (a) | Mersacidin | mrsA | MRSA-F1 MRSA-R1 | GGGTATATGCGGTATAAACTTATG GTTTCCCCAATGATTTACCCTC | [67] |
| (b) | Mersacidin | mrsM | MRSM-F1 MRSM-R1 | AAATGACCCGGCATATGTTC TGCTGACTAACTGGAATTGGAA | |
| 8. | Mycosubtilin | ||||
| (a) | Mycosubtilin | fenF | ITUD-F1 ITUD-R1 | TTGAAYGTCAGYGCSCCTTT TGCGMAAATAATGGSGTCGT | [68] |
| (b) | Mycosubtilin | mycC | MYCC-F1 MYCC-R1 | AATCAATTGGCACGAACCTT ATCGCCCGTTTTGTACATTC | |
| 9. | Zwittermicins | ||||
| (a) | Zwittermicin A | Zwit | ZWITF2 ZWITR1 | TTGGGAGAATATACAGCTCT GACCTTTTGAAATGGGCGTA | [61] |
| 10. | Kurstakins | ||||
| (a) | Kurstakins | Kur | Aks-F Tks-R | TCHACWGGRAATCCAAAGGG CCACCDKTCAAAKAARKWATC | [69] |
| S. No. | Source Organism | Lipopeptide Class/Type | Activity/Action | References (Not to Be Attended) |
|---|---|---|---|---|
| 1. | Actinoplanes friuliensis | Friulimicin | Broad range of multi-resistant Gram-positive bacteria | [101] |
| 2. | Arthrobacter spp. MIS38 | Arthrofactin | Bio-surfactant | [102] |
| 3. | Bacillus subtilis | Iturin A, Bacillomycin, Fengycin, Bacillomycin | Antifungal | [57] |
| 4. | B. subtilis HC8 | Surfactin, Fengycin A, Fengycin B, Iturin A, | Antifungal | [52] |
| 5. | B. subtilis K1 | Surfactin, Iturin, Fengycin A and B, Fengycin A2 and B2 | Antifungal | [103] |
| 6. | B. subtilis GA1 | Iturins, Fengycins, Surfactins | Antifungal | [104] |
| 7. | B. subtilis M4 | Fengycin A and B | Antifungal | [30] |
| 8. | B. subtilis B-FS01 | Fengycin | Antifungal | [105] |
| 9. | B. subtilis and B. amyloliquefaciens | Fengycin, Iturins, Surfactins, Bacillomycin | Antibaterial | [60] |
| 10. | B. subtilis SPB1 | Surfactin, Fengycin, Iturins | Antifungal | [106] |
| 11. | B. subtilis EBS05 | Surfactin A | Antifungal | [107] |
| 12. | B. subtilis B49 | Fengycin, Bacillomycin D | Antifungal | [61] |
| 13. | B. subtillis ATCC 13952 | Fengycin | Antifungal | [61] |
| 14. | B. subtillis DF-HO8 | Fengycin | Antifungal | [61] |
| 15. | B. subtilis CMB32 | Iturin A, Fengycin, Surfactin A | Antifungal | [108] |
| 16. | B. subtilis (Marine) | Surfactins and Fengycins | Delayed Germination | [109] |
| 17. | B. subtilis B1 | Iturin C, Surfactin, Fengycin A and B, Bacillomycin D, Bacilysin, Mycobacillin | Antifungal | [110] |
| 18. | B. subtilis JA | Surfactin, Iturin, and Fengycin | Antifungal | [111] |
| 19. | B. subtilis 9407 | Fengycin | Antifungal | [112] |
| 20. | B. subtilis HC8 | Fengycin A and Fengycin B | Antifungal | [52] |
| 21. | B. subtilis S499 | Surfactin, Fengycin A, and Fengycin B | Antifungal | [30] |
| 22. | B. subtilis fmbj | Fengycin A and Fengycin B | Antifungal | [113] |
| 23. | B. subtilis EPCO16 | Iturin A, Surfactin, Zwittwermicin A, Bacillomycin D | Antifungal | [114] |
| 24. | B. subtilis 6051 | Surfactin | Antibacterial activity against P. Syringae | [79] |
| 25. | B. subtilis M4 | Iturin/Fengycin | Antifungal activity against Pythium ultimum causing Damping-off disease of Beans | [30] |
| 26. | B. subtilis M4 | Fengycin | Antifungal activity against Botrytis cinerea causing Gray mold disease of Apples | [30] |
| 27. | B. subtilis | Iturin/Fengycin | Antifungal activity against podosphaera fusca causing Powdery mildew of Cucurbits | [97] |
| 28. | B. subtilis JA; JA026 | Fengycin | Antifungal activity against Gibberella zeae (anamorph of Fusarium graminearum) Fusarium causing head blight (FHB) in Wheat and Barley and Ear rot in Corn | [115] |
| 29. | B. subtilis B-FS01 | Fengycin | Antifungal activity against Fusarium moniliforme causing Seedlingblight, Stalk rot, and Ear rot | [105] |
| 30. | B. subtilis BBG127 and BBG131 | Cyclic lipopeptides | Antifungal activity against Botrytis cinerea 630 causing Necrosis of Grapevines | [116] |
| 31. | B. subtilis 9407 | Fengycin | Antifungal activity against Botryosphaeria dothidea causing Apple ring rot | [112] |
| 32. | B. subtilis GA1 | Iturin, Fengycin, Surfactin | Antifungal activity against Botrytis cinerea causing Grey mould disease of Apples | [104] |
| 33. | B. amyloliquefaciens ES-2 | Fengycin, Surfactin | Antibacterial/anti-fungal | [53] |
| 34. | B. amyloliquefaciens TF28 | Iturin A | Antifungal | [54] |
| 35. | B. amyloliquefaciens ARP23 and MEP218 | Surfactin C15, Fengycins A, Iturin A | Antifungal activity against Sclerotinia sclerotiorum | [59] |
| 36. | B. amylolequifaciens S499 | Fengycin, Iturins, Surfactin | ISR | [117] |
| 37. | B. amyloliquefaciens 32a | Surfactin, Iturin A, Bacillomycin D, Fengycin | Antimicrobial | [118] |
| 38. | B. amyloliquefaciens CC09 | Fengycin, Iturin, Surfactin, Bacillomycin | Antifungal | [18] |
| 39. | B. amyloliquefaciens BO7 | Surfactin | Antifungal | [119] |
| 40. | B. amyloliquefaciens subsp. plantarum SV65 | Fengycin | Antifungal activity | [120] |
| 41. | B. amyloliquefaciens MEP218 | Iturin, Fengycin, Surfactin | Antibacterial, Antifungal | [121] |
| 42. | B. amyloliquefaciens KPS46 | Surfactin | Antibacterial activity against Xanthomonas axonopodis pv. glycines | [122] |
| 43. | B. amylolequifaciens | Lipopeptides, Surfactin, Iturin, Fengycin | Antiviral ActivityagainstRhizomania, an important disease of Sugarbeet | [117] |
| 44. | B. mojavensis RRC101 | Leu7-Surfactin | Antifungal | [123] |
| 45. | B. mojavensis A21 | Surfactin, Fengycin, Pumalicidin | Antimicrobial, Antifungal | [124] |
| 46. | B. mojavensis RRC101 | Surfactin, Fengycin | Antifungal | [125] |
| 47. | B. licheniformis | Lichenysin | Bio-surfactant | [124] |
| 48. | B. licheniformis | Surfactins, Lichenysins | Bio-surfactant | [126] |
| 49. | B. pumilus HY1 | Iturins | Antifungal | [100] |
| 50. | B. pumilus (Marine) | Pumilacidin | Antibacterial activityagainst Staphylococcus aureus | [127] |
| 51. | B. thuringiensis CMB26 | Fengycins | Fungicidal, Bactericidal, andInsecticidal activity | [128] |
| 52. | B. thuringiensis kurstaki HD-1 | Kurstakins | Antifungal activity against Stachybotrys charatum | [129] |
| 53. | B. thuringiensis kurstaki | Kurstakin | Antifungal activity | [130] |
| 54. | B. vallismortis R2 | Surfactins, Iturins, Fengycins | Antifungal activity against Alternaria alternate causing Black point disease of Wheat | [131] |
| 55. | B. cereus DFE4 | Surfactin, Iturin A, Bacillomycin D | Antifungal | [132] |
| 56. | B. methyltrophicus TEB1 | Iturin A, Fengycin, Surfactin | Antifungal | [133] |
| 57. | B. methylotrophicus HC51 | Iturin A, Fengycin | Antifungal | [134] |
| 58. | Bacillus sp.C3 | Iturin A, Surfactin, Subtilosin, Subtilin | Antifungal | [135] |
| 59. | Bacillus sp.BmB9 | Surfactin, Iturin, Fengycin | Antifungal, Antibacterial | [136] |
| 60. | Bacillus sp. FJAT-14262 | Surfactin | Antifungal | [137] |
| 61. | Bacillus sp. CY22 | Iturin | Antifungal | [138] |
| 62. | Bacillus sp. NH-100 | Surfactin A | Antifungal | [139] |
| 63. | Bacillus sp. | Iturin A, Surfactin, Zwittermicin A, Bacillomycin D | Antifungal activity against Fusarium oxysporum f.sp. lycopersici causing Wilt in Tomato | [114] |
| 64. | Bacillus sp. (Marine) | Mixirins A, B, and C | Cytotoxic | [77] |
| 65. | Chromobacterium sp. C61 | Chromobactomycin | Antifungal activity against Magnoporthe grisea causing Rice Blast | [140] |
| 66. | Fusarium sp. YG-45 | Fusaristatins A and B | Antimicrobial | [141] |
| 67. | Fusarium decemcellulare LG53 | Fusaristatin A | Mildantimicrobial | [142] |
| 68. | Geitlerinema sp.(Marine cyanobacterium) | Mitsoamide | Cytotoxic activities | [77] |
| 69. | Herbaspirillum seropedicae Z67 | Serobactins A, B, and C | As aniron source | [143] |
| 70. | Pseudomonas fluorescens 96.578 | Tensin | Antifungal activity against Rhizoctonia solani causing Sugarbeet seed infection | [144] |
| 71. | P. fluorescens BD5 | Pseudofactin II | Anti-adhesive activity | [89] |
| 72. | P. fluorescens SS101 | Massetolide A | Systemic resistance (Late Blight) | [45] |
| 73. | Pseudomonas poae RE*1-1-14 | Poaeamide | Antifungal activity against Rhizoctonia solani causing Damping off and Rootrot in Sugarbeet | [93] |
| 74. | Pseudomonas sp. UCMA 17,988 (Isolated from Bovine raw milk) | Milkisin | Antimicrobial activity against Listeria monocytogenes, Staphylococcus aureus, and Salmonella enteric | [145] |
| 75. | Paenibacillus polymyxa M-1 | Polymyxin, Fusaricidin | Suppress phytopathogenic Erwinia spp. | [146] |
| 76. | Paenibacillus sp. IIRAC-30 | Surfactin | Antifungal | [147] |
| 77. | Scopulariopsis brevicaulis (Marine sponge-derived) | Scopularides A and B | Cytotoxic activities | [77] |
| 78. | Streptomyces canus | Amphomycins | Inhibit bacterial cell wall synthesis | [148,149] |
| 79. | Streptomyces viridochromogenes | Laspartomycins | Antibacterial, Antiherpes activity | [150] |
| 80. | Variovorax boronicumulanss BAM-48 | Variochelins A and B | Siderophore production | [151] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malviya, D.; Sahu, P.K.; Singh, U.B.; Paul, S.; Gupta, A.; Gupta, A.R.; Singh, S.; Kumar, M.; Paul, D.; Rai, J.P.; et al. Lesson from Ecotoxicity: Revisiting the Microbial Lipopeptides for the Management of Emerging Diseases for Crop Protection. Int. J. Environ. Res. Public Health 2020, 17, 1434. https://doi.org/10.3390/ijerph17041434
Malviya D, Sahu PK, Singh UB, Paul S, Gupta A, Gupta AR, Singh S, Kumar M, Paul D, Rai JP, et al. Lesson from Ecotoxicity: Revisiting the Microbial Lipopeptides for the Management of Emerging Diseases for Crop Protection. International Journal of Environmental Research and Public Health. 2020; 17(4):1434. https://doi.org/10.3390/ijerph17041434
Chicago/Turabian StyleMalviya, Deepti, Pramod Kumar Sahu, Udai B. Singh, Surinder Paul, Amrita Gupta, Abhay Raj Gupta, Shailendra Singh, Manoj Kumar, Diby Paul, Jai P. Rai, and et al. 2020. "Lesson from Ecotoxicity: Revisiting the Microbial Lipopeptides for the Management of Emerging Diseases for Crop Protection" International Journal of Environmental Research and Public Health 17, no. 4: 1434. https://doi.org/10.3390/ijerph17041434
APA StyleMalviya, D., Sahu, P. K., Singh, U. B., Paul, S., Gupta, A., Gupta, A. R., Singh, S., Kumar, M., Paul, D., Rai, J. P., Singh, H. V., & Brahmaprakash, G. P. (2020). Lesson from Ecotoxicity: Revisiting the Microbial Lipopeptides for the Management of Emerging Diseases for Crop Protection. International Journal of Environmental Research and Public Health, 17(4), 1434. https://doi.org/10.3390/ijerph17041434








