Simultaneous Analysis and Dietary Exposure Risk Assessment of Fomesafen, Clomazone, Clethodim and Its Two Metabolites in Soybean Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Solution 0reparation
2.3. Sample Preparation by QuEChERS
2.4. UPLC-ESI-MS/MS Condition
2.5. Method Validation
2.6. Field Trials
2.7. Residue Definition of the Three Herbicides
2.8. Dietary Risk Assessment
3. Results and Discussion
3.1. Optimization of Instrument Conditions
3.2. Optimization of Sample Preparation
3.3. Method Validation
3.4. Terminal Residues of the Studied Herbicides in Soybean Ecosystems
3.5. Dietary Risk Exposure Assessment for Three Pesticides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADI | acceptable daily intake |
| CSO2 | clethodim sulfone |
| CSO | clethodim sulfoxide |
| DOS | dispersible oil suspension |
| GAP | good agricultural practices |
| GCB | graphitized carbon black |
| LOQs | limits of quantification |
| LODs | limits of detection |
| ME | Matrix effect |
| MWCNT | multi-walled carbon nanotubes |
| MRLs | maximum residue limits |
| NEDI | national estimated daily intake |
| PSA | Primary and Secondary Amine |
| QuEChERS | quick, easy, cheap, effective, rugged, and safe |
| RQ | risk quotient |
| RQc | chronic dietary exposure risk probability |
| RSDs | relative standard deviations |
| STMR | supervised trials median residue |
| UPLC-ESI-MS/MS | ultra-high performance liquid chromatography electro-spray ionization tandem mass spectrometry |
References
- FAO. Food and Agriculture Organization of the United Nations Statistics Division. 2017. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 28 July 2019).
- Balaji, A.P.B.; Sastry, T.P.; Manigandan, S.; Mukherjee, A.; Chandrasekaran, N. Environmental benignity of a pesticide in soft colloidal hydrodispersive nanometric form with improved toxic precision towards the target organisms than non-target organisms. Sci. Total. Environ. 2017, 579, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.; Du, P.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Impact of fomesafen on the soil microbial communities in soybean fields in Northeastern China. Ecotox. Environ. Safe. 2018, 148, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Di Gioia, F.; Hwang, J.; Hong, J.; Ozores-Hampton, M.; Zhao, X.; Pisani, C.; Rosskopf, E.; Wilson, P.C. Dissipation of fomesafen in fumigated, anaerobic soil disinfestation-treated, and organic-amended soil in Florida tomato production systems. Pest. Manag. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Sun, T.; Li, M.; Saleem, M.; Zhang, Q.; Wang, C. Soil-applied biochar increases microbial diversity and wheat plant performance under herbicide fomesafen stress. Ecotox. Envron. Safe. 2019, 171, 75–83. [Google Scholar] [CrossRef]
- Reed, T.V.; Boyd, N.S.; Wilson, P.C.; Dittmar, P.J.; Sharpe, S.M. Persistence and Movement of Fomesafen in Florida Strawberry Production. Weed. Sci. 2018, 66, 773–779. [Google Scholar] [CrossRef]
- Khorram, M.S.; Zheng, Y.; Lin, D.; Zhang, Q.; Fang, H.; Yu, Y. Dissipation of fomesafen in biochar-amended soil and its availability to corn (Zea mays L.) and earthworm (Eisenia fetida). J Soil. Sedimet. 2016, 16, 2439–2448. [Google Scholar] [CrossRef]
- Khorram, M.S.; Wang, Y.; Jin, X.X.; Fang, H.; Yu, Y.L. Reduced mobility of fomesafen through enhanced adsorption in biochar-amended soil. Environ. Toxicol. Chem. 2015, 34, 1258–1266. [Google Scholar] [CrossRef]
- Dong, X.; Wang, L.; Feng, R.; Ren, Z.; Zhang, L.; Lu, H. Insights into the binding mechanism of a model protein with fomesafen: Spectroscopic studies, thermodynamics and molecular modeling exploration. J. Mol. Struct. 2019, 1195, 892–903. [Google Scholar] [CrossRef]
- Driver, K.E.; Brunharo, C.A.C.G.; Al-Khatib, K. Mechanism of clomazone resistance in Leptochloa fusca spp. fasicularis to clomazone. Pestic. Biochem. Phys. 2020, 162, 1–5. [Google Scholar] [CrossRef]
- Troxler, S.C.; Askew, S.D.; Wilcut, J.W.; Smith, W.D.; Paulsgrove, M.D. Clomazone, fomesafen, and bromoxynil systems for bromoxynil-resistant cotton (Gossypium hirsutum). Weed. Thechnol. 2002, 16, 838–844. [Google Scholar] [CrossRef]
- Piveta, L.B.; Pinto, J.J.O.; Avila, L.A.; Noldin, J.A.; Santos, L.O. Selectivity of imazapic plus imazapyr herbicides on irrigated rice as affected by seed treatment with dietholate and clomazone applied in preemergence. Planta Daninha 2018, 36, e018149361. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.Y.; Cao, D.; Deng, Z.B. Determination of clomazone residues in soybean and soil by high performance liquid chromatography with DAD detection. Environ. Contam. Tox. 2011, 86, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Hussan, H.N.M.; He, H.R.; Du, P.Q.; Wu, X.H.; Liu, X.G.; Xu, J.; Dong, F.S.; Zheng, Y.Q. Determination of clomazone and acetochlor residues in soybean (Glycine max (L.) Merr.). Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Flower, K.C. Clomazone for control of grasses in flue-cured tobacco (Nicotiana tabacum) seedbeds. Weed. Thechnol. 2001, 31, 617–622. [Google Scholar] [CrossRef]
- Artus, N.N.; Ryberg, M.; Lindsten, A.; Ryberg, H.; Sundqvist, C. The Shibata Shift and the Transformation of Etioplasts to Chloroplasts in Wheat with Clomazone (FMC 57020) and Amiprophos-Methyl (Tokunol M). Plant. Physiol. 1992, 98, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, E.A.; De Andrade, V.C.; Viana, D.J.S.; Dos Santos, A.A.; Da Silva, A.J.M.; Fialho, C.M.T. Greenhouse Assessment of Differences in Clomazone Tolerance among Sweetpotato Cultivars. Weed Technol. 2011, 25, 501–505. [Google Scholar]
- Ferhatoglu, Y.; Barrett, M. Studies of clomazone mode of action. Pestic. Biochem. Phys. 2006, 85, 7–14. [Google Scholar] [CrossRef]
- Escoto, D.F.; Gayer, M.C.; Bianchini, M.C.; Da Cruz Pereira, G.; Roehrs, R.; Denardin, E.L.G. Use of Pistia stratiotes for phytoremediation of water resources contaminated by clomazone. Chemosphere 2019, 227, 299–304. [Google Scholar] [CrossRef]
- Sandín-España, P.; Sevilla-Moran, B.; López-Goti, C.; Mateo-Miranda, M.M.; Alonso-Prados, J.L. Rapid photodegradation of clethodim and sethoxydim herbicides in soil and plant surface model systems. Arab. J. Chem. 2016, 9, 694–703. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, P.T.; Johnson, W.G. Influence of Clethodim Application Timing on Control of Volunteer Corn in Soybean. Weed. Technol. 2013, 27, 645–648. [Google Scholar] [CrossRef]
- You, X.; Liang, L.; Liu, F. Dissipation and residues of clethodim and its oxidation metabolites in a rape-field ecosystem using QuEChERS and liquid chromatography/tandem mass spectrometry. Food Chem. 2014, 43, 170–174. [Google Scholar] [CrossRef] [PubMed]
- EFSA. EFSA Conclusion on the peer review of the pesticide risk assessment of the active substance clethodim. EFSA J. 2011, 9, 2417. [Google Scholar] [CrossRef]
- USEPA. USEPA Pesticide Fact Sheet: Clethodim, 540/FS-92-170; United States Environmental Protection Agency: Washington, DC, USA, 1992; pp. 1–8.
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Calvo, L.; Alonso-Prados, J.L.; Sandín-España, P. Photolysis of clethodim herbicide and a formulation in aquatic environments: Fate and ecotoxicity assessment of photoproducts by QSAR models. Sci. Total. Environ. 2018, 615, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Sevilla-Moran, B.; Alonso-Prados, J.L.; Garcia-Baudin, J.M.; Sandin-Espana, P. Indirect Photodegradation of Clethodim in Aqueous Media. Byproduct Identification by Quadrupole Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2010, 58, 3068–3076. [Google Scholar] [CrossRef]
- Tian, H.Z. Determination of chloramphenicol, enrofloxacin and 29 pesticides residues in bovine milk by liquid chromatography-tandem mass spectrometry. Chemosphere 2011, 83, 349–355. [Google Scholar] [CrossRef]
- Demir, E.; Inam, R. Square Wave Voltammetric Determination of Fomesafen Herbicide Using Modified Nanostructure Carbon Paste Electrode as a Sensor and Applicaion to Food Samples. Food Anal. Method 2017, 10, 74–82. [Google Scholar] [CrossRef]
- Ishimitsu, S.; Kaihara, A.; Yoshii, K.; Tsumura, Y.; Nakamura, Y.; Tonogai, Y. Determination of clethodim and its oxidation metabolites in crops by liquid chromatography with confirmation by LC/MS. J. Aoac. Int. 2001, 84, 1172–1178. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Yang, G.Q.; Xu, J.; Yu, W.W.; Shi, L.H.; Zeng, S.; Chen, L.Z.; Hu, D.Y.; Zhang, K.K. Simultaneous determination and method validation of clethodim and its metabolites clethodim sulfoxide and clethodim sulfone in tobacco by LC-MS/MS. Biomed. Chromatogr. 2018, 32, e4148. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. Aoac. Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Liu, X.; Ma, Y.; Pang, K.; Hu, J. Residue analysis and dietary exposure risk assessment of tebufenozide in stem lettuce (Lactuca sativa L. var. angustana Irish). Food Chem. Toxicol. 2018, 120, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Malhat, F.; Badawy, H.; Barakat, D.; Saber, A.N. Residues, dissipation and safety evaluation of chromafenozide in strawberry under open field conditions. Food Chem. 2014, 152, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhang, D.; Wu, X.; Pan, D.; Shi, T.; Hua, R. Simultaneous determination of neonicotinoid insecticides and metabolites in rice by dispersive solid-liquid microextraction based on an in situ acid-base effervescent reaction and solidification of a floating organic droplet. Anal. Bioanal. Chem. 2019, 411, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Di Corcia, A.; Nazzari, M.; Rao, R.; Samperi, R.; Sebastiani, E. Simultaneous determination of acidic and non-acidic pesticides in natural waters by liquid chromatography-mass spectrometry. J. Chromatogr. A 2000, 878, 87–98. [Google Scholar] [CrossRef]
- Crescenzi, C.; Corcia, A.D.; Márchese, S.; Samperi, R. Determination of Acidic Pesticides in Water by a Benchtop Electrospray Liquid Chromatography Mass Spectrometer. Anal. Chem. 1995, 67, 1968–1975. [Google Scholar] [CrossRef]
- Mantzos, N.; Karakitsou, A.; Zioris, I.; Leneti, E.; Konstantinou, I. QuEChERS and solid phase extraction methods for the determination of energy crop pesticides in soil, plant and runoff water matrices. Int. J. Environ. Anal. Chem. 2013, 93, 1566–1584. [Google Scholar] [CrossRef]
- Ghani, S.B.A.; Hanafi, A.H. QuEChERS method combined with GC-MS for pesticide residues determination in water. J. Anal. Chem. 2016, 71, 508–512. [Google Scholar] [CrossRef]
- Walorczyk, S. Validation and use of a QuEChERS-based gas chromatographic–tandem mass spectrometric method for multiresidue pesticide analysis in black currants including studies of matrix effects and estimation of measurement uncertainty. Talanta 2014, 120, 106–113. [Google Scholar] [CrossRef]
- Kaczynski, P. Clean-up and matrix effect in LC-MS/MS analysis of food of plant origin for high polar herbicides. Food Chem. 2017, 230, 524–531. [Google Scholar] [CrossRef]
- Buhrman, D.L.; Price, P.I.; Rudewicz, P.J. Quantitation of SR 27417 in Human Plasma Using Electrospray Liquid Chromatography-Tandem Mass Spectrometry: A Study of Ion Suppression. J. Am. Soc. Mass Spectrom. 1996, 7, 1099. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.codeofchina.com/standard/GB2763-2019.html (accessed on 30 July 2019).
- Available online: http://www.m5.ws001.squarestart.ne.jp/foundation/agrdtl.php?a_inq=19100 (accessed on 28 January 2020).
- Boxall, A.; Sinclair, C.J.; Fenner, K.; Kolpin, D.; Maud, S.J. When synthetic chemicals degrade in the environment. Environ. Sci. Technol. 2004, 38, 368A–375A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCS. The International Programme on Chemical Safety, Principles and Methods for the Risk Assessment of Chemicals in Food: Environmental Health Criteria 240; A joint publication of the Food and Agriculture Organization of the United Nations and the World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticide-detail/en/ (accessed on 30 July 2019).
- Chinese Nutrition Society. The Chinese Dietary Guidelines; People’s Publishing House: Beijing, China, 2012. [Google Scholar]
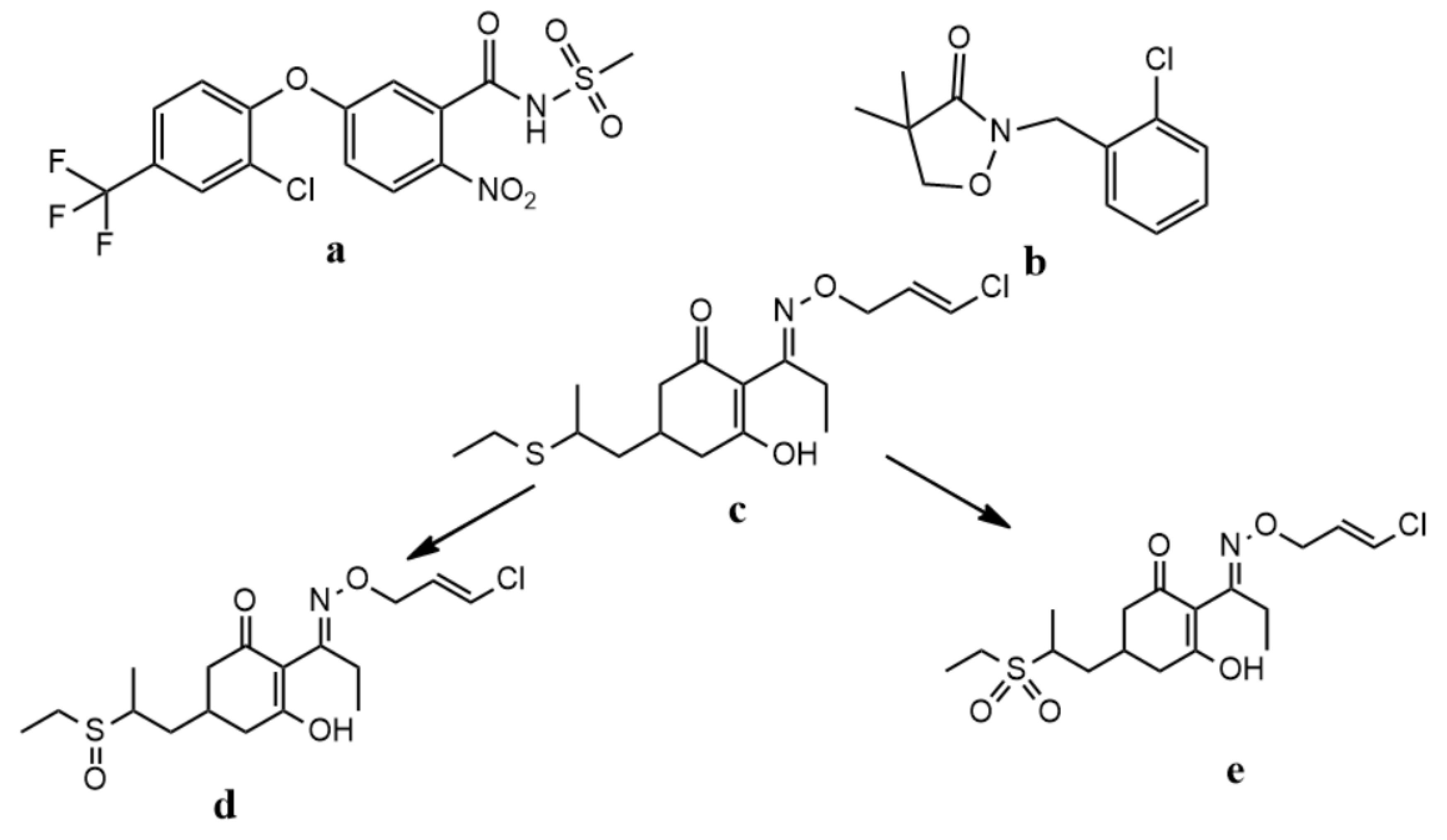

| Compound (Molecular Formula) | Retention Time (min) | Transition m/z (Quantitative IonQualitative Ion) | Fragmentor Voltage (V) | Collision Energy (eV) | Polar |
|---|---|---|---|---|---|
| Fomesafen | 0.75 | 437→286.1 437→315.9 | 150 | 20 25 | Negative |
| Clomazone | 0.96 | 240.1→125 240.1→89 | 85 | 21 55 | Positive |
| Clethodim | 1.31 | 360.1→164 360.1→206.1 | 85 | 17 15 | Positive |
| CSO | 0.78 | 376.1→206.1 376.1→164.1 | 100 | 15 25 | Positive |
| CSO2 | 0.82 | 392.1→164.1 392.1→300.2 | 110 | 35 5 | Positive |
| Matrix | Spiked Level (mg/kg) | Soybean | Green Soybean | Soybean Straw | LOQs (mg/kg) | LODs (μg/kg) | |||
|---|---|---|---|---|---|---|---|---|---|
| Recoveries (%) | RSDs (%) | Recoveries (%) | RSDs (%) | Recoveries (%) | RSDs (%) | ||||
| Fomesafen | 0.01 0.1 1.0 | 110 94 98 | 8.4 7.2 9.3 | 93 103 93 | 2.6 1.9 5.9 | 106 92 108 | 7.6 7.6 5.6 | 0.01 | 0.083 |
| Clomazone | 0.01 0.1 1.0 | 105 94 101 | 8.7 2.9 3.2 | 100 90 94 | 9.9 2.6 3.9 | 91 97 87 | 9.2 3.3 1.8 | 0.01 | 0.018 |
| Clethodim | 0.01 0.1 1.0 | 108 100 93 | 6.4 8.8 6.7 | 105 91 96 | 7.9 1.6 1.9 | 99 99 90 | 9.6 3.0 3.3 | 0.01 | 0.042 |
| CSO | 0.01 0.1 1.0 | 92 91 86 | 3.4 4.9 9.7 | 97 90 98 | 5.9 1.8 1.6 | 92 100 98 | 1.5 2.1 1.6 | 0.01 | 0.077 |
| CSO2 | 0.01 0.1 1.0 | 89 86 91 | 12.7 9.8 9.8 | 111 93 94 | 1.9 3.8 3.0 | 89 98 88 | 4.3 1.8 3.8 | 0.01 | 0.125 |
| Herbicides | Crops | Food Classification | Fi (kg) | STMRi (mg/kg) | Sources [43,47] | NEDI (mg/kg bw) | ADI (mg/kg bw) | RQc |
|---|---|---|---|---|---|---|---|---|
| Fomesafen | Soybean | Vegetable oil | 0.016 | 0.01 | STMR | 0.00016 | 0.0025 | |
| Peanut | Vegetable oil | 0.0327 | 0.2 | China | 0.00654 | 4.3 | ||
| Clomazone | Soybean | Vegetable oil | 0.016 | 0.01 | STMR | 0.00016 | 0.133 | |
| Potato | Tubers | 0.0495 | 0.02 | China | 0.00099 | |||
| Sugarcane | Sugar, starch | 0.0044 | 0.1 | China | 0.00044 | |||
| Pumpkin | Light vegetables | 0.0915 | 0.05 | China | 0.004575 | |||
| Rice | Rice and its products | 0.2399 | 0.02 | China | 0.004798 | 0.13 | ||
| Clethodim | Soybean | Vegetable oil | 0.016 | 0.03 | STMR | 0.00048 | 0.01 | |
| Garlic | Sauce | 0.009 | 0.5 | European Union | 0.0045 | |||
| Tomato | Dark vegetables | 0.0915 | 1 | European Union | 0.0915 | |||
| Sugarbeet | Sugar, starch | 0.0044 | 0.1 | European Union | 0.00044 | |||
| Potato | Tubers | 0.0495 | 0.5 | European Union | 0.02475 | 19.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, K.; Hu, J. Simultaneous Analysis and Dietary Exposure Risk Assessment of Fomesafen, Clomazone, Clethodim and Its Two Metabolites in Soybean Ecosystem. Int. J. Environ. Res. Public Health 2020, 17, 1951. https://doi.org/10.3390/ijerph17061951
Pang K, Hu J. Simultaneous Analysis and Dietary Exposure Risk Assessment of Fomesafen, Clomazone, Clethodim and Its Two Metabolites in Soybean Ecosystem. International Journal of Environmental Research and Public Health. 2020; 17(6):1951. https://doi.org/10.3390/ijerph17061951
Chicago/Turabian StylePang, Kyongjin, and Jiye Hu. 2020. "Simultaneous Analysis and Dietary Exposure Risk Assessment of Fomesafen, Clomazone, Clethodim and Its Two Metabolites in Soybean Ecosystem" International Journal of Environmental Research and Public Health 17, no. 6: 1951. https://doi.org/10.3390/ijerph17061951
APA StylePang, K., & Hu, J. (2020). Simultaneous Analysis and Dietary Exposure Risk Assessment of Fomesafen, Clomazone, Clethodim and Its Two Metabolites in Soybean Ecosystem. International Journal of Environmental Research and Public Health, 17(6), 1951. https://doi.org/10.3390/ijerph17061951





