Treatment of Gaseous Ammonia Emissions Using Date Palm Pits Based Granular Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Granular Activated Carbon (GAC) Production
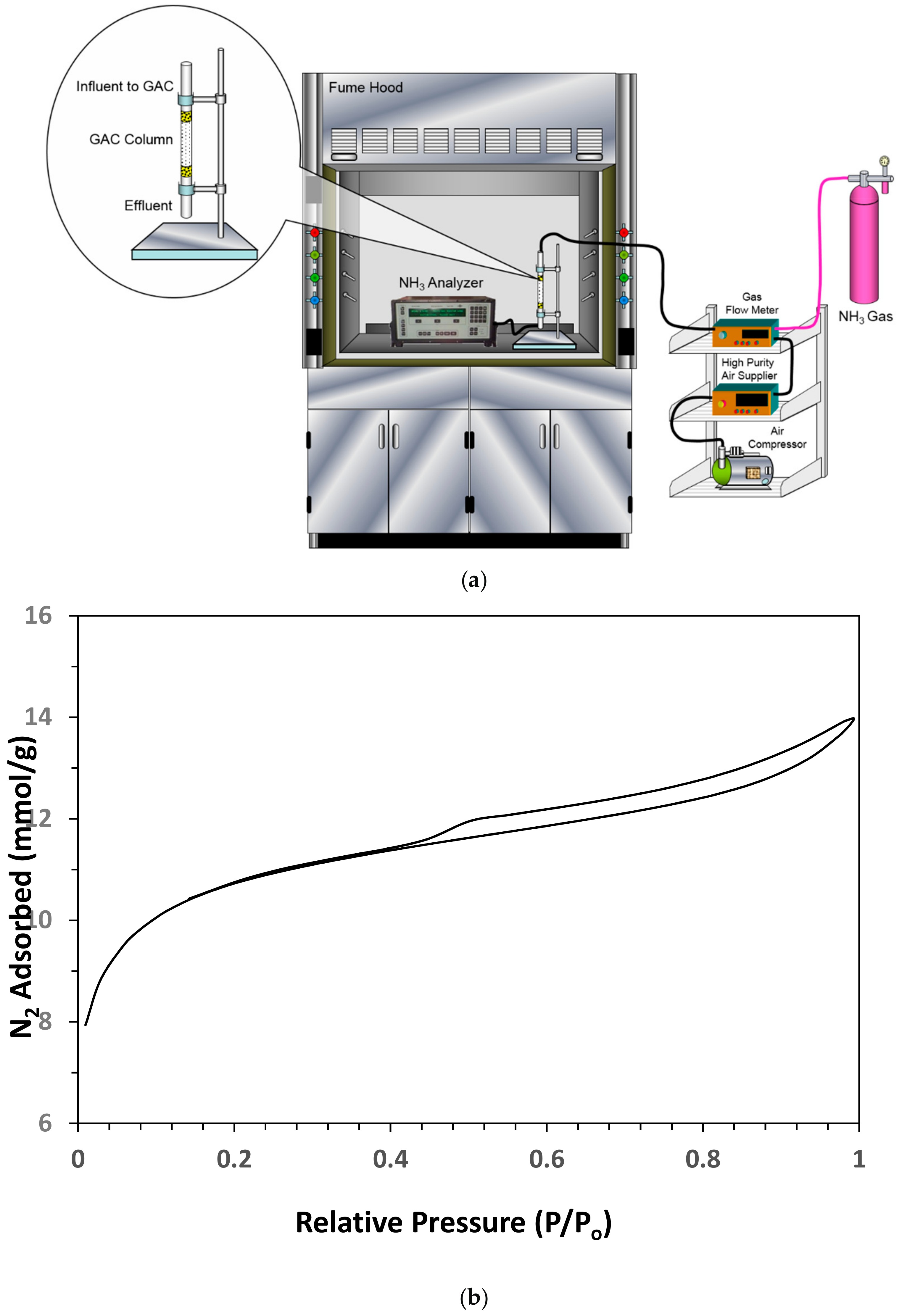
2.3. Gaseous Ammonia Dynamic Adsorption Studies
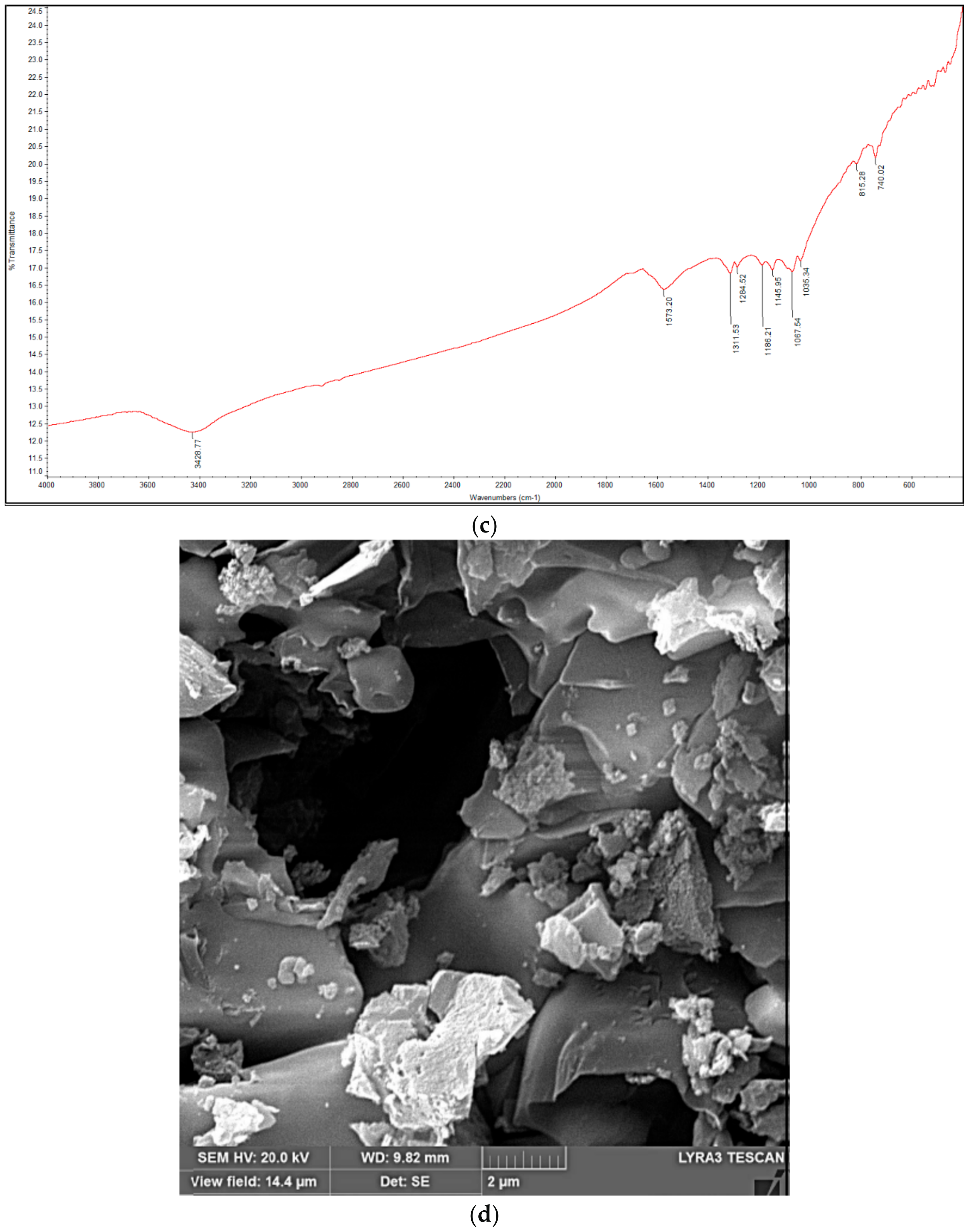
2.4. Analytical Methods
3. Results and Discussion
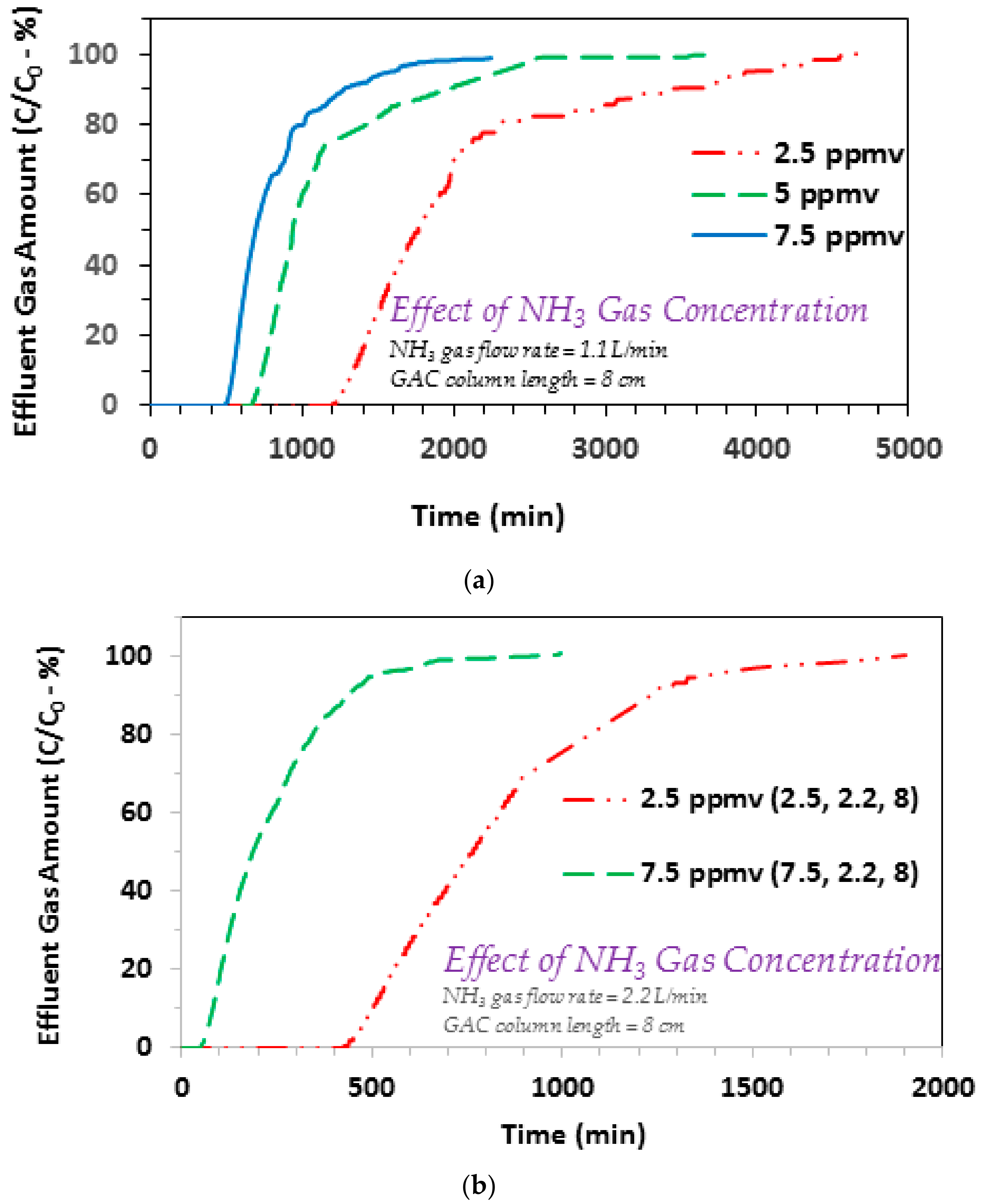
3.1. Effect of Ammonia Gas Concentration
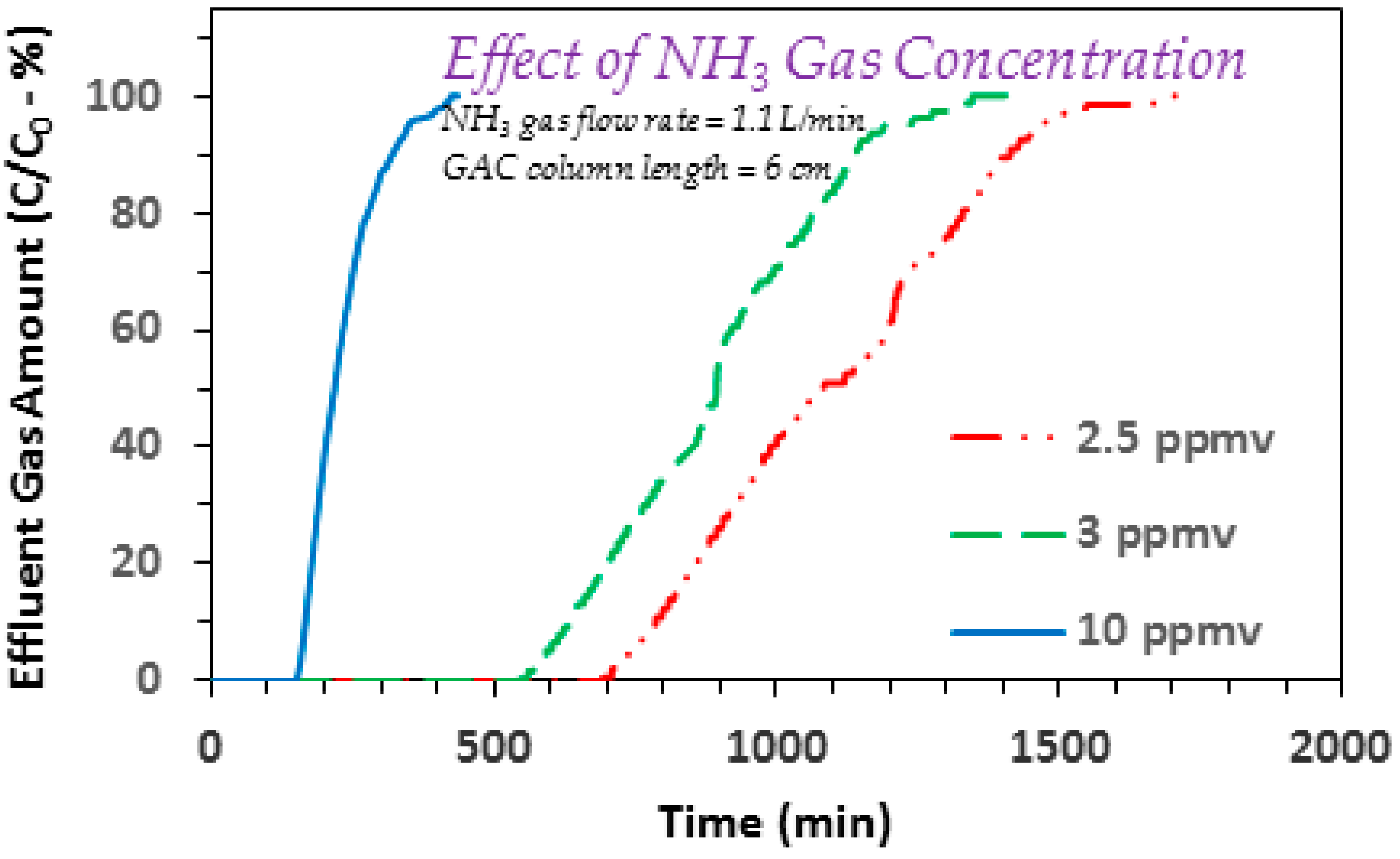
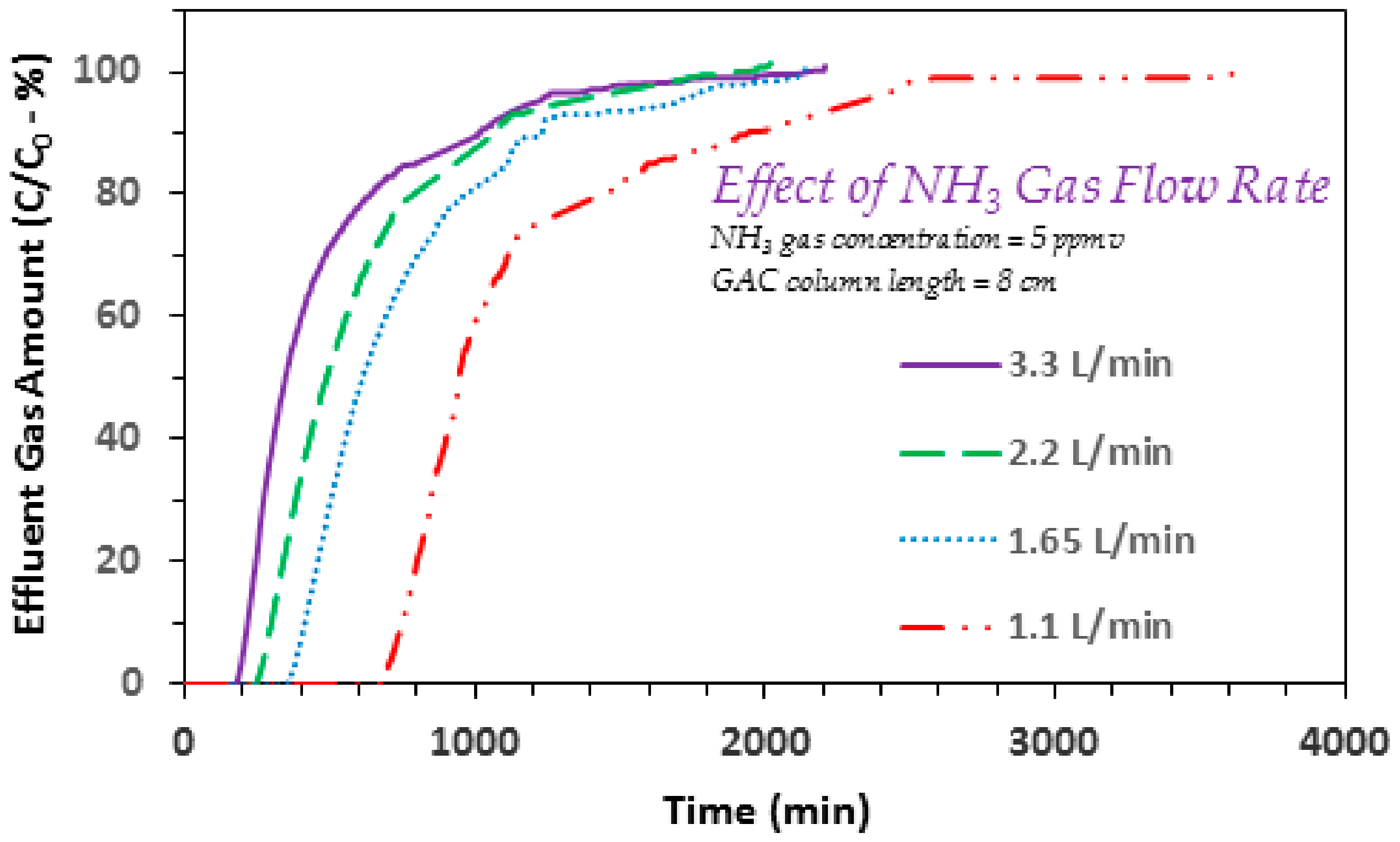
3.2. Effect of Ammonia Gas Flow Rate
3.3. Effect of GAC Column Length
3.4. Comparison with a Commercial GAC
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Iyobe, T.; Asada, T.; Kawata, K.; Oikawa, K. Comparison of removal efficiencies for ammonia and amine gases between woody charcoal and activated carbon. J. Health Sci. 2004, 50, 148–153. [Google Scholar]
- Yang, X.; Fraser, T.; Myat, D.; Smart, S.; Zhang, J.; Da Costa, J.C.D.; Liubinas, A.; Duke, M. A pervaporation study of ammonia solutions using molecular sieve silica membranes. Membranes 2014, 4, 40–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, M.-S.; Wang, C.-H. Treatment of Ammonia in Air Stream by Biotrickling Filter. Aerosol Air Qual. Res. 2007, 7, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Manakasettharn, S.; Takahashi, A.; Kawamoto, T.; Noda, K.; Sugiyama, Y.; Nakamura, T. Differences in NH3 gas adsorption behaviors of metal-hexacyanoferrate nanoparticles (Mx[FeII(CN)6]y·zH2O:M = In3+, Fe3+, and Mn2+). J. Solid State Chem. 2019, 270, 112–117. [Google Scholar] [CrossRef]
- Couvert, A.; Sanchez, C.; Laplanche, A.; Renner, C. Scrubbing intensification for sulphur and ammonia compounds removal. Chemosphere 2008, 70, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Bai, Z.H.; Winiwarter, W.; Kiesewetter, G.; Heyes, C.; Ma, L. Mitigating ammonia emission from agriculture reduces PM2.5 pollution in the Hai River Basin in China. Sci. Total Environ. 2017, 609, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Cole, N.A.; Gruber, S.; Kube, J.; Teeter, J.S. Modeling and prediction accuracy of ammonia gas emissions from feedlot cattle. Appl. Anim. Sci. 2019, 35, 347–356. [Google Scholar] [CrossRef]
- Sun, J.; Bai, M.; Shen, J.; Griffith, D.W.T.; Denmead, O.T.; Hill, J.; Lam, S.K.; Mosier, A.R.; Chen, D. Effects of lignite application on ammonia and nitrous oxide emissions from cattle pens. Sci. Total Environ. 2016, 565, 148–154. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Van Zwieten, L.; Singh, B.P.; Smillie, R.; Harden, S.; Fornasier, F. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 2017, 61, 129–137. [Google Scholar] [CrossRef]
- Drewry, J.L.; Choi, C.Y.; Powell, J.M.; Luck, B.D. Computational model of methane and ammonia emissions from dairy barns: Development and validation. Comput. Electron. Agric. 2018, 149, 80–89. [Google Scholar] [CrossRef]
- Kanagawa, T.; Qi, H.W.; Okubo, T.; Tokura, N. Biological treatment of ammonia gas at high loading. Water Sci. Technol. 2004, 50, 283–290. [Google Scholar] [CrossRef]
- Santos, C.; Goufo, P.; Fonseca, J.; Pereira, J.L.S.; Ferreira, L.; Coutinho, J.; Trindade, H. Effect of lignocellulosic and phenolic compounds on ammonia, nitric oxide and greenhouse gas emissions during composting. J. Clean. Prod. 2018, 171, 548–556. [Google Scholar] [CrossRef]
- Oyarzun, P.; Alarcón, L.; Calabriano, G.; Bejarano, J.; Nuñez, D.; Ruiz-Tagle, N.; Urrutia, H. Trickling filter technology for biotreatment of nitrogenous compounds emitted in exhaust gases from fishmeal plants. J. Environ. Manag. 2019, 232, 165–170. [Google Scholar] [CrossRef]
- Li, S.; Lang, J.; Zhou, Y.; Liang, X.; Chen, D.; Wei, P. Trends in ammonia emissions from light-duty gasoline vehicles in China, 1999–2017. Sci. Total Environ. 2020, 700, 134359. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Activated carbons modified with aluminium-zirconium polycations as adsorbents for ammonia. Microporous Mesoporous Mater. 2008, 114, 137–147. [Google Scholar] [CrossRef]
- Lee, S.H.; Li, C.; Heber, A.J.; Ni, J.; Huang, H. Biofiltration of a mixture of ethylene, ammonia, n-butanol, and acetone gases. Bioresour. Technol. 2013, 127, 366–377. [Google Scholar] [CrossRef]
- Ramírez, M.; Gómez, J.M.; Aroca, G.; Cantero, D. Removal of ammonia by immobilized Nitrosomonas europaea in a biotrickling filter packed with polyurethane foam. Chemosphere 2009, 74, 1385–1390. [Google Scholar] [CrossRef]
- Maxima, M.; Hiraiwa, C.; Yamaguchi, A.; Fukunaga, A.; Awazu, T.; Ueda, T.; Nishizuka, K.; Mitsuhashi, F.; Kuramoto, T. Development of ammonia gas removal device. SEI Tech. Rev. 2012, 91–94. [Google Scholar]
- Du, C.; Xue, Y.; Wu, Z.; Wu, Z. Microwave-assisted one-step preparation of macadamia nut shell-based activated carbon for efficient adsorption of Reactive Blue. New J. Chem. 2017, 41, 15373–15383. [Google Scholar] [CrossRef]
- He, X.; Wu, Z.; Sun, Z.; Wei, X.; Wu, Z.; Ge, X.; Cravotto, G. A novel hybrid of β -cyclodextrin grafted onto activated carbon for rapid adsorption of naphthalene from aqueous solution. J. Mol. Liq. 2018, 255, 160–167. [Google Scholar] [CrossRef]
- Xue, Y.; Du, C.; Wu, Z.; Zhang, L. Relationship of cellulose and lignin contents in biomass to the structure and RB-19 adsorption behavior of activated carbon. New J. Chem. 2018, 42, 16493–16502. [Google Scholar] [CrossRef]
- Wu, Z.; Wei, X.; Xue, Y.; He, X.; Yang, X. Removal effect of atrazine in co-solution with Bisphenol A or humic acid by different activated carbons. Materials 2018, 11, 2558. [Google Scholar] [CrossRef] [Green Version]
- Faisal, A.A.H.; Al-Wakel, S.F.A.; Assi, H.A.; Naji, L.A.; Naushad, M. Waterworks sludge-filter sand permeable reactive barrier for removal of toxic lead ions from contaminated groundwater. J. Water Process Eng. 2020, 33, 101112. [Google Scholar] [CrossRef]
- Naushad, M.; Alqadami, A.A.; AlOthman, Z.A.; Alsohaimi, I.H.; Algamdi, M.S.; Aldawsari, A.M. Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated carbon. J. Mol. Liq. 2019, 293, 111442. [Google Scholar] [CrossRef]
- Guo, J.; Xu, W.S.; Chen, Y.L.; Lua, A.C. Adsorption of NH3 onto activated carbon prepared from palm shells impregnated with H2SO4. J. Colloid Interface Sci. 2005, 281, 285–290. [Google Scholar] [CrossRef]
- Gu, L.; Wang, D.; Deng, R.; Liu, H.; Ai, H. Effect of surface modification of activated carbon on its adsorption capacity for bromate. Desalin. Water Treat. 2013, 51, 2592–2601. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; Gonalves, M.; Rodríguez-Reinoso, F. Oxidation of activated carbon with aqueous solution of sodium dichloroisocyanurate: Effect on ammonia adsorption. Microporous Mesoporous Mater. 2011, 142, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.C.; Li, H.S.; Chen, C.H. Effect of surface acidic oxides of activated carbon on adsorption of ammonia. J. Hazard. Mater. 2008, 159, 523–527. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Petit, C. On the reactive adsorption of ammonia on activated carbons modified by impregnation with inorganic compounds. J. Colloid Interface Sci. 2009, 338, 329–345. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, S.Y. Effect of ozone treatment on ammonia removal of activated carbons. J. Colloid Interface Sci. 2005, 286, 417–419. [Google Scholar] [CrossRef]
- De la Cruz-Lovera, C.; Manzano-Agugliaro, F.; Salmerón-Manzano, E.; De la Cruz-Fernández, J.L.; Perea-Moreno, A.J. Date seeds (Phoenix dactylifera L.) valorization for boilers in the mediterranean climate. Sustainability 2019, 11, 711. [Google Scholar] [CrossRef] [Green Version]
- Hai, A.; Bharath, G.; Babu, K.R.; Taher, H.; Naushad, M.; Banat, F. Date seeds biomass-derived activated carbon for efficient removal of NaCl from saline solution. Process Saf. Environ. Prot. 2019, 129, 103–111. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Alshehri, S.M. Ultra-fast spill oil recovery using a mesoporous lignin based nanocomposite prepared from date palm pits (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2019, 130, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jamil, F.; Al-Muhtaseb, A.H.; Naushad, M.; Baawain, M.; Al-Mamun, A.; Saxena, S.K.; Viswanadham, N. Evaluation of synthesized green carbon catalyst from waste date pits for tertiary butylation of phenol. Arab. J. Chem. 2020, 13, 298–307. [Google Scholar] [CrossRef]
- Naushad, M.; Khan, M.R.; AlOthman, Z.A.; AlSohaimi, I.; Rodriguez-Reinoso, F.; Turki, T.M.; Ali, R. Removal of BrO3− from drinking water samples using newly developed agricultural waste-based activated carbon and its determination by ultra-performance liquid chromatography-mass spectrometry. Environ. Sci. Pollut. Res. 2015, 22, 15853–15865. [Google Scholar] [CrossRef]
- Jibril, B.; Houache, O.; Al-maamari, R.; Al-rashidi, B. Effects of H3PO4 and KOH in carbonization of lignocellulosic material. J. Anal. Appl. Pyrolysis 2008, 83, 151–156. [Google Scholar] [CrossRef]
- Alhamed, Y.A. Adsorption kinetics and performance of packed bed adsorber for phenol removal using activated carbon from dates’ stones. J. Hazard. Mater. 2009, 170, 763–770. [Google Scholar] [CrossRef]
- Al-muhtaseb, S.A.; El-naas, M.H.; Abdallah, S. Removal of aluminum from aqueous solutions by adsorption on date-pit and BDH activated carbons. J. Hazard. Mater. 2008, 158, 300–307. [Google Scholar] [CrossRef]
- Belhachemi, M.; Rios, R.V.R.A.; Addoun, F.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. Preparation of activated carbon from date pits: Effect of the activation agent and liquid phase oxidation. J. Anal. Appl. Pyrolysis 2009, 86, 168–172. [Google Scholar] [CrossRef]
- Bouchelta, C.; Salah, M.; Bertrand, O.; Bellat, J. Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis 2008, 82, 70–77. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Girgis, B.S. Impact of surface characteristics of acti v ated carbon on adsorption of BTEX. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 181–193. [Google Scholar] [CrossRef]
- El-naas, M.H.; Al-zuhair, S.; Alhaija, M.A. Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J. Hazard. Mater. 2010, 173, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Girgis, B.S. Porosity development in activated carbons obtained from date pits under chemical activation with phosphoric acid. Microporous Mesoporous Mater. 2002, 52, 105–117. [Google Scholar] [CrossRef]
- Hameed, B.H.; Salman, J.M.; Ahmad, A.L. Adsorption isotherm and kinetic modeling of 2, 4-D pesticide on activated carbon derived from date stones. J. Hazard. Mater. 2009, 163, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Merzougui, Z.; Addoun, F. Effect of oxidant treatment of date pit activated carbons application to the treatment of waters. Desalination 2008, 222, 394–403. [Google Scholar] [CrossRef]
- Labaran, B.A.; Vohra, M.S. Application of activated carbon produced from phosphoric acid-based chemical activation of oil fly ash for the removal of some charged aqueous phase dyes: Role of surface charge, adsorption kinetics, and modeling. Desalin. Water Treat. 2016, 57, 16034–16052. [Google Scholar] [CrossRef]
- Vohra, M.S. Adsorption-Based Removal of Gas-Phase Benzene Using Granular Activated Carbon (GAC) Produced from Date Palm Pits. Arab. J. Sci. Eng. 2015, 40, 3007–3017. [Google Scholar] [CrossRef]
- Le Leuch, L.M.; Bandosz, T.J. The role of water and surface acidity on the reactive adsorption of ammonia on modified activated carbons. Carbon 2007, 45, 568–578. [Google Scholar] [CrossRef]
- Fortier, H.; Westreich, P.; Selig, S.; Zelenietz, C.; Dahn, J.R. Ammonia, cyclohexane, nitrogen and water adsorption capacities of an activated carbon impregnated with increasing amounts of ZnCl2, and designed to chemisorb gaseous NH3 from an air stream. J. Colloid Interface Sci. 2008, 320, 423–435. [Google Scholar] [CrossRef]
- Lim, E.; Yao, J. Modeling and simulation of the polymeric nanocapsule formation process. IFAC Proc. Vol. 2009, 7, 405–410. [Google Scholar]
- Held, A.; Pratt, D.W. Ammonia as a Hydrogen Bond Donor and Acceptor in the Gas Phase. Structures of 2-Pyridone-NH3 and 2-Pyridone-(NH3)2 in Their S0 and S1 Electronic States. J. Am. Chem. Soc. 1993, 115, 9718–9723. [Google Scholar] [CrossRef]
- Weng, Y.; Qiu, S.; Ma, L.; Liu, Q.; Ding, M.; Zhang, Q.; Zhang, Q.; Wang, T. Jet-Fuel range hydrocarbons from biomass-derived sorbitol over Ni-HZSM-5/SBA-15 catalyst. Catalysts 2015, 5, 2147–2160. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vohra, M. Treatment of Gaseous Ammonia Emissions Using Date Palm Pits Based Granular Activated Carbon. Int. J. Environ. Res. Public Health 2020, 17, 1519. https://doi.org/10.3390/ijerph17051519
Vohra M. Treatment of Gaseous Ammonia Emissions Using Date Palm Pits Based Granular Activated Carbon. International Journal of Environmental Research and Public Health. 2020; 17(5):1519. https://doi.org/10.3390/ijerph17051519
Chicago/Turabian StyleVohra, Muhammad. 2020. "Treatment of Gaseous Ammonia Emissions Using Date Palm Pits Based Granular Activated Carbon" International Journal of Environmental Research and Public Health 17, no. 5: 1519. https://doi.org/10.3390/ijerph17051519
APA StyleVohra, M. (2020). Treatment of Gaseous Ammonia Emissions Using Date Palm Pits Based Granular Activated Carbon. International Journal of Environmental Research and Public Health, 17(5), 1519. https://doi.org/10.3390/ijerph17051519




