Decreased Risk of Renal Calculi in Patients Receiving Androgen Deprivation Therapy for Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Design
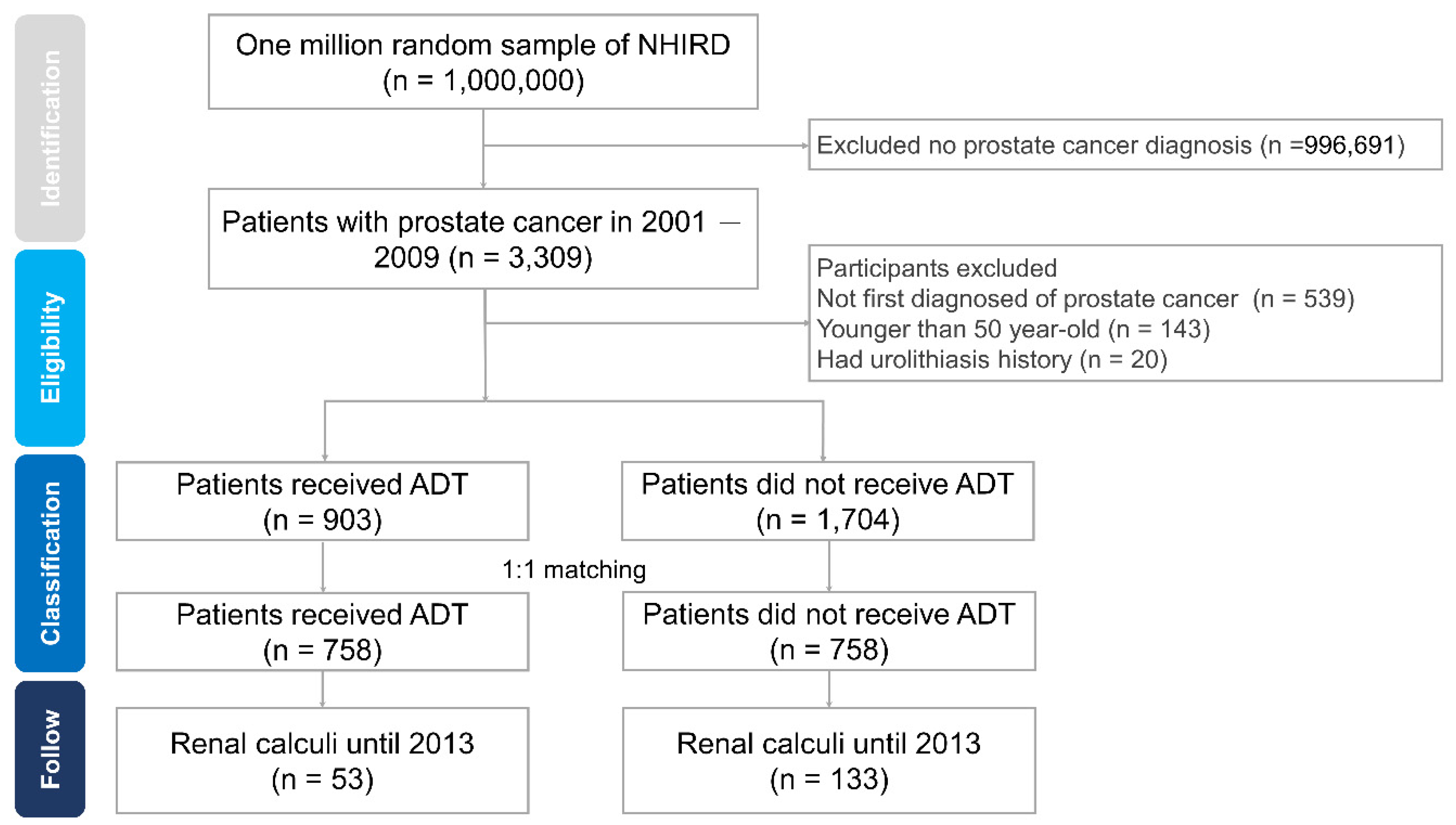
2.2. Study Population
2.3. Study Outcomes and Covariates
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Di Santi, A.; Cernera, G.; Rossi, V.; Abbondanza, C.; Moncharmont, B.; Sinisi, A.A.; et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget 2015, 7, 193–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragon-Ching, J.B.; Williams, K.M.; Gulley, J. Impact of androgen-deprivation therapy on the immune system: Implications for combination therapy of prostate cancer. Front. Biosci. 2007, 12, 4957–4971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalina, J.L.; Neilson, D.S.; Comber, A.P.; Rauw, J.; Alexander, A.; Vergidis, J.; Lum, J.J. Immune Modulation by Androgen Deprivation and Radiation Therapy: Implications for Prostate Cancer Immunotherapy. Cancers 2017, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-M.; Lin, C.-Y.; Chuang, H.-C.; Hsu, R.-J. No increased risk of psoriasis in patients receiving androgen deprivation therapy for prostate cancer: A 17-year population-based study. Ther. Clin. Risk Manag. 2018, 14, 1831–1837. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-M.; Liu, D.-W.; Chuang, H.-C.; Wu, C.-T.; Lin, C.-Y.; Hsu, R.-J. Androgen deprivation therapy and the risk of tenosynovitis in prostate cancer patients. Int. Urol. Nephrol. 2019, 51, 1113–1119. [Google Scholar] [CrossRef]
- Liu, J.-M.; Yu, C.-P.; Chuang, H.-C.; Wu, C.-T.; Hsu, R.-J. Androgen deprivation therapy for prostate cancer and the risk of autoimmune diseases. Prostate Cancer Prostatic Dis. 2019, 22, 475–482. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Liao, B.; Luo, D.; Wang, K.-J.; Li, H.; Zeng, G. Epidemiology of urolithiasis in Asia. Asian J. Urol. 2018, 5, 205–214. [Google Scholar] [CrossRef]
- Raheem, O.A.; Khandwala, Y.S.; Sur, R.L.; Ghani, K.R.; Denstedt, J.D. Burden of Urolithiasis: Trends in Prevalence, Treatments, and Costs. Eur. Urol. Focus 2017, 3, 18–26. [Google Scholar] [CrossRef]
- Trinchieri, A. Epidemiology of urolithiasis: An update. Clin. Cases Miner. Bone Metab. 2008, 5, 101–106. [Google Scholar]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.-G. Kidney stones. Nat. Rev. Dis. Prim. 2016, 2, 16009. [Google Scholar] [CrossRef] [PubMed]
- Moe, O.W. Kidney stones: Pathophysiology and medical management. Lancet 2006, 367, 333–344. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Lin, C.-L.; Chang, Y.-J.; Hsu, W.-H.; Wang, I.-K.; Chang, C.-T.; Chang, C.-H.; Lin, M.-C.; Kao, C.-H. Association between Kidney Stones and Risk of Stroke. Medicine 2016, 95, e2847. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-P.; Lin, C.-C.; Lin, H.-C.; Chen, Y.-H.; Yu, H.-J. Association between Schizophrenia and Urinary Calculi: A Population-Based Case-Control Study. PLoS ONE 2013, 8, e56942. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-Y.; Zhou, T.; Gao, X.; Xu, C.; Sun, Y.; Peng, Y.; Chang, Z.; Zhang, Y.; Jiang, J.; Wang, L.; et al. Testosterone and Androgen Receptor in Human Nephrolithiasis. J. Urol. 2010, 184, 2360–2363. [Google Scholar] [CrossRef]
- Liang, L.; Li, L.; Tian, J.; Lee, S.O.; Dang, Q.; Huang, C.-K.; Yeh, S.; Erturk, E.; Bushinsky, D.; Chang, L.S.; et al. Androgen Receptor Enhances Kidney Stone-CaOx Crystal Formation via Modulation of Oxalate Biosynthesis & Oxidative Stress. Mol. Endocrinol. 2014, 28, 1291–1303. [Google Scholar]
- Naghii, M.R.; Babaei, M.; Hedayati, M. Androgens Involvement in the Pathogenesis of Renal Stones Formation. PLoS ONE 2014, 9, e93790. [Google Scholar] [CrossRef]
- Gupta, K.; Gill, G.S.; Mahajan, R. Possible role of elevated serum testosterone in pathogenesis of renal stone formation. Int. J. Appl. Basic Med Res. 2016, 6, 241–244. [Google Scholar] [CrossRef]
- Arrabal-Polo, M.A.; Cano-García, M.D.C.; Arrabal-Martín, M. Βones, stones and androgen deprivation therapy. Hormones 2015, 14, 668–669. [Google Scholar] [CrossRef] [Green Version]
- Convalía, E.J.D.; Cano-García, M.D.C.; Miján-Ortiz, J.L.; Arrabal-Martín, M.; Arrabal-Polo, M.A.; Cózar-Olmo, J.M. Androgen deprivation therapy in prostate cancer and risk of developing renal calculi: Results of a case–control study. Med. Clín. 2017, 148, 495–497. [Google Scholar] [CrossRef]
- Shakhssalim, N.; Gilani, K.R.; Parvin, M.; Torbati, P.M.; Kashi, A.H.; Azadvari, M.; Golestan, B.; Basiri, A. An assessment of parathyroid hormone, calcitonin, 1,25 (OH)2 vitamin D3, estradiol and testosterone in men with active calcium stone disease and evaluation of its biochemical risk factors. Urol. Res. 2010, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Changtong, C.; Peerapen, P.; Khamchun, S.; Fong-Ngern, K.; Chutipongtanate, S.; Thongboonkerd, V. In vitro evidence of the promoting effect of testosterone in kidney stone disease: A proteomics approach and functional validation. J. Proteom. 2016, 144, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-T.; Yang, Y.-H.; Chen, P.-C.; Chen, M.-F.; Chen, W.-C. Androgen deprivation increases the risk of fracture in prostate cancer patients: A population-based study in Chinese patients. Osteoporos. Int. 2015, 26, 2281–2290. [Google Scholar] [CrossRef]
- Lemann, J.; Worcester, E.M.; Gray, R.W. Hypercalciuria and Stones. Am. J. Kidney Dis. 1991, 17, 386–391. [Google Scholar] [CrossRef]
- Sakhaee, K.; Maalouf, N.M.; Kumar, R.; Pasch, A.; Moe, O.W. Nephrolithiasis-associated bone disease: Pathogenesis and treatment options. Kidney Int. 2010, 79, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Ramaswamy, K.; Shah, O. Metabolic syndrome and nephrolithiasis. Transl. Androl. Urol. 2014, 3, 285–295. [Google Scholar]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005, 68, 1230–1235. [Google Scholar] [CrossRef] [Green Version]
- Nerli, R.; Jali, M.; Guntaka, A.K.; Patne, P.; Patil, S.; Hiremath, M.B. Type 2 diabetes mellitus and renal stones. Adv. Biomed. Res. 2015, 4, 180. [Google Scholar]
- Daudon, M.; Traxer, O.; Conort, P.; Lacour, B.; Jungers, P. Type 2 Diabetes Increases the Risk for Uric Acid Stones. J. Am. Soc. Nephrol. 2006, 17, 2026–2033. [Google Scholar] [CrossRef] [Green Version]
- Kovshilovskaya, B.; Chi, T.; Miller, J.; Stoller, M.L. Systemic implications of urinary stone disease. Transl. Androl. Urol. 2012, 1, 89–96. [Google Scholar] [PubMed]

| Characteristics | ADT Patients, n (%) | Non-ADT Patients, n (%) | p Value |
|---|---|---|---|
| No. of cases | 758 | 758 | |
| Gender | |||
| Male | 758 (100.0%) | 758 (100.0%) | |
| Age | 0.994 | ||
| 50–59 | 33 (4.4%) | 35 (4.6%) | |
| 60–69 | 143 (18.9%) | 141 (18.6%) | |
| 70–79 | 352 (46.4%) | 351 (46.3%) | |
| ≥80 | 230 (30.3%) | 231 (30.5%) | |
| Insured region | <0.001 * | ||
| Northern Taiwan | 395 (52.1%) | 470 (62.0%) | |
| Middle Taiwan | 110 (14.5%) | 101 (13.3%) | |
| Southern Taiwan | 218 (28.8%) | 171 (22.6%) | |
| Other (Eastern Taiwan | 35 (4.6%) | 16 (2.1%) | |
| and outlying islands) | |||
| Urbanization | <0.05 * | ||
| 1 (highest) | 341 (45.0%) | 393 (51.8%) | |
| 2 | 151 (19.9%) | 161 (21.2%) | |
| 3 | 170 (22.4%) | 122 (16.1%) | |
| 4 (lowest) | 96 (12.7%) | 82 (10.9%) | |
| Insured amount NTD a | 0.681 | ||
| <20,000 | 679 (89.6%) | 669 (88.3%) | |
| 20,000–39,999 | 30 (4.0%) | 39 (5.1%) | |
| 40,000–59,999 | 24 (3.2%) | 22 (2.9%) | |
| ≥60,000 | 25 (3.2%) | 28 (3.7%) | |
| Comorbidity | |||
| Diabetes Mellitus | 232 (30.6%) | 211 (27.8%) | 0.236 |
| Hypertension | 477 (62.9%) | 443 (58.4%) | 0.074 |
| Hyperlipidemia | 187 (24.7%) | 194 (25.6%) | 0.679 |
| Coronary heart disease | 278 (36.7%) | 273 (36%) | 0.789 |
| Cerebral vascular accident | 219 (28.9%) | 196 (25.9%) | 0.185 |
| COPD | 279 (36.8%) | 227 (29.9%) | <0.05 * |
| Alcoholism | 4 (0.5%) | 3 (0.4%) | 0.705 |
| Obesity | 2 (0.3%) | 4 (0.5%) | 0.413 |
| Tobacco use disorder | 245 (32.3%) | 201 (26.5%) | <0.05 * |
| Number of Patients | ||
|---|---|---|
| ADT Patients n = 758 | Non-ADT Patients n = 758 | |
| Renal calculi | 53 (7.0%) | 133 (17.5%) |
| Without renal calculi | 705 (93.0%) | 625 (82.5%) |
| Crude HR | 0.37 (0.27 to 0.52) ** | |
| Crude HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|
| Prostate cancer | ||
| non-ADT | 1 | 1 |
| ADT | 0.37 (0.27 to 0.52) ** | 0.38 (0.28 to 0.53) ** |
| Age | ||
| 50–59 | 1 | 1 |
| 60–69 | 1.84 (0.84 to 4.04) | 1.96 (0.87 to 4.39) |
| 70–79 | 1.14 (0.53 to 2.46) | 1.18 (0.52 to 2.67) |
| ≥80 | 0.87 (0.39 to 1.93) | 0.81 (0.35 to 1.89) |
| Insured region | ||
| Northern Taiwan | 1 | 1 |
| Middle Taiwan | 1.18 (0.79 to 1.76) | 1.17 (0.77 to 1.77) |
| Southern Taiwan | 0.72 (0.50 to 1.05) | 0.80 (0.53 to 1.19) |
| Other (Eastern Taiwan | 1.03 (0.48 to 2.21) | 1.56 (0.70 to 3.46) |
| and outlying islands) | ||
| Urbanization | ||
| 1 (highest) | 1 | 1 |
| 2 | 0.87 (0.59 to 1.27) | 0.85 (0.58 to 1.26) |
| 3 | 0.95 (0.65 to 1.39) | 1.10 (0.74 to 1.65) |
| 4 (lowest) | 0.71 (0.43 to 1.20) | 0.81 (0.47 to 1.43) |
| Insured amount NTD a | ||
| <20,000 | 1 | 1 |
| 20,000–39,999 | 0.80 (0.38 to 1.71) | 0.53 (0.24 to 1.15) |
| 40,000–59,999 | 1.26 (0.59 to 2.67) | 0.94 (0.42 to 2.08) |
| ≥60,000 | 0.75 (0.31 to 1.82) | 0.54 (0.22 to 1.34) |
| Comorbidity disease | ||
| Diabetes Mellitus | 0.56 (0.39 to 0.80) * | 0.64 (0.44 to 0.95) * |
| Hypertension | 0.72 (0.54 to 0.96) * | 1.02 (0.73 to 1.41) |
| Hyperlipidemia | 0.71 (0.49 to 1.01) | 0.76 (0.51 to 1.11) |
| Coronary heart disease | 0.78 (0.57 to 1.07) | 1.04 (0.74 to 1.46) |
| Cerebral vascular accident | 0.56 (0.39 to 0.82) * | 0.67 (0.45 to 0.99) * |
| COPD | 0.55 (0.39 to 0.78) * | 0.93 (0.52 to 1.65) |
| Alcoholism | 0.05 (0 to 469.73) | NA |
| Obesity | 0.05 (0 to 977.5) | NA |
| Tobacco use disorder | 0.51 (0.35 to 0.74) ** | 0.66 (0.36 to 1.23) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Liu, J.-M.; Wu, C.-T.; Hsu, R.-J.; Hsu, W.-L. Decreased Risk of Renal Calculi in Patients Receiving Androgen Deprivation Therapy for Prostate Cancer. Int. J. Environ. Res. Public Health 2020, 17, 1762. https://doi.org/10.3390/ijerph17051762
Lin C-Y, Liu J-M, Wu C-T, Hsu R-J, Hsu W-L. Decreased Risk of Renal Calculi in Patients Receiving Androgen Deprivation Therapy for Prostate Cancer. International Journal of Environmental Research and Public Health. 2020; 17(5):1762. https://doi.org/10.3390/ijerph17051762
Chicago/Turabian StyleLin, Chien-Yu, Jui-Ming Liu, Chun-Te Wu, Ren-Jun Hsu, and Wen-Lin Hsu. 2020. "Decreased Risk of Renal Calculi in Patients Receiving Androgen Deprivation Therapy for Prostate Cancer" International Journal of Environmental Research and Public Health 17, no. 5: 1762. https://doi.org/10.3390/ijerph17051762






