External Validation of the ‘PHYT in Dementia’, a Theoretical Model Promoting Physical Activity in People with Dementia
Abstract
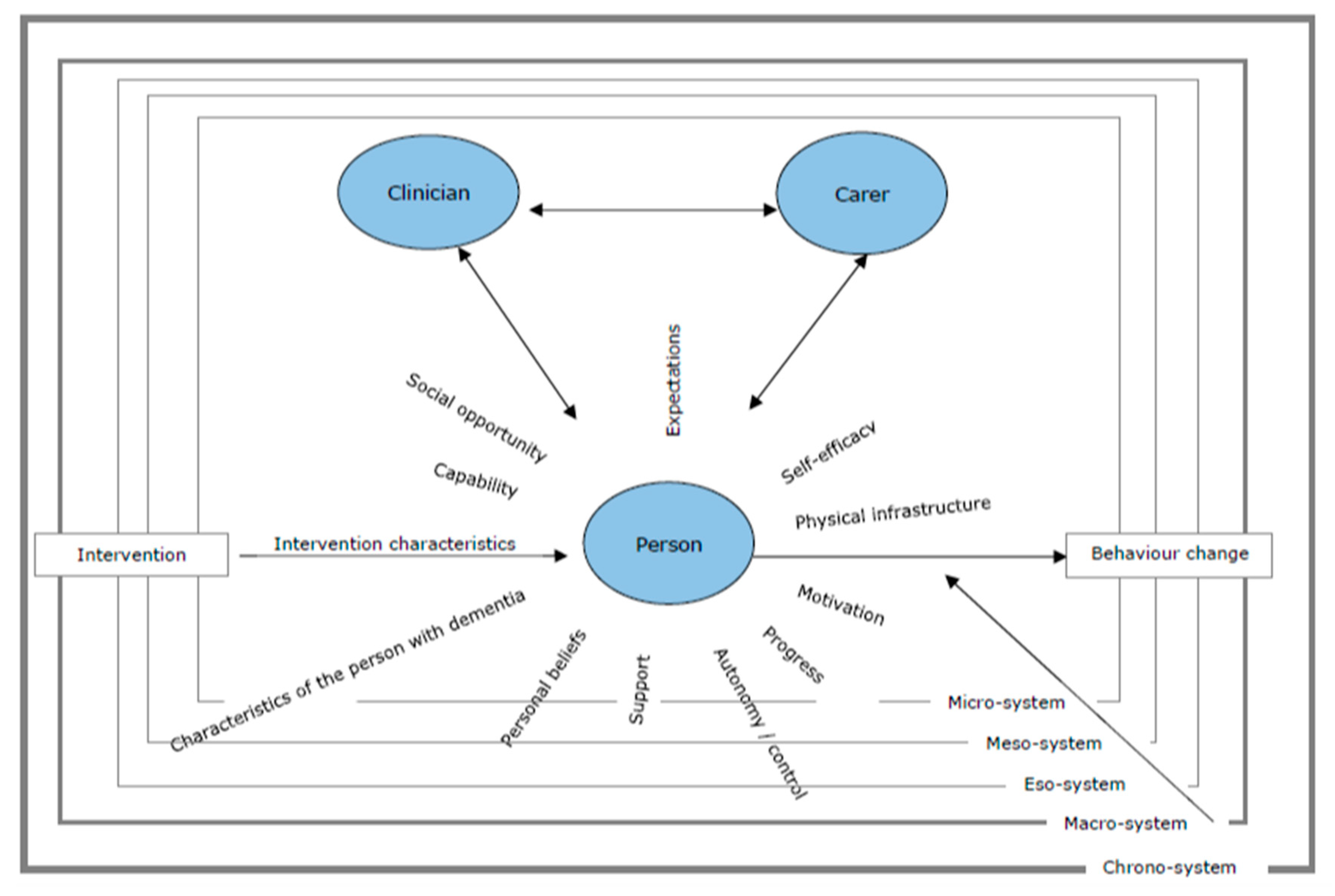
:1. Introduction
2. Materials and Methods
2.1. Participants and Setting
2.2. Data Collection
2.3. Data Analysis
2.4. Ethic Approval
3. Results
3.1. Characteristics of the Person with Dementia
3.2. Support
3.3. Expectations/Goals
3.4. The Carer(s)
3.5. Progress
3.6. Social Opportunity
3.7. Self-Efficacy
3.8. Capability
3.9. Ideas around the Activity/Intervention
3.10. Autonomy/Control
3.11. Physical Infrastructure
3.12. Personal History
3.13. Information/Knowledge
3.14. The Professional(s)
3.15. Personal Beliefs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Research UK (2019). About Dementia. Available online: https://www.alzheimersresearchuk.org/about-dementia/?gclid=Cj0KCQjw3JXtBRC8ARIsAEBHg4myuYeahFMLCcSGLr-flQxznmdB-dObW2gXc5MUN9o_dfNw5wwI5EwaAvH6EALw_wcB (accessed on 26 October 2019).
- Muir, S.W.; Gopaul, K.; Montero Odasso, M.M. The role of cognitive impairment in fall risk among older adults: A systematic review and meta-analysis. Age Ageing 2012, 41, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Delbaere, K.; Kochan, N.A.; Close, J.C.; Menant, J.C.; Sturnieks, D.L.; Brodaty, H.; Lord, S.R. Mild cognitive impairment as a predictor of falls in community-dwelling older people. Am. J. Geriatr. Psychiatry 2012, 20, 845–853. [Google Scholar] [CrossRef]
- Kallin, K.; Gustafson, Y.; Sandman, P.O.; Karlsson, S. Factors associated with falls among older, cognitively impaired people in geriatric care settings: A population-based study. Am. J. Geriatr. Psychiatry 2005, 13, 501–509. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Forbes, D.; Forbes, S.C.; Blake, C.M.; Thiessen, E.J.; Forbes, S. Exercise programs for people with dementia. Cochrane Libr. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, R.; Ellard, D.; Rees, K.; Thorogood, M. A systematic review of the effects of physical activity on physical functioning, quality of life and depression in older people with dementia. Int. J. Geriatr. Psychiatry 2011, 26, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Blankevoort, C.G.; Van Heuvelen, M.J.; Boersma, F.; Luning, H.; De Jong, J.; Scherder, E.J. Review of effects of physical activity on strength, balance, mobility and ADL performance in elderly subjects with dementia. Dement. Geriatr. Cogn. Disord. 2010, 30, 392–402. [Google Scholar] [CrossRef]
- Heyn, P.; Abreu, B.C.; Ottenbacher, K.J. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis1. Arch. Phys. Med. Rehabil. 2004, 85, 1694–1704. [Google Scholar] [CrossRef]
- Pitkala, K.H.; Raivio, M.M.; Laakkonen, M.L.; Tilvis, R.S.; Kautiainen, H.; Strandberg, T.E. Exercise rehabilitation on home-dwelling patients with Alzheimer’s disease-a randomized, controlled trial. Study protocol. Trials 2010, 11, 92. [Google Scholar] [CrossRef] [Green Version]
- Pitkala, K.H.; Raivio, M.M.; Laakkonen, M.L.; Tilvis, R.S.; Kautiainen, H.; Strandberg, T.E. Exercise rehabilitation on home-dwelling patients with Alzheimer disease: A randomized, controlled trial. Baseline findings and feasibility. Eur. Geriatr. Med. 2011, 2, 338–343. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126. [Google Scholar] [PubMed]
- Zanetti, O.; Binetti, G.; Magni, E.; Rozzini, L.; Bianchetti, A.; Trabucchi, M. Procedural memory stimulation in Alzheimer’s disease: Impact of a training programme. Acta Neurol. Scandin. 1997, 95, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kasl-Godley, J.; Gatz, M. Psychosocial interventions for individuals with dementia: An integration of theory, therapy, and a clinical understanding of dementia. Clin. Psychol. Rev. 2000, 20, 755–782. [Google Scholar] [CrossRef]
- Josephsson, S.; Bäckman, L.; Borell, L.; Bernspång, B.; Nygård, L.; Rönnberg, L. Supporting everyday activities in dementia: An intervention study. Int. J. Geriatr. Psychiatry 1993, 8, 395–400. [Google Scholar] [CrossRef]
- Law, L.L.; Barnett, F.; Yau, M.K.; Gray, M.A. Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: A systematic review. Ageing Res. Rev. 2014, 15, 61–75. [Google Scholar] [CrossRef]
- Öhman, A.; Nygård, L. Meanings and motives for engagement in self-chosen daily life occupations among individuals with Alzheimer’s disease. OTJR Occup. Particip. Health 2005, 25, 89–97. [Google Scholar] [CrossRef]
- Graff, M.J.; Vernooij-Dassen, M.J.; Thijssen, M.; Dekker, J.; Hoefnagels, W.H.; Rikkert, M.G.O. Community based occupational therapy for patients with dementia and their care givers: Randomised controlled trial. BMJ 2006, 333, 1196. [Google Scholar] [CrossRef] [Green Version]
- Graff, M.J.; Adang, E.M.; Vernooij-Dassen, M.J.; Dekker, J.; Jönsson, L.; Thijssen, M.; Rikkert, M.G.O. Community occupational therapy for older patients with dementia and their care givers: Cost effectiveness study. BMJ 2008, 336, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Di Lorito, C.; Bosco, A.; Booth, V.; Goldberg, H.R.; van der Wardt, V. Adherence to exercise and physical activity interventions in older people with mild cognitive impairment and dementia: A systematic review and meta-analysis. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2019, 15, 804–805. [Google Scholar] [CrossRef]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new medical research council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef] [Green Version]
- Clarke, D.J.; Godfrey, M.; Hawkins, R.; Sadler, E.; Harding, G.; Forster, A.; Farrin, A. Implementing a training intervention to support caregivers after stroke: A process evaluation examining the initiation and embedding of programme change. Implement. Sci. 2013, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, J.J.; Eccles, M.P.; Johnston, M.; Whitty, P.; Grimshaw, J.M.; Kaner, E.F.; Walker, A. Explaining the effects of an intervention designed to promote evidence-based diabetes care: A theory-based process evaluation of a pragmatic cluster randomised controlled trial. Implement. Sci. 2008, 3, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorito, C.; Pollock, K.; Harwood, R.; das Nair, R.; Logan, P.; Goldberg, S.; van der Wardt, V. A scoping review of behaviour change theories in adults without dementia to adapt and develop the ′PHYT in dementia′, a model promoting physical activity in people with dementia. Maturitas 2019, 121, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Wardt, V.; Hancox, J.; Gondek, D.; Logan, P.; das Nair, R.; Pollock, K.; Harwood, R. Adherence support strategies for exercise interventions in people with mild cognitive impairment and dementia: A systematic review. Prev. Med. Rep. 2017, 7, 38–45. [Google Scholar] [CrossRef]
- Mcintyre, A.; Reynolds, F. There’s no apprenticeship for Alzheimer’s: The caring relationship when an older person experiencing dementia falls. Ageing Soc. 2012, 32, 873–896. [Google Scholar] [CrossRef] [Green Version]
- Faes, M.C.; Reelick, M.F.; Joosten-Weyn Banningh, L.W.; Gier, M.D.; Esselink, R.A.; Olde Rikkert, M.G. Qualitative study on the impact of falling in frail older persons and family caregivers: Foundations for an intervention to prevent falls. Aging Ment. Health 2010, 14, 834–842. [Google Scholar] [CrossRef]
- Kuzuya, M.; Masuda, Y.; Hirakawa, Y.; Iwata, M.; Enoki, H.; Hasegawa, J.; Iguchi, A. Falls of the elderly are associated with burden of caregivers in the community. Int. J. Geriatr. Psychiatry J. Psychiatry Late Life Allied Sci. 2006, 21, 740–745. [Google Scholar] [CrossRef]
- Apóstolo, J.; Cooke, R.; Bobrowicz-Campos, E.; Santana, S.; Marcucci, M.; Cano, A.; Vollenbroek-Hutten, M.; Germini, F.; D’avanzo, B.; Gwyther, H.; et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 140. [Google Scholar]
- Di Lorito, C.; Bosco, A.; Booth, V.; Goldberg, H.R.; van der Wardt, V. Adherence to exercise and physical activity interventions in older people with mild cognitive impairment and dementia: a systematic review. Prev. Med. Rep. under review.
- Cooper, B.S. Confronting models with data. J. Hosp. Infect. 2007, 65, 88–92. [Google Scholar] [CrossRef]
- Eddy, D.M.; Hollingworth, W.; Caro, J.J. Model transparency and validation: A report of the ISPOR-SMDM modeling good research practices task force-7. Med. Decis. Mak. 2012, 32, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Di Lorito, C.; Pollock, K.; Harwood, R.; das Nair, R.; Logan, P.; Goldberg, S.; van der Wardt, V. Protocol for the process evaluation of the promoting activity, independence and stability in early dementia and mild cognitive impairment (PrAISED 2) randomised controlled trial. Maturitas 2019, 122, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, R.K.; Goldberg, S.E.; van der Wardt, V.; Burgon, C.; Di Lorito, C.; Godfrey, M.; Smith, H. A randomised controlled trial of an exercise intervention promoting activity, independence and stability in older adults with mild cognitive impairment and early dementia (PrAISED)-A Protocol. Trials 2019, 20, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J. Using conceptual depth criteria: Addressing the challenge of reaching saturation in qualitative research. Qual. Res. 2017, 17, 554–570. [Google Scholar] [CrossRef] [Green Version]
- QSR International Pty Ltd. NVivo Qualitative Data Analysis Software; QSR International Pty Ltd.: Melbourne, Australia, 2018. [Google Scholar]
- Mayring, P. Qualitative Content Analysis. A companion to Qualitative Research. Forum Qual. Soc. Res. 2000, 1, 159–176. [Google Scholar]
- Pope, C.; Ziebland, S.; Mays, N. Analysing qualitative data. In Qualitative Research in Health Care, 3rd ed.; Pope, C., Mays, N., Eds.; Oxford: Blackwell Publishing: Hoboken, NJ, USA, 2006; pp. 63–81. [Google Scholar]
- Potter, W.J.; Levine-Donnerstein, D. Rethinking validity and reliability in content analysis. J. Appl. Commun. Res. 1999, 27, 258–284. [Google Scholar] [CrossRef]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Kondracki, N.L.; Wellman, N.S.; Amundson, D.R. Content analysis: Review of methods and their applications in nutrition education. J. Nutr. Educ. Behav. 2002, 34, 224–230. [Google Scholar] [CrossRef]
- Bronfenbrenner, U. Experiments by Nature and Design. In The Ecology of Human Development; Harvard University Press: Cambridge, MA, USA, 1979. [Google Scholar]
- 38 Burke, N.J.; Joseph, G.; Pasick, R.J.; Barker, J.C. Theorizing social context: Rethinking behavioral theory. Health Educ. Behav. 2009, 36, 55S–70S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyman, S.R.; Adamczewska, N.; Howlett, N. Systematic review of behaviour change techniques to promote participation in physical activity among people with dementia. Br. J. Health Psychol. 2018, 23, 148–170. [Google Scholar] [CrossRef] [Green Version]
- Ryan, R.M.; Deci, E.L. Self-Determination Theory: Basic Psychological Needs in Motivation, Development, and Wellness; Guilford Publishing: New York, NY, USA, 2017. [Google Scholar]
- Livingston, G.; Leavey, G.; Manela, M.; Livingston, D.; Rait, G.; Sampson, E.; Cooper, C. Making decisions for people with dementia who lack capacity: Qualitative study of family carers in UK. BMJ 2010, 341, c4184. [Google Scholar] [CrossRef] [Green Version]
- Peach, T.; Pollock, K.; van der Wardt, V.; das Nair, R.; Logan, P.; Harwood, R.H. Attitudes of older people with mild dementia and mild cognitive impairment and their relatives about falls risk and prevention: A qualitative study. PLoS ONE 2017, 12, e0177530. [Google Scholar] [CrossRef]



| Participant ID * | Gender | Age | Ethnicity | Living Arrangement | Relationship to Carer |
|---|---|---|---|---|---|
| P01 | M | 75 | White | Lives independently | Brother |
| P02 | M | 83 | White | Lives with carer | Spouse |
| P03 | M | 76 | White | Lives with carer | Spouse |
| P04 | M | 73 | White | Lives with carer | Spouse |
| P05 | M | 90 | White | Lives with carer | Spouse |
| P06 | M | 80 | White | Lives with carer | Spouse |
| P07 | M | 78 | White | Lives with carer | Spouse |
| P08 | M | 83 | White | Lives with carer | Spouse |
| P09 | M | 75 | White | Lives with carer | Spouse |
| P10 | M | 70 | White | Lives with carer | Spouse |
| P11 | M | 77 | White | Lives with carer | Spouse |
| P12 | M | 85 | White | Lives with carer | Spouse |
| P13 | M | 81 | White | Lives with carer | Spouse |
| P14 | M | 80 | White | Lives with carer | Spouse |
| P15 | M | 85 | White | Lives with carer | Spouse |
| P16 | M | 74 | White | Lives with carer | Spouse |
| P17 | M | 77 | White | Lives with carer | Spouse |
| P18 | F | 85 | White | Lives independently | Mother |
| P19 | F | 83 | Black | Lives independently | Mother |
| P20 | M | 86 | White | Lives with carer | Spouse |
| C01 | F | 80 | White | ||
| C02 | F | 81 | White | ||
| C03 | F | 73 | White | ||
| C04 | F | 73 | White | ||
| C05 | F | 88 | White | ||
| C06 | F | 72 | White | ||
| C07 | F | 75 | White | ||
| C08 | F | 82 | White | ||
| C09 | F | 76 | White | ||
| C10 | F | 70 | White | ||
| C11 | F | 71 | White | ||
| C12 | F | 72 | White | ||
| C13 | F | 78 | White | ||
| C14 | F | 75 | White | ||
| C15 | F | 83 | White | ||
| C16 | F | 71 | White | ||
| C17 | F | 78 | White | ||
| C18 | M | 58 | White | ||
| C19 | M | 60 | Black | ||
| C20 | F | 78 | White | ||
| Construct | Operational Definition | Example of How the Construct Might Mediate Behaviour Change |
|---|---|---|
| Characteristics of the person with dementia | Characteristics of the person affecting behaviour change, which include personality, temperament and identity | Risk-takers might be more willing to challenge themselves in a physical activity programme than overly cautious subjects, thus potentially obtaining more positive outcomes |
| Support | Practical and emotional support from others (e.g., carer, therapist, society) which affects behaviour change | People might need an initial external push to initiate behaviour change, which may be provided by family members |
| Expectations/goals | Expectationsgoals around the behaviour, including benefits, barriers and facilitators | A person with dementia will sign up to an intervention delivering home-based physical exercise, if they believe that it will improve their health |
| Carer(s) | Any aspect, behaviour and attitude of the carer, which mediates behaviour change and maintenance | A carer might have risk-averse attitudes toward physical exercise and developing gate-keeping behaviour toward the person with dementia |
| Progress | Perceived or actual improvement in the person’s physical or mental health, following the behaviour | A person will find motivation to initiate/maintain behaviour change if they see progress/improvements |
| Social opportunity | Social contacts and networking opportunities (or lack thereof) granted through engaging in the behaviour | The opportunity for socialisation presented by a physical activity group in the community might encourage a person with dementia to sign up |
| Self-efficacy | Confidence in one’s ability to execute a given behaviour, including (perceived) physical, cognitive ability and competence | People with dementia might be reluctant to sign up for a walking group in the community, as they fear they might fall |
| Capability | One’s actual ability to perform a behaviour through essential skills, including (actual) physical, chronic conditions, cognitive ability, competence psychological/personal and social capability | People who have extensive memory impairment might struggle to remember the potential benefits that behaviour change might generate |
| Activity/intervention characteristics | Characteristics of the activity or intervention which influence participants’ engagement in it. They include how much the participant felt they are tailored to their needs, goal, preferences and aspirations, how helpful, enjoyable and challenging they are and how they fit into their routine | If a person finds an activity enjoyable, they will be more willing to engage |
| Autonomy/control | Being causal agents of one’s behaviour | A person will engage in behaviour change more easily when they have made an autonomous decision |
| Physical infrastructure | Environment and its characteristics, where the behaviour change occurs | A person with dementia who gives up their driving license might struggle to travel to an activity group organised in the community |
| Personal history | Personal history of a person, which affects present behaviour change | People who have been always very physically active are more motivated to engage in physical rehabilitation after hospitalisation |
| Information/knowledge | Information and knowledge that the person needs to change their behaviour | People who are informed about the benefits of behaviour change are more willing to initiate it |
| Professional | Any aspect, behaviour and attitude of the professional, which mediates behaviour change and maintenance | A person might be encouraged to exercise, if information on the benefits of exercising comes from a professional who is held in high esteem |
| Personal beliefs | The self-regulated mechanisms that the person uses in relation to initiation, adherence and withdrawal from behaviour change | A person might think that going to the gym could expose them to a higher risk of injury than spending more time at home |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorito, C.; Bosco, A.; Pollock, K.; H. Harwood, R.; das Nair, R.; Logan, P.; Goldberg, S.; Booth, V.; Vedhara, K.; Godfrey, M.; et al. External Validation of the ‘PHYT in Dementia’, a Theoretical Model Promoting Physical Activity in People with Dementia. Int. J. Environ. Res. Public Health 2020, 17, 1544. https://doi.org/10.3390/ijerph17051544
Di Lorito C, Bosco A, Pollock K, H. Harwood R, das Nair R, Logan P, Goldberg S, Booth V, Vedhara K, Godfrey M, et al. External Validation of the ‘PHYT in Dementia’, a Theoretical Model Promoting Physical Activity in People with Dementia. International Journal of Environmental Research and Public Health. 2020; 17(5):1544. https://doi.org/10.3390/ijerph17051544
Chicago/Turabian StyleDi Lorito, Claudio, Alessandro Bosco, Kristian Pollock, Rowan H. Harwood, Roshan das Nair, Pip Logan, Sarah Goldberg, Vicky Booth, Kavita Vedhara, Maureen Godfrey, and et al. 2020. "External Validation of the ‘PHYT in Dementia’, a Theoretical Model Promoting Physical Activity in People with Dementia" International Journal of Environmental Research and Public Health 17, no. 5: 1544. https://doi.org/10.3390/ijerph17051544
APA StyleDi Lorito, C., Bosco, A., Pollock, K., H. Harwood, R., das Nair, R., Logan, P., Goldberg, S., Booth, V., Vedhara, K., Godfrey, M., Dunlop, M., & van der Wardt, V. (2020). External Validation of the ‘PHYT in Dementia’, a Theoretical Model Promoting Physical Activity in People with Dementia. International Journal of Environmental Research and Public Health, 17(5), 1544. https://doi.org/10.3390/ijerph17051544





