Urinary Mercapturic Acids to Assess Exposure to Benzene and Other Volatile Organic Compounds in Coke Oven Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Mercapturic Acid Analysis
2.3. Data Elaboration and Statistical Analysis
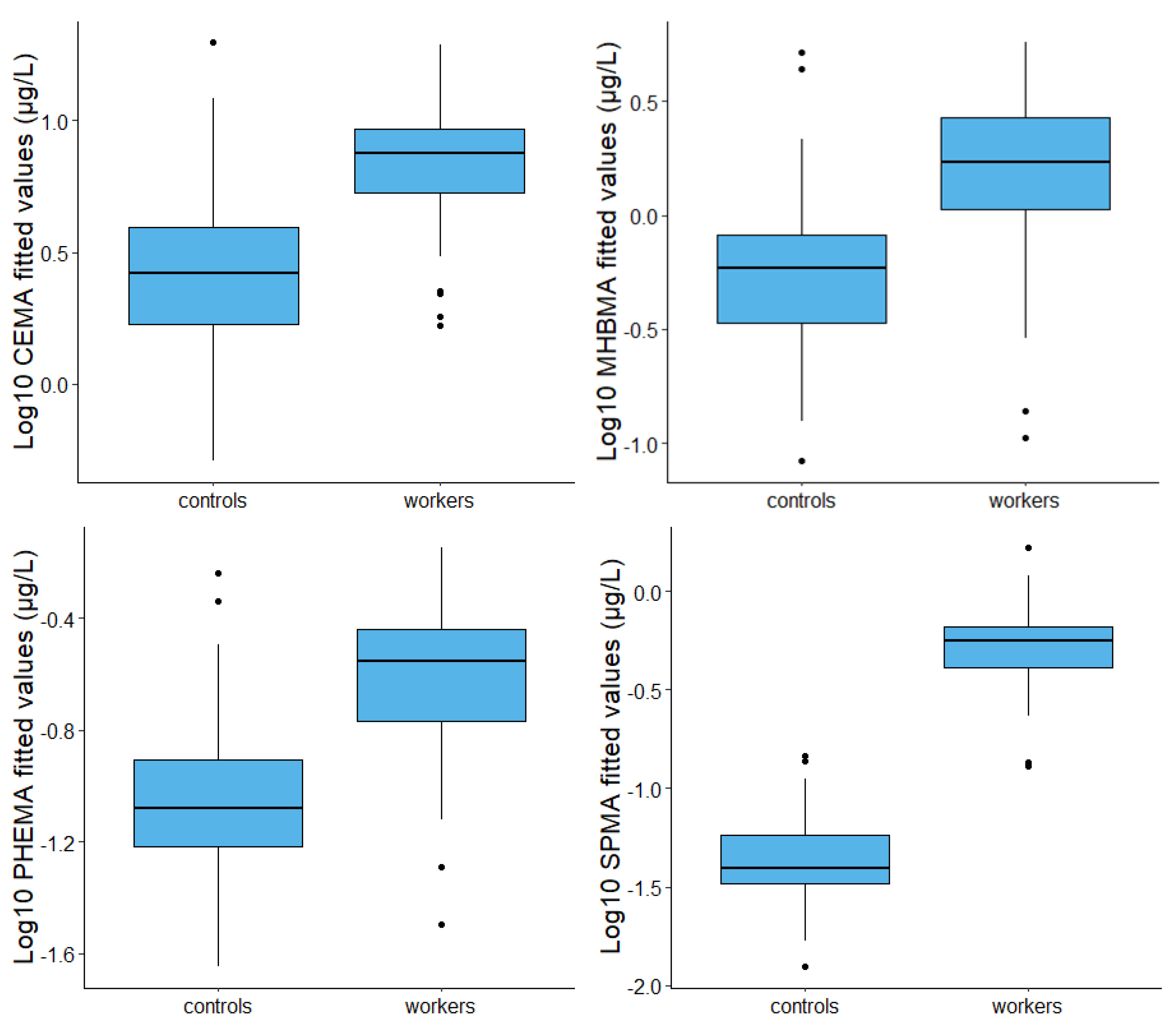
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- EU. Directive 2004/42/CE of the European Parliament and of the Council of 21 April 2004 on the limitation of emissions of volatile organic compounds due to the use of organic solvents in certain paints and varnishes and vehicle refinishing products and amending Directive 1999/13/EC. Off. J. Eur. Union L 2004, 143, 87–96. [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; Chemical Agents and Related Occupations; IARC Scientific Publications: Lyon, France, 2012; Volume 100 Pt F. [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; Benzene; IARC Scientific Publications: Lyon, France, 2018; Volume 120. [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; Some Industrial Chemicals; IARC Scientific Publications: Lyon, France, 1994; Volume 60. [Google Scholar]
- International Agency for Research on Cancer. Carcinogenicity of quinoline, styrene, and styrene-7,8-oxide. Lancet Oncol 2018, 19, 728–729. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide; IARC Scientific Publications: Lyon, France, 1999; Volume 71. [Google Scholar]
- ATSDR. Toxicological Profile for Benzene. 2007. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp3.pdf (accessed on 29 February 2020).
- ATSDR. Toxicological Profile for Styrene. 2010. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp53.pdf (accessed on 29 February 2020).
- ATSDR. Toxicological Profile for 1,3-Butadiene. 2012. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp28.pdf (accessed on 29 February 2020).
- ATSDR. Toxicological Profile for Acrylonitrile. 1990. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp125.pdf (accessed on 29 February 2020).
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; Personal habits and indoor combustions; IARC Scientific Publications: Lyon, France, 2012; Volume 100 Pt E. [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; Tobacco Smoke and Involuntary Smoking; IARC Scientific Publications: Lyon, France, 2004; Volume 83. [Google Scholar]
- De Rooij, B.M.; Commandeur, J.N.M.; Vermeulen, N.P.E. Mercapturic acids as biomarkers of exposure to electrophilic chemicals:applications to environmental and industrial chemicals. Biomarkers 1998, 3, 239–303. [Google Scholar]
- Parkinson, A.; Ogilvie, W. Biotransformation of Xenobiotics. In Essentials of Toxicology, 2nd ed.; Hill, M.G., Ed.; McGraw-Hill Education: New York, NY, USA, 2010. [Google Scholar]
- ACGIH. Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2017. [Google Scholar]
- DFG. List of MAK and BAT Values. 2018. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/9783527818402 (accessed on 20 January 2020).
- ECHA. Opinion on Scientific Evaluation of Occupational Exposure Limits for Benzene. 2018. Available online: https://echa.europa.eu/documents/10162/13641/benzene_opinion_en.pdf/4fec9aac-9ed5-2aae-7b70-5226705358c7 (accessed on 20 January 2020).
- Zając, J.; Gomółka, E.; Maziarz, B.; Szot, W. Occupational Exposure to Polycyclic Aromatic Hydrocarbons in Polish Coke Plant Workers. Ann. Occup. Hyg. 2016, 60, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Campo, L.; Vimercati, L.; Carrus, A.; Bisceglia, L.; Pesatori, A.C.; Bertazzi, P.A.; Assennato, G.; Fustinoni, S. Environmental and biological monitoring of PAHs exposure in coke-oven workers at the Taranto plant compared to two groups from the general population of Apulia, Italy. Med. Lav. 2012, 103, 347–360. [Google Scholar] [PubMed]
- Dehghani, F.; Omidi, F.; Heravizadeh, O.; Barati Chamgordani, S.; Gharibi, V.; Sotoudeh Manesh, A. Occupational health risk assessment of volatile organic compounds emitted from the coke production unit of a steel plant. Int J. Occup. Saf. Ergon. 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Deng, H.; Bai, Z.; Kong, S.; Wang, X.; Hao, J.; Han, X.; Ning, P. Emission and profile characteristic of volatile organic compounds emitted from coke production, iron smelt, heating station and power plant in Liaoning Province, China. Sci. Total Environ. 2015, 515–516, 101–108. [Google Scholar] [CrossRef]
- Bieniek, G.; Łusiak, A. Occupational exposure to aromatic hydrocarbons and polycyclic aromatic hydrocarbons at a coke plant. Ann. Occup. Hyg. 2012, 56, 796–807. [Google Scholar]
- Chang, E.E.; Wei-Chi, W.; Li-Xuan, Z.; Hung-Lung, C. Health risk assessment of exposure to selected volatile organic compounds emitted from an integrated iron and steel plant. Inhal. Toxicol. 2010, 22 (Suppl. 2), 117–125. [Google Scholar] [CrossRef]
- Ciaparra, D.; Aries, E.; Booth, M.; Anderson, D.R.; Almeida, S.M.; Harrad, S. Characterisation of volatile organic compounds and polycyclic aromatic hydrocarbons in the ambient air of steelworks. Atmos. Environ. 2009, 43, 2070–2079. [Google Scholar] [CrossRef]
- Tsai, J.H.; Lin, K.H.; Chen, C.Y.; Lai, N.; Ma, S.Y.; Chiang, H.L. Volatile organic compound constituents from an integrated iron and steel facility. J. Hazard. Mater. 2008, 157, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, G.; Kurkiewicz, S.; Wilczok, T.; Klimek, K.; Swiatkowska, L.; Lusiak, A. Occupational exposure to aromatic hydrocarbons at a coke plant: Part II. Exposure assessment of volatile organic compounds. J. Occup. Health 2004, 46, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, G. Aromatic and polycyclic hydrocarbons in air and their urinary metabolites in coke plant workers. Am. J. Ind. Med. 1998, 34, 445–454. [Google Scholar] [CrossRef]
- Lovreglio, P.; De Palma, G.; Barbieri, A.; Andreoli, R.; Drago, I.; Greco, L.; Gallo, E.; Diomede, L.; Scaramuzzo, P.; Ricossa, M.C.; et al. Biological monitoring of exposure to low concentrations of benzene in workers at a metallurgical coke production plant: New insights into S-phenylmercapturic acid and urinary benzene. Biomarkers 2018, 23, 70–77. [Google Scholar] [CrossRef]
- Hotz, P.; Carbonnelle, P.; Haufroid, V.; Tschopp, A.; Buchet, J.P.; Lauwerys, R. Biological monitoring of vehicle mechanics and other workers exposed to low concentrations of benzene. Int. Arch. Occup. Environ. Health 1997, 70, 29–40. [Google Scholar] [CrossRef]
- Kivistö, H.; Pekari, K.; Peltonen, K.; Svinhufvud, J.; Veidebaum, T.; Sorsa, M.; Aitio, A. Biological monitoring of exposure to benzene in the production of benzene and in a cokery. Sci. Total Environ. 1997, 199, 49–63. [Google Scholar] [CrossRef]
- Fan, R.; Li, J.; Chen, L.; Xu, Z.; He, D.; Zhou, Y.; Zhu, Y.; Wei, F. Biomass fuels and coke plants are important sources of human exposure to polycyclic aromatic hydrocarbons, benzene and toluene. Environ. Res. 2014, 135, 1–8. [Google Scholar] [CrossRef]
- Colman, R.; Coleman, A. Unexpected cause of raised benzene absorption in coke oven by-product workers. Occup. Med. (Lond.) 2006, 56, 269–271. [Google Scholar] [CrossRef][Green Version]
- Pople, J.E.; Ball, R.L.; Padgett, M.J.; Aston, J.P. Construction of a database of benzene biological monitoring. Toxicol. Lett. 2002, 134, 301–304. [Google Scholar] [CrossRef]
- Frigerio, G.; Mercadante, R.; Polledri, E.; Missineo, P.; Campo, L.; Fustinoni, S. An LC-MS/MS method to profile urinary mercapturic acids, metabolites of electrophilic intermediates of occupational and environmental toxicants. J. Chromatogr. B 2019, 1117, 66–76. [Google Scholar] [CrossRef]
- Pavanello, S.; Kapka, L.; Siwinska, E.; Mielzyñska, D.; Bolognesi, C.; Clonfero, E. Micronuclei related to anti-B[a]PDE-DNA adduct in peripheral blood lymphocytes of heavily polycyclic aromatic hydrocarbon-exposed nonsmoking coke-oven workers and controls. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2795–2799. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campo, L.; Fustinoni, S.; Consonni, D.; Pavanello, S.; Kapka, L.; Siwinska, E.; Mielzyňska, D.; Bertazzi, P. Urinary carcinogenic 4-6 ring polycyclic aromatic hydrocarbons in coke oven workers and in subjects belonging to the general population: Role of occupational and environmental exposure. Int. J. Hyg. Environ. Health 2014, 217, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Fustinoni, S.; Campo, L.; Polledri, E.; Mercadante, R.; Erspamer, L.; Ranzi, A.; Lauriola, P.; Goldoni, C.A.; Bertazzi, P.A. A validated method for urinary cotinine quantification used to classify active and environmental tobacco smoke exposure. Curr. Anal. Chem. 2013, 9, 447–456. [Google Scholar] [CrossRef]
- Kroll, M.H.; Chesler, R.; Hagengruber, C.; Blank, D.W.; Kestner, J.; Rawe, M. Automated determination of urinary creatinine without sample dilution: Theory and practice. Clin. Chem. 1986, 32, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Haufroid, V.; Lison, D. Urinary cotinine as a tobacco-smoke exposure index: A minireview. Int. Arch. Occup. Environ. Health 1998, 71, 162–168. [Google Scholar] [CrossRef] [PubMed]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 20 January 2020).
- Wickham, H. Tidyverse: Easily Install and Load the ‘Tidyverse’. R Package Version 1.2.1. 2017. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 20 January 2020).
- SIVR. Società Italiana Valori di Riferimento—Quarta Lista dei Valori di Riferimento per Elementi, Composti Organici e loro Metaboliti; SIVR: Siena, Italy, 2017. [Google Scholar]
- NHANES. Fourth National Report on Human Exposure to Environmental Chemicals; Updated Tables; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017; Volume 1.
- Pluym, N.; Gilch, G.; Scherer, G.; Scherer, M. Analysis of 18 urinary mercapturic acids by two high-throughput multiplex-LC-MS/MS methods. Anal. Bioanal. Chem. 2015, 407, 5463–5476. [Google Scholar] [CrossRef] [PubMed]
- Schettgen, T.; Musiol, A.; Alt, A.; Ochsmann, E.; Kraus, T. A method for the quantification of biomarkers of exposure to acrylonitrile and 1,3-butadiene in human urine by column-switching liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 393, 969–981. [Google Scholar] [CrossRef]
- Schettgen, T.; Musiol, A.; Kraus, T. Simultaneous determination of mercapturic acids derived from ethylene oxide (HEMA), propylene oxide (2-HPMA), acrolein (3-HPMA), acrylamide (AAMA) and N,N-dimethylformamide (AMCC) in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 2629–2638. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Smith, D.M.; Edwards, K.C.; Blount, B.C.; Caldwell, K.L.; Feng, J.; Wang, L.; Christensen, C.; Ambrose, B.; Borek, N.; et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open 2018, 1, e185937. [Google Scholar] [CrossRef]
- Keith, R.J.; Fetterman, J.L.; Orimoloye, O.A.; Dardari, Z.; Lorkiewicz, P.; Hamburg, N.M.; DeFilippis, A.P.; Blaha, M.J.; Bhatnagar, A. Characterization of Volatile Organic Compound (VOC) metabolites in Cigarette smokers, Electronic Nicotine Device Users, Dual Users and Non- users of tobacco. Nicotine Tob. Res. 2019. [Google Scholar] [CrossRef]
- Ji, K.; Kang, S.; Lee, G.; Lee, S.; Jo, A.; Kwak, K.; Kim, D.; Kho, D.; Kim, S.; Hiuang, Y.F.; et al. Urinary levels of N-acetyl-S-(2-carbamoylethyl)-cysteine (AAMA), an acrylamide metabolite, in Korean children and their association with food consumption. Sci. Total Environ. 2013, 456–457, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Goerke, K.; Ruenz, M.; Lampen, A.; Abraham, K.; Bakuradze, T.; Eisenbrand, G.; Richling, E. Biomonitoring of nutritional acrylamide intake by consumers without dietary preferences as compared to vegans. Arch. Toxicol. 2019, 93, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Borgie, M.; Garat, A.; Cazier, F.; Delbende, A.; Allorge, D.; Ledoux, F.; Courcot, D.; Shirali, P.; Dagher, Z. Traffic-related air pollution. A pilot exposure assessment in Beirut, Lebanon. Chemosphere 2014, 96, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Manuela, C.; Francesco, T.; Tiziana, C.; Assunta, C.; Lara, S.; Nadia, N.; Giorgia, A.; Barbara, S.; Maria, F.; Carlotta, C.; et al. Environmental and biological monitoring of benzene in traffic policemen, police drivers and rural outdoor male workers. J. Environ. Monit. 2012, 14, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
| Chemical | CAS Number | Organization | Biomarker | Sampling Time | Biological Value | Value | |
|---|---|---|---|---|---|---|---|
| Acrolein | 107-02-8 | DFG | 3-HPMA | End of shift/for long-term exposures: at the end of the shift after several shifts | BAR (NS) | 600 µg/g creatinine | |
| Acrylamide | 79-06-1 | DFG | AAMA | End of shift | BAR (NS) | 100 µg/g creatinine | |
| Benzene | 71-43-2 | ACGIH | SPMA | End of shift | BEI | 25 µg/g creatinine | |
| ECHA | End of shift, after several shifts | BLV | 2 µg/g creatinine | ||||
| BGV | 0.5 µg/g creatinine | ||||||
| DFG | End of shift | EKA | Air (mg/m3) | Biomarker (µg/g creatinine) | |||
| 0.1 | 1.5 (NS) | ||||||
| 0.2 | 3 (NS) | ||||||
| 0.5 | 5 | ||||||
| 1.0 | 12 | ||||||
| 2.0 | 25 | ||||||
| 3.3 | 45 | ||||||
| 6.5 | 90 | ||||||
| BAR (NS) | 0.3 | ||||||
| 1,3-butadiene | 106-99-0 | DFG | DHBMA | End of shift/for long-term exposures: at the end of the shift after several shifts | EKA | Air (mg/m3) | Biomarker (µg/g creatinine) |
| 0.45 | 600 | ||||||
| 1.1 | 1000 | ||||||
| 2.3 | 1600 | ||||||
| 4.5 | 2900 | ||||||
| 6.8 | 4200 | ||||||
| BAR (NS) | 400 | ||||||
| MHBMA | End of shift/for long-term exposures: at the end of the shift after several shifts | EKA | Air (mg/m3) | Biomarker (mg/g creatinine) | |||
| 0.45 | 10 | ||||||
| 1.1 | 20 | ||||||
| 2.3 | 40 | ||||||
| 4.5 | 80 | ||||||
| 6.8 | 120 | ||||||
| BAR (NS) | <2 | ||||||
| N,N-dimethylformamide | 68-12-2 | ACGIH | AMCC | End of shift at end of work week | BEI | 30 mg/L | |
| DFG | BAT | 25 µg/g creatinine | |||||
| Mercapturic Acid | Metabolite of | LOQ (µg/L) | Statistics | Controls (n = 49) (µg/g Creatinine) | Workers (n = 49) (µg/g Creatinine) | T-Test on Log10-Transformed Data (p-Value) |
|---|---|---|---|---|---|---|
| 2-HPMA | propylene oxide | 0.5 | Median | 3.5 | 4.8 | 0.293 |
| 5th–95th | 0.9–11.6 | 1.1–11.1 | ||||
| %>LOQ | 100 | 100 | ||||
| 3-HPMA | acrolein | 0.2 | Median | 219.1 | 215.7 | 0.092 |
| 5th–95th | 81.2–1109.1 | 26.7–841.1 | ||||
| %>LOQ | 100 | 100 | ||||
| AAMA | acrylamide | 3.2 | Median | 21.3 | 25.8 | 0.385 |
| 5th–95th | 11.5–117.5 | 8.9–97.5 | ||||
| %>LOQ | 100 | 100 | ||||
| AMCC | N,N-dimethylformamide | 2 | Median | 112 | 119 | 0.272 |
| 5th–95th | 40–214 | 34–256 | ||||
| %>LOQ | 100 | 100 | ||||
| CEMA | acrylonitrile | 0.9 | Median | 1.4 | 3.7 | <0.001 |
| 5th–95th | <LOQ–16.6 | 1.4–9.6 | ||||
| %>LOQ | 88 | 98 | ||||
| CMEMA | crotonaldehyde | 2 | Median | 300 | 265 | 0.613 |
| 5th–95th | 99–809 | 97–1080 | ||||
| %>LOQ | 100 | 100 | ||||
| DHBMA | 1,3-butadiene | 1.0 | Median | 177.2 | 212.7 | 0.222 |
| 5th–95th | 109.7–345.4 | 96.8–413.5 | ||||
| %>LOQ | 100 | 100 | ||||
| EMA | ethylating agents | 0.01 | Median | 0.04 | 0.03 | 0.153 |
| 5th–95th | <LOQ–0.32 | <LOQ–0.11 | ||||
| %>LOQ | 82 | 90 | ||||
| GAMA | acrylamide | 1.0 | Median | 5.0 | 6.0 | 0.092 |
| 5th–95th | 2.5–13.3 | 3.3–11.8 | ||||
| %>LOQ | 100 | 100 | ||||
| HEMA | acrylonitrileethylene oxide | 0.3 | Median | 0.5 | 0.6 | 0.658 |
| 5th–95th | <LOQ–1.6 | <LOQ–1.6 | ||||
| %>LOQ | 86 | 86 | ||||
| HMPMA | crotonaldehyde | 2 | Median | 109 | 101 | 0.262 |
| 5th–95th | 56–270 | 46–278 | ||||
| %>LOQ | 100 | 98 | ||||
| MHBMA | 1,3-butadiene | 0.04 | Median | 0.42 | 1.10 | 0.001 |
| 5th–95th | <LOQ–2.47 | 0.18–3.35 | ||||
| %>LOQ | 90 | 96 | ||||
| MMA | methylating agents | 0.09 | Median | 3.53 | 2.95 | 0.175 |
| 5th–95th | 0.66–11.28 | <LOQ–12.61 | ||||
| %>LOQ | 100 | 92 | ||||
| NANPC | 4-chloronitrobenze | 0.11 | Median | <LOQ | <LOQ | NA |
| 5th–95th | <LOQ | <LOQ | ||||
| %>LOQ | 4 | 4 | ||||
| PHEMA | styrene | 0.01 | Median | 0.07 | 0.15 | <0.001 |
| 5th–95th | <LOQ–0.23 | 0.04–0.4 | ||||
| %>LOQ | 88 | 100 | ||||
| SBMA | toluene | 0.02 | Median | 0.62 | 0.80 | 0.316 |
| 5th–95th | 0.22–2.00 | 0.25–3.58 | ||||
| %>LOQ | 100 | 100 | ||||
| SPMA | benzene | 0.01 | Median | 0.02 | 0.31 | <0.001 |
| 5th–95th | <LOQ–0.25 | 0.04–2.98 | ||||
| %>LOQ | 71 | 100 |
| Mercapturic Acids | Group = Workers (Reference = Controls) | Log10 Creatinine (g/L) | Age (years) | Log10 Cotinine (µg/L) | Exposure Last 3 Days = Yes (Reference = No) | R2 Adj p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| GMR (95%CI) p-Value | GMR (95%CI) p-Value | r (95%CI) p-Value | GMR (95%CI) p-Value | r (95%CI) p-Value | GMR (95%CI) p-Value | r (95%CI) p-Value | GMR (95%CI) p-Value | ||
| 2-HPMA2 | 1.05 | 20.20 | 0.54 | 1.00 | −0.01 | 1.12 | 0.11 | 1.36 | 0.31 |
| 0.75–1.46 | 7.39–55.17 | 0.37–0.67 | 0.98–1.02 | −0.21–0.19 | 0.90–1.39 | −0.10–0.31 | 0.92–1.99 | <0.001 | |
| 0.77 | <0.001 | <0.001 | 0.906 | 0.904 | 0.305 | 0.294 | 0.117 | ||
| 3-HPMA3 | 0.63 | 26.01 | 0.43 | 1.01 | 0.12 | 1.19 | 0.12 | 1.00 | 0.16 |
| 0.39–1.02 | 6.05–111.85 | 0.25–0.58 | 0.99–1.04 | −0.08–0.32 | 0.87–1.62 | −0.09–0.31 | 0.57–1.74 | <0.001 | |
| 0.06 | <0.001 | <0.001 | 0.257 | 0.247 | 0.28 | 0.269 | 0.998 | ||
| AAMA | 0.98 | 14.22 | 0.52 | 1.01 | 0.15 | 1.28 | 0.26 | 1.08 | 0.29 |
| 0.72–1.33 | 5.62–35.98 | 0.35–0.65 | 1.00–1.03 | −0.06–0.34 | 1.05–1.57 | 0.06–0.44 | 0.76–1.54 | <0.001 | |
| 0.889 | <0.001 | <0.001 | 0.164 | 0.154 | 0.014 | 0.012 | 0.677 | ||
| AMCC | 1.00 | 10.09 | 0.56 | 1.02 | 0.30 | 1.20 | 0.24 | 1.61 | 0.39 |
| 0.79–1.27 | 4.8820.85 | 0.40–0.68 | 1.01–1.03 | 0.11–0.48 | 1.03–1.40 | 0.04–0.42 | 1.22–2.13 | <0.001 | |
| 0.989 | <0.001 | <0.001 | 0.004 | 0.003 | 0.022 | 0.019 | <0.001 | ||
| CEMA | 1.75 | 7.98 | 0.41 | 1.01 | 0.08 | 1.88 | 0.53 | 1.63 | 0.52 |
| 1.26–2.42 | 2.97–21.43 | 0.22–0.56 | 0.99–1.02 | -0.12–0.28 | 1.52–2.32 | 0.37–0.66 | 1.12–2.38 | <0.001 | |
| <0.001 | <0.001 | <0.001 | 0.455 | 0.444 | <0.001 | <0.001 | 0.011 | ||
| CMEMA | 0.95 | 18.73 | 0.53 | 1.02 | 0.22 | 0.94 | −0.06 | 0.98 | 0.25 |
| 0.68–1.31 | 6.96–50.41 | 0.37–0.66 | 1.00–1.03 | 0.02–0.40 | 0.76–1.16 | −0.26–0.14 | 0.67–1.43 | <0.001 | |
| 0.741 | <0.001 | <0.001 | 0.039 | 0.035 | 0.563 | 0.555 | 0.907 | ||
| DHBMA | 1.05 | 21.62 | 0.73 | 1.01 | 0.25 | 1.13 | 0.2 | 1.06 | 0.54 |
| 0.86–1.28 | 11.77–39.74 | 0.62–0.81 | 1.00–1.02 | 0.05–0.43 | 0.99–1.29 | 0.00–0.39 | 0.84–1.33 | <0.001 | |
| 0.662 | <0.001 | <0.001 | 0.019 | 0.016 | 0.061 | 0.055 | 0.632 | ||
| EMA | 0.75 | 9.02 | 0.32 | 1.02 | 0.14 | 0.84 | −0.12 | 1.22 | 0.08 |
| 0.48–1.19 | 2.23–36.51 | 0.12–0.49 | 0.99–1.04 | −0.06–0.34 | 0.63–1.14 | −0.31–0.08 | 0.71–2.07 | 0.027 | |
| 0.226 | 0.002 | 0.002 | 0.176 | 0.167 | 0.261 | 0.25 | 0.469 | ||
| GAMA | 1.10 | 14.62 | 0.68 | 1.01 | 0.16 | 1.19 | 0.28 | 0.99 | 0.49 |
| 0.90–1.34 | 7.95–26.88 | 0.56–0.78 | 1.00–1.02 | −0.05–0.35 | 1.05–1.36 | 0.08–0.45 | 0.78–1.25 | <0.001 | |
| 0.363 | <0.001 | <0.001 | 0.136 | 0.127 | 0.009 | 0.007 | 0.926 | ||
| HEMA | 1.00 | 6.7 | 0.38 | 0.99 | −0.09 | 0.98 | –0.03 | 1.33 | 0.15 |
| 0.73–1.38 | 2.55–17.63 | 0.20–0.54 | 0.98–1.01 | −0.29–0.11 | 0.79–1.20 | −0.23–0.18 | 0.92–1.93 | 0.001 | |
| 0.994 | <0.001 | <0.001 | 0.378 | 0.368 | 0.814 | 0.81 | 0.128 | ||
| HMPMA | 0.85 | 13.6 | 0.49 | 1 | 0.01 | 1.11 | 0.11 | 0.99 | 0.21 |
| 0.62–1.18 | 5.03–36.79 | 0.31–0.63 | 0.98–1.02 | −0.19–0.21 | 0.90–1.38 | −0.10–0.30 | 0.68–1.45 | <0.001 | |
| 0.341 | <0.001 | <0.001 | 0.922 | 0.921 | 0.315 | 0.304 | 0.96 | ||
| MHBMA | 2.06 | 145.01 | 0.53 | 1.02 | 0.14 | 1.2 | 0.11 | 1.01 | 0.33 |
| 1.19–3.57 | 27.31–769.98 | 0.37–0.66 | 0.99–1.05 | −0.06–0.34 | 0.84–1.72 | −0.10–0.30 | 0.54–1.92 | <0.001 | |
| 0.010 | <0.001 | <0.001 | 0.181 | 0.171 | 0.313 | 0.302 | 0.966 | ||
| MMA | 0.63 | 86.08 | 0.52 | 1.01 | 0.09 | 0.91 | −0.06 | 1.31 | 0.24 |
| 0.38–1.05 | 18.39–402.96 | 0.36–0.66 | 0.99–1.04 | −0.11–0.29 | 0.66–1.27 | −0.26–0.15 | 0.73–2.36 | <0.001 | |
| 0.078 | <0.001 | <0.001 | 0.375 | 0.364 | 0.591 | 0.583 | 0.364 | ||
| PHEMA | 2.15 | 36.9 | 0.54 | 0.99 | −0.09 | 1.19 | 0.15 | 0.99 | 0.43 |
| 1.46–3.16 | 11.38–119.66 | 0.38–0.67 | 0.97–1.01 | −0.29–0.11 | 0.93–1.53 | −0.06–0.34 | 0.63–1.55 | <0.001 | |
| <0.001 | <0.001 | <0.001 | 0.38 | 0.37 | 0.168 | 0.159 | 0.965 | ||
| SBMA | 1.09 | 33.41 | 0.59 | 1.03 | 0.33 | 1.01 | 0.01 | 1.37 | 0.36 |
| 0.78–1.52 | 12.10–92.27 | 0.44–0.71 | 1.01–1.04 | 0.14–0.50 | 0.81–1.25 | −0.20–0.21 | 0.93–2.02 | <0.001 | |
| 0.606 | <0.001 | <0.001 | 0.001 | 0.001 | 0.962 | 0.961 | 0.108 | ||
| SPMA | 9.53 | 9.9 | 0.3 | 1.01 | 0.06 | 1.28 | 0.15 | 2.09 | 0.56 |
| 5.71–15.91 | 2.09–46.93 | 0.10–0.47 | 0.98–1.03 | −0.15–0.26 | 0.91–1.78 | −0.05–0.35 | 1.15–3.79 | <0.001 | |
| <0.001 | 0.004 | 0.004 | 0.582 | 0.574 | 0.149 | 0.14 | 0.016 | ||
| Mercapturic Acids | Company = D (Reference = J) GMR (95%CI) p-Value | Company = R (Reference = J) GMR (95%CI) p-Value | Job Title = Engine Operators (Reference = Foremen) GMR (95%CI) p-Value | Job Title = Coke Makers (Reference = Foremen) GMR (95%CI) p-Value | Dirty Skin = Yes (Reference = No) GMR (95%CI) p-Value | R2 Adj p-Value |
|---|---|---|---|---|---|---|
| 2-HPMA | 1.18 | 1.06 | 1.29 | 0.70 | 0.73 | 0.23 0.015 |
| 0.71–1.96 | 0.51–2.19 | 0.62–2.71 | 0.39–1.26 | 0.28–1.90 | ||
| 0.504 | 0.882 | 0.487 | 0.230 | 0.516 | ||
| 3-HPMA | 1.49 | 1.15 | 0.86 | 1.15 | 15.48 | 0.31 0.002 |
| 0.67–3.27 | 0.37–3.57 | 0.27–2.71 | 0.46–2.86 | 3.51–68.30 | ||
| 0.317 | 0.807 | 0.786 | 0.755 | <0.001 | ||
| AAMA | 1.04 | 2.50 | 1.17 | 0.85 | 1.24 | 0.26 0.007 |
| 0.63–1.70 | 1.23–5.11 | 0.57–2.42 | 0.48–1.50 | 0.49–3.15 | ||
| 0.888 | 0.013 | 0.656 | 0.562 | 0.646 | ||
| AMCC | 1.10 | 1.04 | 0.79 | 1.06 | 2.23 | 0.15 0.065 |
| 0.69–1.76 | 0.53–2.04 | 0.40–1.55 | 0.62–1.82 | 0.93–5.35 | ||
| 0.673 | 0.900 | 0.481 | 0.821 | 0.073 | ||
| CEMA | 1.55 | 0.91 | 0.80 | 1.30 | 1.09 | 0.35 <0.001 |
| 0.97–2.46 | 0.46–1.77 | 0.40–1.57 | 0.76–2.22 | 0.46–2.63 | ||
| 0.066 | 0.772 | 0.505 | 0.331 | 0.836 | ||
| CMEMA | 1.38 | 0.87 | 1.10 | 1.29 | 2.67 | 0.14 0.072 |
| 0.78–2.46 | 0.38–1.99 | 0.48–2.56 | 0.67–2.50 | 0.90–7.89 | ||
| 0.264 | 0.730 | 0.815 | 0.441 | 0.074 | ||
| DHBMA | 1.39 | 1.36 | 1.21 | 1.32 | 2.35 | 0.50 <0.001 |
| 0.99–1.94 | 0.84–2.20 | 0.74–1.98 | 0.89–1.94 | 1.24–4.42 | ||
| 0.058 | 0.209 | 0.443 | 0.161 | 0.010 | ||
| EMA | 1.27 | 0.61 | 1.55 | 1.00 | 1.32 | 0.02 0.362 |
| 0.68–2.37 | 0.25–1.49 | 0.62–3.88 | 0.49–2.06 | 0.41–4.29 | ||
| 0.452 | 0.266 | 0.336 | 0.997 | 0.636 | ||
| GAMA | 0.88 | 1.08 | 1.18 | 1.27 | 1.78 | 0.56 <0.001 |
| 0.67–1.16 | 0.73–1.60 | 0.79–1.75 | 0.93–1.73 | 1.06–2.96 | ||
| 0.362 | 0.684 | 0.414 | 0.134 | 0.029 | ||
| HEMA | 0.93 | 0.60 | 0.92 | 1.13 | 0.62 | 0.05 0.274 |
| 0.51–1.68 | 0.25–1.40 | 0.39–2.18 | 0.57–2.25 | 0.20–1.88 | ||
| 0.803 | 0.229 | 0.841 | 0.711 | 0.385 | ||
| HMPMA | 1.62 | 2.05 | 0.99 | 1.26 | 6.70 | 0.34 0.001 |
| 0.94–2.81 | 0.93–4.51 | 0.44–2.21 | 0.67–2.38 | 2.38–18.81 | ||
| 0.082 | 0.075 | 0.983 | 0.461 | <0.001 | ||
| MHBMA | 1.81 | 1.98 | 1.04 | 1.39 | 2.76 | 0.23 0.014 |
| 0.88–3.71 | 0.71–5.57 | 0.37–2.97 | 0.61–3.17 | 0.72–10.65 | ||
| 0.103 | 0.187 | 0.939 | 0.428 | 0.136 | ||
| MMA | 1.90 | 1.44 | 1.07 | 0.96 | 2.86 | 0.22 0.019 |
| 0.74–4.91 | 0.37–5.65 | 0.27–4.29 | 0.32–2.86 | 0.48–17.03 | ||
| 0.179 | 0.591 | 0.919 | 0.940 | 0.242 | ||
| PHEMA | 0.71 | 0.75 | 1.30 | 2.04 | 0.76 | 0.32 0.002 |
| 0.41–1.20 | 0.35–1.60 | 0.60–2.82 | 1.11–3.77 | 0.28–2.06 | ||
| 0.193 | 0.444 | 0.505 | 0.023 | 0.577 | ||
| SBMA | 0.95 | 0.57 | 0.83 | 1.01 | 4.13 | 0.18 0.036 |
| 0.51–1.77 | 0.24–1.40 | 0.34–2.05 | 0.49–2.07 | 1.29–13.29 | ||
| 0.873 | 0.216 | 0.679 | 0.976 | 0.018 | ||
| SPMA | 3.00 | 1.21 | 2.38 | 2.71 | 0.92 | 0.22 0.019 |
| 1.36–6.59 | 0.39–3.76 | 0.75–7.50 | 1.10–6.72 | 0.21–4.04 | ||
| 0.008 | 0.732 | 0.136 | 0.032 | 0.909 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frigerio, G.; Campo, L.; Mercadante, R.; Mielżyńska-Švach, D.; Pavanello, S.; Fustinoni, S. Urinary Mercapturic Acids to Assess Exposure to Benzene and Other Volatile Organic Compounds in Coke Oven Workers. Int. J. Environ. Res. Public Health 2020, 17, 1801. https://doi.org/10.3390/ijerph17051801
Frigerio G, Campo L, Mercadante R, Mielżyńska-Švach D, Pavanello S, Fustinoni S. Urinary Mercapturic Acids to Assess Exposure to Benzene and Other Volatile Organic Compounds in Coke Oven Workers. International Journal of Environmental Research and Public Health. 2020; 17(5):1801. https://doi.org/10.3390/ijerph17051801
Chicago/Turabian StyleFrigerio, Gianfranco, Laura Campo, Rosa Mercadante, Danuta Mielżyńska-Švach, Sofia Pavanello, and Silvia Fustinoni. 2020. "Urinary Mercapturic Acids to Assess Exposure to Benzene and Other Volatile Organic Compounds in Coke Oven Workers" International Journal of Environmental Research and Public Health 17, no. 5: 1801. https://doi.org/10.3390/ijerph17051801
APA StyleFrigerio, G., Campo, L., Mercadante, R., Mielżyńska-Švach, D., Pavanello, S., & Fustinoni, S. (2020). Urinary Mercapturic Acids to Assess Exposure to Benzene and Other Volatile Organic Compounds in Coke Oven Workers. International Journal of Environmental Research and Public Health, 17(5), 1801. https://doi.org/10.3390/ijerph17051801








