Maternal Obesity Programs Offspring Development and Resveratrol Potentially Reprograms the Effects of Maternal Obesity
Abstract
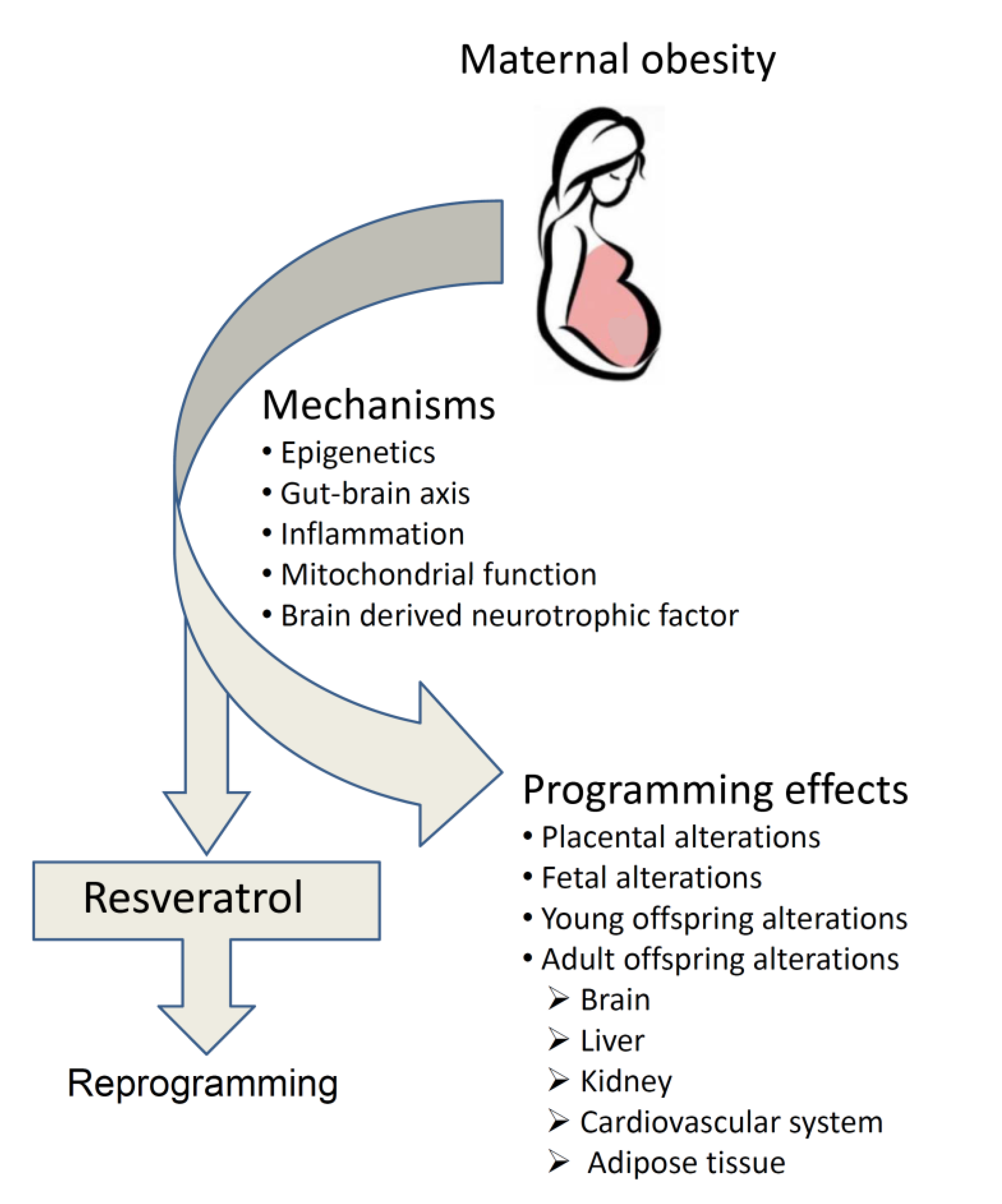
:1. Introduction
2. Long-Term Public Health Issues of Maternal Obesity
3. Specific Mechanisms Underlying How Maternal Obesity Programs Offspring
3.1. Epigenome
3.2. Gut-Brain Axis
3.3. Inflammation
3.4. Mitochondrial Function
3.5. Brain-Derived Neurotrophic Factor
4. Programming Effects of Maternal Obesity Across Life-Span
4.1. Placental Alterations
4.2. Fetal Alterations
4.3. Young Offspring Alterations
4.4. Adult Offspring Alterations
4.4.1. Brain
4.4.2. Liver
4.4.3. Kidney
4.4.4. Cardiovascular System
4.4.5. Adipose Tissue
5. Reprogramming the Adversities Encountered in Maternal Obesity
6. Resveratrol Safety Profiles
7. Maternal Resveratrol Administration in the Context of Maternal Adversities
8. Possible Beneficial Mechanisms of Maternal Resveratrol Administration in the Context of Maternal Obesity
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afshin, A.; Reitsma, M.B.; Murray, C.J.L. Health effects of overweight and obesity in 195 countries. N. Engl. J. Med. 2017, 377, 1496–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Obesity and Overweight: Media Center; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Johnson, C.L. Prevalence and trends in obesity among US adults 1999–2000. JAMA 2002, 288, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Kanagalingam, M.G.; Forouhi, N.G.; Greer, I.A.; Sattar, N. Changes in booking body mass index over a decade: Retrospective analysis from a Glasgow Maternity Hospital. BJOG 2005, 112, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Yaktine, K.M.; Editors, A.L. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Bann, D.; Cooper, R.; Wills, A.K.; Adams, J.; Kuh, D. NSHD scientific and data collection team. Socioeconomic position across life and body composition in early old age: Findings from a British birth cohort study. J. Epidemiol. Community Health. 2014, 68, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Galtier-Dereure, F.; Boegner, C.; Bringer, J. Obesity and pregnancy: Complications and cost. Am. J. Clin. Nutr. 2000, 71, 1242S–1248S. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Twinn, D.S.; Hjort, L.; Novakovic, B.; Ozanne, S.E.; Saffery, R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 2019, 62, 1789–1801. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Zheng, Q.; Ford, S.P.; Nathanielsz, P.W.; Ren, J. Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. J. Mol. Cell Cardiol. 2013, 55, 111–116. [Google Scholar] [CrossRef]
- Gaillard, R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur. J. Epidemiol. 2015, 30, 1141–1152. [Google Scholar] [CrossRef] [Green Version]
- Edlow, A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- Iozzo, P.; Holmes, M.; Schmidt, M.V.; Cirulli, F.; Guzzardi, M.A.; Berry, A.; Balsevich, G.; Andreassi, M.G.; Wesselink, J.J.; Liistro, T.; et al. Developmental ORIgins of healthy and unhealthy ageiNg: The role of maternal obesity-introduction to DORIAN. Obes. Facts 2014, 7, 130–151. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Chillarón, J.C.; Díaz, R.; Martínez, D.; Pentinat, T.; Ramón-Krauel, M.; Ribó, S. The role of nutrition on epigenetic modifications and their implications on health. Biochimie 2012, 94, 2242–2263. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.L.; Jima, D.; Sharp, G.C.; McCullough, L.E.; Park, S.S.; Gowdy, K.M.; Skaar, D.; Cowley, M.; Maguire, R.L.; Fuemmeler, B.; et al. Maternal pre-pregnancy obesity, offspring cord blood DNA methylation, and offspring cardiometabolic health in early childhood: An epigenome-wide association study. Epigenetics 2019, 14, 325–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogues, P.; Dos Santos, E.; Jammes, H.; Berveiller, P.; Arnould, L.; Vialard, F.; Dieudonné, M.N. Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clin. Epigenetics 2019, 11, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z.J.; Liang, Q.X.; Hou, Y.; Han, Z.M.; Schatten, H.; Sun, Q.Y.; Zhang, C.L. Maternal obesity and diabetes may cause DNA methylation alteration in the spermatozoa of offspring in mice. Reprod. Biol. Endocrinol. 2014, 12, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, O.; Goodrich, J.K.; Cullende, R.T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Zacarías, M.F.; Collado, M.C.; Gómez-Gallego, C.; Flinck, H.; Aittoniemi, J.; Isolauri, E. Pregestational overweight and obesity are associated with differences in gut microbiota composition and systemic inflammation in the third trimester. PLoS ONE 2018, 13, e0200305. [Google Scholar] [CrossRef] [Green Version]
- Santacruz, A. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1030. [Google Scholar] [CrossRef]
- Steegenga, W.T.; Mischke, M.; Lute, C.; Boekschoten, M.V.; Lendvai, A.; Pruis, M.G. Maternal exposure to a Western-style diet causes differences in intestinal microbiota composition and gene expression of suckling mouse pups. Mol. Nutr. Food Res. 2017, 61, 1. [Google Scholar] [CrossRef] [Green Version]
- Gohir, W.; Ratcliffe, E.M.; Sloboda, D.M. Of the bugs that shape us: Maternal obesity, the gut microbiome, and long-term disease risk. Pediatr. Res. 2015, 77, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Rusterholz, C.; Hahn, S.; Holzgreve, W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin. Immunopathol. 2007, 29, 151–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantham, P.; Aye, I.L.; Powell, T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aye, I.L.; Lager, S.; Ramirez, V.I.; Gaccioli, F.; Dudley, D.J.; Jansson, T. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol. Reprod. 2014, 90, 1–9. [Google Scholar] [CrossRef]
- Challier, J.C.; Basu, S.; Bintein, T.; Minium, J.; Hotmire, K.; Catalano, P.M. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008, 29, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Roberts, K.A.; Riley, S.C.; Reynolds, R.M.; Barr, S.; Evans, M.; Statham, A. Placental structure and inflammation in pregnancies associated with obesity. Placenta 2011, 32, 247–254. [Google Scholar] [CrossRef]
- Kim, D.W.; Young, S.L.; Grattan, D.R.; Jasoni, C.L. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol. Reprod. 2014, 90, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.J.; Du, M.; Nathanielsz, P.W.; Ford, S.P. Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta 2010, 31, 387–391. [Google Scholar] [CrossRef]
- Radaelli, T.; Uvena-Celebrezze, J.; Minium, J.; Huston-Presley, L.; Catalano, P.; Hauguel-de Mouzon, S. Maternal interleukin-6: Marker of fetal growth and adiposity. J. Soc. Gynecol. Investig. 2006, 13, 53–57. [Google Scholar] [CrossRef]
- Denison, F.C.; Roberts, K.A.; Barr, S.M.; Norman, J.E. Obesity, pregnancy, inflammation, and vascular function. Reproduction 2010, 140, 373–385. [Google Scholar] [CrossRef]
- Igosheva, N.; Abramov, A.Y.; Poston, L.; Eckert, J.J.; Fleming, T.P.; Duchen, M.R.; McConnell, J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS. ONE 2010, 5, e10074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, C.S.; Maloyan, A.; Myat, T.L. Maternal obesity alters brain derived neurotrophic factor (BDNF) signaling in the placenta in a sexually dimorphic manner. Placenta 2017, 49, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusco, S.; Spinelli, M.; Cocco, S.; Ripoli, C.; Mastrodonato, A.; Natale, F.; Rinaudo, M.; Livrizzi, G.; Grassi, C. Maternal insulin resistance multigenerationally impairs synaptic plasticity and memory via gametic mechanisms. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briana, D.D.; Malamitsi-Puchner, A. Developmental origins of adult health and disease: The metabolic role of BDNF from early life to adulthood. Metabolism 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Lewis, R.M.; Desoye, G. Placental lipid and fatty acid transfer in maternal overnutrition. Ann. Nutr. Metab. 2017, 70, 228–231. [Google Scholar] [CrossRef]
- Myatt, L.; Maloyan, A. Obesity and placental function. Semin. Reprod. 2016, 34, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Hayward, C.E.; Higgins, L.; Cowley, E.J.; Greenwood, S.L.; Mills, T.A.; Sibley, C.P. Chorionic plate arterial function is altered in maternal obesity. Placenta 2013, 34, 281–287. [Google Scholar] [CrossRef]
- Hayes, E.K.; Lechowicz, A.; Petrik, J.J.; Storozhuk, Y.; Paez-Parent, S.; Dai, Q. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: Role of altered development of the placental vasculature. PLoS. ONE 2012, 7, e33370. [Google Scholar] [CrossRef] [Green Version]
- Stachowiak, E.K.; Srinivasan, M.; Stachowiak, M.K.; Patel, M.S. Maternal obesity induced by a high fat diet causes altered cellular development in fetal brains suggestive of a predisposition of offspring to neurological disorders in later life. Metab. Brain Dis. 2013, 28, 721–725. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ma, H.; Tong, C.; Zhang, H.; Lawlis, G.B.; Li, Y.; Zang, M.; Ren, J.; Nijland, M.J.; Ford, S.P.; et al. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J. 2010, 24, 2066–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhlhausler, B.; Smith, S.R. Early-life origins of metabolic dysfunction: Role of the adipocyte. Trends Endocrinol. Metab. 2009, 2, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Borengasser, S.J.; Zhong, Y.; Kang, P.; Lindsey, F.; Ronis, M.J.; Badger, T.M. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 2013, 154, 4113–4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: A systematic review and meta-analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.Q.; Gaysinskaya, V.; Karatayev, O.; Leibowitz, S.F. Maternal high-fat diet and fetal programming: Increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J. Neurosci. 2008, 28, 12107–12119. [Google Scholar] [CrossRef] [Green Version]
- Britto, P.R.; Lye, S.J.; Proulx, K.; Yousafzai, A.K.; Matthews, S.G.; Vaivada, T.; Perez-Escamilla, R.; Rao, N.; Ip, P.; Fernald, L.C.H.; et al. Early Childhood Development Interventions Review Group, for the Lancet Early Childhood Development Series Steering Committee. Lancet 2017, 389, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Alfaradhi, M.Z.; Fernandez-Twinn, D.S.; Martin-Gronert, M.S.; Musial, B.; Fowden, A.; Ozanne, S.E. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R26–R34. [Google Scholar] [CrossRef]
- Henry, S.L.; Barzel, B.; Wood-Bradley, R.J.; Burke, S.L.; Head, G.A.; Armitage, J.A. Developmental origins of obesity-related hypertension. Clin. Exp. Pharmacol. Physiol. 2012, 39, 799–806. [Google Scholar] [CrossRef]
- Glastras, S.J.; Tsang, M.; Teh, R.; Chen, H.; McGrath, R.T.; Zaky, A.A.; Pollock, C.A.; Saad, S. Maternal obesity promotes diabetic nephropathy in rodent offspring. Sci. Rep. 2016, 6, 27769. [Google Scholar] [CrossRef]
- Samuelsson, A.M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.; Piersma, A.H.; Ozanne, S.E.; Twinn, D.F.; Remacle, C.; et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: A novel murine model of developmental programming. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, M.; Ross, M.G. Fetal programming of adipose tissue: Effects of intrauterine growth restriction and maternal obesity/high-fat diet. Semin. Reprod. Med. 2011, 29, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.J.; Chen, H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int. J. Obes. 2009, 33, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tozuka, Y.; Wada, E.; Wada, K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009, 23, 1920–1934. [Google Scholar] [CrossRef]
- Tozuka, Y.; Kumon, M.; Wada, E. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int. 2010, 57, 235–247. [Google Scholar] [CrossRef]
- Page, K.C.; Jones, E.K.; Anday, E.K. Maternal and postweaning high-fat diets disturb hippocampal gene expression, learning, and memory function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R527–R537. [Google Scholar] [CrossRef] [Green Version]
- Shankar, K.; Kang, P.; Harrell, A.; Zhong, Y.; Marecki, J.C.; Ronis, M.J.J.; Badger, T.M. Maternal overweight programs insulin and adiponectin signaling in the offspring. Endocrinology 2010, 151, 2577–2589. [Google Scholar] [CrossRef]
- Armitage, J.A.; Lakasing, L.; Taylor, P.D.; Balachandran, A.A.; Jensen, R.I.; Dekou, V.; Ashton, N.; Nyengaard, J.R.; Poston, L. Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J. Physiol. 2005, 565, 171–184. [Google Scholar] [CrossRef]
- Mamun, A.A.; O’Callaghan, M.; Callaway, L.; Williams, G.; Najman, J.; Lawlor, D.A. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: Evidence from a birth cohort study. Circulation 2009, 119, 1720–1727. [Google Scholar] [CrossRef]
- Reynolds, R.M.; Allan, K.M.; Raja, E.A.; Bhattacharya, S.; McNeill, G.; Hannaford, P.C.; Sarwar, N.; Lee, A.J.; Bhattacharya, S.; Norman, J.E. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: Follow-up of 1 323 275 person years. BMJ 2013, 347, f4539. [Google Scholar] [CrossRef] [Green Version]
- Murabayashi, N.; Sugiyama, T.; Zhang, L.; Kamimoto, Y.; Umekawa, T.; Ma, N. Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Lecoutre, S.; Breton, C. The cellularity of offspring’s adipose tissue is programmed by maternal nutritional manipulations. Adipocyte 2014, 3, 256–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, M.; Han, G.; Ross, M.G. Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite 2016, 99, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N. Developmental programming of adult disease: Reprogramming by melatonin? Int. J. Mol. Sci. 2017, 18, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.L.; Hsu, C.N. Developmental programming of the metabolic syndrome: Can we reprogram with resveratrol? Int. J. Mol. Sci. 2018, 19, 2584. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.N.; Tain, Y.L. The good, the bad, and the ugly of pregnancy nutrients and developmental programming of adult disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef] [Green Version]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer. Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [Green Version]
- la Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharm. 2010, 49, 449–454. [Google Scholar] [CrossRef]
- Williams, L.D.; Burdock, G.A.; Edwards, J.A.; Beck, M.; Bausch, J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 2009, 47, 2170–2182. [Google Scholar] [CrossRef]
- Madhyastha, S.; Sekhar, S.; Rao, G. Resveratrol improves postnatal hippocampal neurogenesis and brain derived neurotrophic factor in prenatally stressed rats. Int. J. Dev. Neurosci. 2013, 31, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zheng, A.; Xu, J.; Li, H.; Liu, J.; Peng, Y.; Long, J.; Zou, X.; Li, Y.; Chen, C.; et al. AMPK activation prevents prenatal stress-induced cognitive impairment: Modulation of mitochondrial content and oxidative stress. Free Radic. Biol. Med. 2014, 75, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Care, A.S.; Sung, M.M.; Panahi, S.; Gragasin, F.S.; Dyck, J.R.; Davidge, S.T.; Bourque, S.L. Perinatal resveratrol supplementation to spontaneously hypertensive rat dams mitigates the development of hypertension in adult offspring. Hypertension 2016, 67, 1038–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.E.; Lin, Y.J.; Lin, I.C.; Yu, H.R.; Sheen, J.M.; Tsai, C.C.; Huang, L.T.; Tain, Y.L. Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019, 70, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Kang, Y.; Fan, Y.; Chen, Q. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr. Connect. 2017, 6, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Darby, J.R.T.; Mohd Dollah, M.H.B.; Regnault, T.R.H.; Williams, M.T.; Morrison, J.L. Systematic review: Impact of resveratrol exposure during pregnancy on maternal and fetal outcomes in animal models of human pregnancy complications-Are we ready for the clinic? Pharmacol. Res. 2019, 144, 264–278. [Google Scholar] [CrossRef]
- Oliveira, A.L.B.; Monteiro, V.V.S.; Navegantes-Lima, K.C.; Reis, J.F.; Gomes, R.S.; Rodrigues, D.V.S. Resveratrol role in autoimmune disease-a mini-review. Nutrients 2017, 9, 1306. [Google Scholar] [CrossRef] [Green Version]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors. 2017, 44, 36–49. [Google Scholar] [CrossRef]
- Bourque, S.L.; Dolinsky, V.W.; Dyck, J.R.; Davidge, S.T. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta 2012, 33, 449–452. [Google Scholar] [CrossRef]
- Kim, O.Y.; Chung, J.Y.; Song, J. Effect of resveratrol on adipokines and myokines involved in fat browning: Perspectives in healthy weight against obesity. Pharmacol. Res. 2019, 148, 104411. [Google Scholar] [CrossRef]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamkova, K.; Yi, Y.J.; Petr, J.; Zalmanova, T.; Hoskova, K.; Jelinkova, P. SIRT1-dependent modulation of methylation and acetylation of histone H3 on lysine 9 (H3K9) in the zygotic pronuclei improves porcine embryo development. J. Anim. Sci. Biotechnol. 2017, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhees, K.; van Schooten, F.J.; Moonen, E.J.; Maas, L.M.; van Waalwijk van Doorn-Khosrovani, S.B.; Godschalk, R.W. Maternal intake of quercetin during gestation alters ex vivo benzo[a]pyrene metabolism and DNA adduct formation in adult offspring. Mutagenesis 2012, 27, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.M.; Yu, H.R.; Tain, Y.L.; Tsai, W.L.; Tiao, M.M.; Lin, I.C.; Tsai, C.C.; Lin, Y.J.; Huang, L.T. Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: A multiple-organ study. Sci. Rep. 2018, 8, 5607. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Carpene, C.; Mercader, J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef]
- Dal-Pan, A.; Blanc, S.; Aujard, F. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol. 2010, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Xie, H.; Lim, B.; Lodish, H.F. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 2009, 58, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheydari, Z.; Nabavi, S.M. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta. 2016, 1860, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.; Haghiac, M.; Minium, J.; Skomorovska-Prokvolit, Y.; Calabuig-Navarro, V.; O’Tierney-Ginn, P. Activation of AMPK in Human Placental Explants Impairs Mitochondrial Function and Cellular Metabolism. Reprod. Sci. 2019, 26, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xie, K.; Yang, X.; Gu, J.; Ge, L.; Wang, X.; Wang, Z. Resveratrol reverses the effects of chronic unpredictable mild stress on behavior, serum corticosterone levels and BDNF expression in rats. Behav. Brain Res. 2014, 264, 9–16. [Google Scholar] [CrossRef]
- Kodomari, I.; Wada, E.; Nakamura, S.; Wada, K. Maternal supply of BDNF to mouse fetal brain through the placenta. Neurochem. Int. 2009, 54, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Zheng, S.; Feng, Q.; Cheng, J.; Zheng, J. Maternal resveratrol consumption and its programming effects on metabolic health in offspring mechanisms and potential implications. Biosci. Rep. 2018, 38, BSR20171741. [Google Scholar] [CrossRef] [Green Version]
- Roberts, V.H.; Pound, L.D.; Thorn, S.R.; Gillingham, M.B.; Thornburg, K.L.; Friedman, J.E.; Frias, A.E.; Grove, K.L. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014, 28, 2466–2477. [Google Scholar] [CrossRef] [Green Version]
- Pound, L.D.; Comstock, S.M.; Grove, K.L. Consumption of a Western-style diet during pregnancy impairs offspring islet vascularization in a Japanese macaque model. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E115–E123. [Google Scholar] [CrossRef]
- Zou, T.; Chen, D.; Yang, Q.; Wang, B.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef]
- Ros, P.; Díaz, F.; Freire-Regatillo, A.; Argente-Arizón, P.; Barrios, V.; Argente, J.; Chowen, J.A. Resveratrol intake during pregnancy and lactation modulates the early metabolic effects of maternal nutrition differently in male and female offspring. Endocrinology 2018, 159, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Malvasi, A.; Kosmas, I.; Mynbaev, O.A.; Sparic, R.; Gustapane, S.; Guido, M. Can trans resveratrol plus d-chiro-inositol and myo-inositol improve maternal metabolic profile in overweight pregnant patients? Clin. Ther. 2017, 168, e240–e247. [Google Scholar] [CrossRef]
- Hanson, M.; Barker, M.; Dodd, J.M.; Kumanyika, S.; Norris, S.; Steegers, E.; Stephenson, J.; Thangaratinam, S.; Yang, H. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017, 5, 65–76. [Google Scholar] [CrossRef]
| Gender/Species | Diet Module Obesity | Dose and Period of Resveratrol Supplementation | Maternal Obesity/Over-Weight Offspring Obesity Group Size | Age at Evaluation Group Size | Major Beneficial Findings in Offspring | Reference |
|---|---|---|---|---|---|---|
| Japanese macaques | Maternal Western-style (36% fat) diet | 0.37% w/w resveratrol in diet from 3 months before breeding and until gestational day 130 | +/−N = 6 | Gestational day 130 | Restored the loss of fetal islet vascularization | [101] |
| Japanese macaques | Maternal Western-style (36% fat) diet | 0.37% w/w resveratrol in diet from 3 months before breeding and until gestational day 130 | +/−N = 6 | Gestational day 130 | 30% maternal weight loss, increased uterine artery blood flow, decreased placenta inflammation, reduced fetal liver triglyceride deposition | [100] |
| Male and female Wistar rats | Maternal high-fat diet (61.6%) | Resveratrol (50 mg/L) in drinking water during pregnancy and lactation | +/+N = 4–6 | 3 weeks | Attenuated hyperglycemia, obesity and hyperlipidemia. Resveratrol reduced body weight, leptin, visceral adipose tissue, and subcutaneous adipose tissue, with females being more affected | [102] |
| Japanese macaques | Maternal Western-style (36% fat) diet | 0.37% w/w resveratrol in diet from 3 months before breeding and until gestational day 130 | +/− N = 6 | Gestational day 130 N = 5–10 | Resveratrol stimulated placental DHA uptake activity, AMPK activation, and transporter expression | [95] |
| Male C57BL/6 J mice | Maternal plus postnatal high-fat diet for 11 weeks | 0.2% w/w (~200 mg/kg/day) resveratrol in diet during pregnancy and lactation | +/+ N = 10 | 14 weeks | Promotes beige adipocyte development in offspring white adipose tissue. Protects offspring against high-fat diet-induced obesity | [101] |
| Italy | Human | Resveratrol 80mg/day | +/N 110 pregnant women | 30 days and 60 days | Supplementation of resveratrol to DCI/MI improves mother glucose and lipid control | [103] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.-H.; Chen, Y.-C.; Sheen, J.-M.; Huang, L.-T. Maternal Obesity Programs Offspring Development and Resveratrol Potentially Reprograms the Effects of Maternal Obesity. Int. J. Environ. Res. Public Health 2020, 17, 1610. https://doi.org/10.3390/ijerph17051610
Hsu M-H, Chen Y-C, Sheen J-M, Huang L-T. Maternal Obesity Programs Offspring Development and Resveratrol Potentially Reprograms the Effects of Maternal Obesity. International Journal of Environmental Research and Public Health. 2020; 17(5):1610. https://doi.org/10.3390/ijerph17051610
Chicago/Turabian StyleHsu, Mei-Hsin, Yu-Chieh Chen, Jiunn-Ming Sheen, and Li-Tung Huang. 2020. "Maternal Obesity Programs Offspring Development and Resveratrol Potentially Reprograms the Effects of Maternal Obesity" International Journal of Environmental Research and Public Health 17, no. 5: 1610. https://doi.org/10.3390/ijerph17051610
APA StyleHsu, M. -H., Chen, Y. -C., Sheen, J. -M., & Huang, L. -T. (2020). Maternal Obesity Programs Offspring Development and Resveratrol Potentially Reprograms the Effects of Maternal Obesity. International Journal of Environmental Research and Public Health, 17(5), 1610. https://doi.org/10.3390/ijerph17051610






