Neonicotinoids: Spreading, Translocation and Aquatic Toxicity
Abstract
:1. Introduction
2. Use of Neonicotinoids as Coating Materials, Dosages
3. The Presence of Neonicotinoids in Soil
4. Excretion via Guttation
5. The Presence of Neonicotinoids in Surface Waters
6. Ecotoxicological Testing of Neonicotinoid Active Ingredients and Their Formulations
6.1. Toxicity of Neonicotinoid Active Ingredients and Formulations on Daphnia magna
6.2. Neuronal Targets of Neonicotinoids in the Snail Nervous System
6.3. Toxicity Tests of Neonicotinoids on other Indicator Organisms
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Shao, X.; Liu, Z.; Xu, X.; Li, Z.; Qian, X. Overall status of neonicotinoid insecticides in China: Production, application and innovation. J. Pestic. Sci. 2013, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Douglas, M.R.; Tooker, J.F. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. field crops. Environ. Sci. Technol. 2015, 49, 5088–5097. [Google Scholar] [CrossRef]
- The European Commission. Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. Off. J. Eur. Union 2013, L 139, 19–26. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013R0485&qid=1582882303243&from=EN (accessed on 26 February 2020).
- Fryday, S.; Tiede, K.; Stein, J. Scientific services to support EFSA systematic reviews: Lot 5 Systematic literature review on the neonicotinoids (namely active substances clothianidin, thiamethoxam and imidacloprid) and the risks to bees. EFSA Support. Publ. 2015, 12, EN-756. [Google Scholar] [CrossRef] [Green Version]
- The European Commission. Commission Implementing Regulation (EU) No 783/2018 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance imidacloprid. Off. J. Eur. Union 2018, L 132, 31–34. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R0783&from=EN (accessed on 26 February 2020).
- The European Commission. Commission Implementing Regulation (EU) No 784/2018 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance clothianidin. Off. J. Eur. Union 2018, L 132, 35–39. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R0784&from=EN (accessed on 26 February 2020).
- The European Commission. Commission Implementing Regulation (EU) 785/2018 of 29 May 2018 amending Implementing Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance thiamethoxam. Off. J. Eur. Union 2018, L 132, 40–44. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R0785&from=EN (accessed on 26 February 2020).
- Goulson, D. Review: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Erickson, B.E. Neonicotinoid Pesticides Can Stay in the US Market, EPA Says. Chem. Eng. News 2020, 98. Available online: https://cen.acs.org/environment/pesticides/Neonicotinoid-pesticides-stay-US-market/98/web/2020/02 (accessed on 26 February 2020).
- Furlan, L.; Pozzebon, A.; Duso, C.; Simon-Delso, N.; Sánchez-Bayo, F.; Marchand, P.A.; Codato, F.; van Lexmond, M.B.; Bonmatin, J.-M. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 3: Alternatives to systemic insecticides. Environ. Sci. Pollut. Res. 2018. [Google Scholar] [CrossRef] [Green Version]
- Gurian-Sherman, D. Alternatives to Neonicotinoid Insecticide-Coated Corn Seed: Agroecological Methods Are Better for Farmers and the Environment; Center for Food Safety: Washington, DC, USA, 2017; Available online: http://www.centerforfoodsafety.org/files/alternatives-to-neonics_v9_23186.pdf (accessed on 26 February 2020).
- Scott, C.; Bilsborrow, P.E. The impact of the EU neonicotinoid seed-dressing ban on oilseed rape production in England. Pest Manag. Sci. 2019, 75, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathage, J.; Castañera, P.; Alonso-Prados, J.L.; Gómez-Barbero, G.; Rodríguez-Cerezo, E. The impact of restrictions on neonicotinoid and fipronil insecticides on pest management in maize, oilseed rape and sunflower in eight European Union regions. Pest Manag. Sci. 2018, 74, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jactel, H.; Verheggen, F.; Thiéry, D.; Escobar-Gutiérrez, A.J.; Gachet, E.; Desneux, N. Alternatives to neonicotinoids. Environ. Int. 2019, 129, 423–429. [Google Scholar] [CrossRef]
- US EPA. Preliminary Bee Risk Assessment to Support the Registration Review of Clothianidin and Thiamethoxam; US EPA: Washington, DC, USA, 2017. Available online: https://www.regulations.gov/document?D=EPA-HQ-OPP-2011-0865-0173 (accessed on 26 February 2020).
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef] [Green Version]
- Pistorius, J.; Bischoff, G.; Heimbach, U.; Stähler, M. Bee poisoning incidents in Germany in spring 2008 caused by abrasion of active substance from treated seeds during sowing of maize. Julius Kühn Arch. 2009, 423, 118–126. Available online: https://ojs.openagrar.de/index.php/JKA/article/view/142 (accessed on 26 February 2020).
- Mörtl, M.; Darvas, B.; Vehovszky, Á.; Győri, J.; Székács, A. Occurrence of neonicotinoids in guttation liquid of maize—soil mobility and cross-contamination. Int. J. Environ. Anal. Chem. 2017, 97, 868–884. [Google Scholar] [CrossRef]
- US EPA. Pesticide—Fact Sheet for Clothianidin; US EPA: Washington, DC, USA, 2003. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-044309_30-May-03.pdf (accessed on 26 February 2020).
- Schaafsma, A.; Limay-Rios, V.; Baute, T.; Smith, J.; Xue, Y. Neonicotinoid insecticide residues in surface water and soil associated with commercial maize (corn) fields in Southwestern Ontario. PLoS ONE 2015, 10, e0118139. [Google Scholar] [CrossRef] [Green Version]
- Giorio, C.; Safer, A.; Sánchez-Bayo, F.; Tapparo, A.; Lentola, A.; Girolami, V.; van Lexmond, M.B.; Bonmatin, J.M. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 1: New molecules, metabolism, fate, and transport. Environ. Sci. Pollut. Res. 2017. [Google Scholar] [CrossRef] [Green Version]
- Hilton, M.J.; Jarvis, T.D.; Ricketts, D.C. The degradation rate of thiamethoxam in European field studies. Pest. Manag. Sci. 2016, 72, 388–397. [Google Scholar] [CrossRef]
- Dankyi, E.; Gordon, C.; Carboo, D.; Apalangya, V.A.; Fomsgaard, I.S. Sorption and degradation of neonicotinoid insecticides in tropical soils. J. Environ. Sci. Health B 2018, 53, 587–594. [Google Scholar] [CrossRef]
- Li, Y.; Su, P.; Li, Y.; Wen, K.; Bi, G.; Cox, M. Adsorption-desorption and degradation of insecticide clothianidin and thiamethoxam in agricultural soils. Chemosphere 2018, 207, 708–714. [Google Scholar] [CrossRef]
- Schaafsma, A.; Limay-Rios, V.; Xue, Y.; Smith, J.; Baute, T. Field-scale examination of neonicotinoid insecticide persistence in soil as a result of seed treatment use in commercial maize (corn) fields in Southwestern Ontario. Environ. Toxicol. Chem. 2016, 35, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Wintermantel, D.; Odoux, J.F.; Decourtye, A.; Henry, M.; Allier, F.; Bretagnolle, V. Neonicotinoid-induced mortality risk for bees foraging on oilseed rape nectar persists despite EU moratorium. Sci. Total Environ. 2020, 704, 135400. [Google Scholar] [CrossRef] [PubMed]
- Mörtl, M.; Kereki, O.; Darvas, B.; Klátyik, S.; Vehovszky, Á.; Győri, J.; Székács, A. Study on soil mobility of two neonicotinoid insecticides. J. Chem. 2016, 2016, 4546584. [Google Scholar] [CrossRef] [Green Version]
- Radolinski, J.; Wu, J.; Xia, K.; Stewart, R. Transport of a neonicotinoid pesticide, thiamethoxam, from artificial seed coatings. Sci. Total Environ. 2018, 618, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Guttation: Mechanism, momentum and modulation. Bot. Rev. 2016, 82, 149–182. [Google Scholar] [CrossRef]
- Girolami, V.; Mazzon, L.; Squartini, A.; Mori, N.; Marzaro, M.; Di Bernardo, A.; Greatti, M.; Giorio, C.; Tapparo, A. Translocation of neonicotinoids insecticides from coated seeds to seedling guttation drops: A novel way of intoxication for bees. J. Econ. Entomol. 2009, 102, 1808–1815. [Google Scholar] [CrossRef]
- Tapparo, A.; Giorio, C.; Marzaro, M.; Marton, D.; Solda, L.; Girolami, V. Rapid analysis of neonicotinoid insecticides in guttation drops of corn seedlings obtained from coated seeds. J. Environ. Monit. 2011, 13, 1564–1568. [Google Scholar] [CrossRef]
- Mörtl, M.; Darvas, B.; Vehovszky, Á.; Győri, J.; Székács, A. Contamination of the guttation liquid of two common weeds with neonicotinoids from coated maize seeds planted in close proximity. Sci. Total Environ. 2019, 649, 1137–1143. [Google Scholar] [CrossRef]
- Schenke, D.; Wirtz, I.P.; Lorenz, S.; Pistorius, J.; Heimbach, U. Two-year field data on neonicotinoid concentrations in guttation drops of seed treated maize (Zea mays). Data Brief 2018, 21, 299–306. [Google Scholar] [CrossRef]
- Marzaro, M.; Vivan, L.; Targa, A.; Mazzon, L.; Mori, N.; Greatti, M.; Petrucco Toffolo, E.; Di Bernardo, A.; Giorio, C.; Marton, D.; et al. Lethal aerial powdering of honey bees with neonicotinoids from fragments of maize seed coat. Bull. Insectol. 2011, 64, 119–126. [Google Scholar]
- Botías, C.; David, A.; Hill, E.M.; Goulson, D. Contamination of wild plants near neonicotinoid seed-treated crops, and implications for non-target insects. Sci. Total Environ. 2016, 566, 269–278. [Google Scholar] [CrossRef]
- The European Commission. Commission Implementing Decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015, L 78, 40–42. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015D0495&from=EN (accessed on 26 February 2020).
- The European Commission. Commission Implementing Decision (EU) 2018/840 of 5 June 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495. Off. J. Eur. Union 2018, L 141, 9–12. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0840&from=EN (accessed on 26 February 2020).
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kania, J.; Malina, G.; Kmiecik, E.; Wator, K. Pesticides from the EU First and Second Watch Lists in the Water Environment. Clean Soil Air Water 2019, 47, 1800376. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance imidacloprid. EFSA J. 2008, 6, 1–120. [Google Scholar] [CrossRef]
- Posthuma-Doodeman, C.J.A.M. Environmental Risk Limits for Imidacloprid; Rijksinstituut voor Volksgezondheid en Milieu (RIVM): Bilthoven, The Nederlands, 2008; Available online: https://www.rivm.nl/bibliotheek/rapporten/601716018.pdf (accessed on 26 February 2020).
- Smit, C.E. Water Quality Standards for Imidacloprid. Proposal for an Update According to the Water Framework Directive. RIVM Letter Report 270006001/2014; Rijksinstituut voor Volksgezondheid en Milieu (RIVM): Bilthoven, The Netherlands, 2014; Available online: https://www.rivm.nl/bibliotheek/rapporten/270006001.pdf (accessed on 26 February 2020).
- US-EPA. Aquatic Life Benchmarks and Ecological Risk Assessments for Registered Pesticides; US-EPA: Washington, DC, USA, 2014. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-and-ecological-risk#use (accessed on 26 February 2020).
- Canadian Council of Ministers of the Environment (CCME). Canadian Environmental Quality Guidelines. Canadian Water Quality Guidelines for the Protection of Aquatic Life; CCME: Hull, QC, Canada, 2014; Available online: http://ceqg-rcqe.ccme.ca/en/index.html (accessed on 26 February 2020).
- King, O.C.; Smith, R.A.; Mannand, R.M.; Warne, M.S.J. Proposed Aquatic Ecosystem Protection Guideline Values for Pesticides Commonly Used in the Great Barrier Reef Catchment Area: Part 1 (amended)–2,4-D, Ametryn, Diuron, Glyphosate, Hexazinone, Imazapic, Imidacloprid, Isoxaflutole, Metolachlor, Metribuzin, Metsulfuron-methyl, Simazine, Tebuthiuron; Department of Environment and Science: Brisbane, Queensland, Australia, 2017. Available online: https://www.publications.qld.gov.au/dataset/proposed-guideline-values-27-pesticides-used-in-the-gbr-catchment/resource/12e1b6af-9b71-40aa-bb50-163fe577a2c1 (accessed on 26 February 2020).
- Australian Government. Australian and New Zealand Guidelines for Fresh and Marine Water Quality. Pathway for Toxicant Default Guideline Value Publication; Australian and New Zealand Environment Conservation Council (ANZECC) and Agriculture and Resource Management Council of Australia and New Zealand (ARMCANZ): Canberra, Australia, 2000. Available online: https://www.waterquality.gov.au/anz-guidelines/guideline-values/default/draft-dgvs#third-party-process-for-proposing-default-guideline-values (accessed on 26 February 2020).
- Hladik, M.L.; Kolpin, D.W. First national-scale reconnaissance of neonicotinoid insecticides in streams across the USA. Environ. Chem. 2016, 13, 12–20. [Google Scholar] [CrossRef]
- Montiel-León, J.M.; Munoz, G.; Vo Duy, S.; Do, D.T.; Vaudreuil, M.A.; Goeury, K.; Guillemette, F.; Amyot, M.; Sauvé, S. Widespread occurrence and spatial distribution of glyphosate, atrazine, and neonicotinoids pesticides in the St. Lawrence and tributary rivers. Environ. Pollut. 2019, 250, 29–39. [Google Scholar] [CrossRef]
- Struger, J.; Grabuski, J.; Cagampan, S.; Sverko, E.; McGoldrick, D.; Marvin, C.H. Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada. Chemosphere 2017, 169, 516–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Challis, J.K.; Cuscito, L.D.; Joudan, S.; Luong, K.H.; Knapp, C.W.; Hanson, M.L.; Wong, C.S. Inputs, source apportionment, and transboundary transport of pesticides and other polar organic contaminants along the lower Red River, Manitoba, Canada. Sci. Total Environ. 2018, 635, 803–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klarich, K.L.; Pflug, N.C.; DeWald, E.M.; Hladik, M.L.; Kolpin, D.W.; Cwiertny, D.M.; LeFevre, G.H. Occurrence of neonicotinoid insecticides in finished drinking water and fate during drinking water treatment. Environ. Sci. Technol. Lett. 2017, 4, 168–173. [Google Scholar] [CrossRef]
- Sultana, T.; Murray, C.; Kleywegt, S.; Metcalfe, C.D. Neonicotinoid pesticides in drinking water in agricultural regions of southern Ontario, Canada. Chemosphere 2018, 202, 506–513. [Google Scholar] [CrossRef]
- Mahai, G.; Wan, Y.; Xia, W.; Yang, S.; He, Z.; Xu, S. Neonicotinoid insecticides in surface water from the central Yangtze River, China. Chemosphere 2019, 229, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.G.C.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.G.C.; Ribeiro, A.R.; Barbosa, M.O.; Ribeiro, C.; Tiritan, M.E.; Pereira, M.F.R.; Silva, A.M.T. Monitoring of the 17 EU Watch List contaminants of emerging concern in the Ave and the Sousa Rivers. Sci. Total Environ. 2019, 649, 1083–1095. [Google Scholar] [CrossRef]
- Székács, A.; Mörtl, M.; Darvas, B. Monitoring pesticide residues in surface and ground water in Hungary—Surveys in 1990–2015. J. Chem. 2015, 2015, 717948. [Google Scholar] [CrossRef] [Green Version]
- Mörtl, M.; Takács, E.; Klátyik, S.; Székács, A. Aquatic toxicity and loss of linear alkylbenzenesulfonates alone and in a neonicotinoid insecticide formulation in surface water. Sci. Total Environ. 2019, 652, 780–787. [Google Scholar] [CrossRef]
- Cox, C.; Surgan, M. Unidentified inert ingredients in pesticides: Implications for human and environmental health. Environ. Health Perspect. 2006, 114, 1803–1806. [Google Scholar] [CrossRef] [Green Version]
- Mesnage, R.; Bernay, B.; Séralini, G.E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 16, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Székács, I.; Fejes, Á.; Klátyik, S.; Takács, E.; Patkó, D.; Pomóthy, J.; Mörtl, M.; Horváth, R.; Madarász, E.; Darvas, B.; et al. Environmental and toxicological impacts of glyphosate with its formulating adjuvant. Int. J. Biol. Vet. Agric. Food Eng. 2014, 8, 213–218. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; Spiroux de Vendomois, J.; Séralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tišler, T.; Jemec, A.; Mozetič, B.; Trebše, P. Hazard identification of imidacloprid to aquatic environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef]
- Vehovszky, Á.; Farkas, A.; Ács, A.; Stoliar, O.; Székács, A.; Mörtl, M.; Győri, J. Neonicotinoid insecticides inhibit cholinergic neurotransmission in a molluscan (Lymnaea stagnalis) nervous system. Aquat. Toxicol. 2015, 167, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Bayo, F.; Goka, K.; Hayasaka, D. Contamination of the aquatic environment with neonicotinoids and its implication for ecosystems. Front. Environ. Sci. 2016, 4, 71. [Google Scholar] [CrossRef]
- OECD. OECD Test No. 202: Daphnia Sp. Acute AQ7 Immobilisation Test; Organisation for Economic Co-operation and Development: Paris, France, 2004. [Google Scholar] [CrossRef]
- Takács, E.; Klátyik, S.; Mörtl, M.; Rácz, G.; Kovács, K.; Darvas, B.; Székács, A. Effects of neonicotinoid insecticide formulations and their components on Daphnia magna—The role of active ingredients and co-formulants. Int. J. Environ. Anal. Chem. 2017, 97, 885–900. [Google Scholar] [CrossRef]
- Defarge, N.; Takács, E.; Lozano, V.L.; Mesnage, R.; Spiroux de Vendomois, J.; Séralini, G.E.; Székács, A. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int. J. Environ. Res. Public Health 2016, 13, 264. [Google Scholar] [CrossRef] [Green Version]
- Klátyik, S.; Takács, E.; Mörtl, M.; Földi, A.; Trábert, Z.; Ács, É.; Darvas, B.; Székács, A. Dissipation of the herbicide active ingredient glyphosate in natural water samples in the presence of biofilms. Int. J. Environ. Anal. Chem. 2017, 90, 901–921. [Google Scholar] [CrossRef] [Green Version]
- Tsui, M.T.K.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- Mesnage, R.; Defrage, N.; Spiroux de Vendomois, J.; Séralini, G.E. Major pesticides are more toxic to human cells than their declared active principles. BioMed Res. Int. 2014, 2014, 179691. [Google Scholar] [CrossRef] [Green Version]
- Brausch, J.M.; Beall, B.; Smith, P.N. Acute and sub-lethal toxicity of three POEA surfactant formulations to Daphnia magna. Bull. Environ. Contam. Toxicol. 2007, 78, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, H.; Pang, X.; Cui, K.; Lin, J.; Liu, F.; Mu, W. Quaternary ammonium cationic surfactants increase bioactivity of indoxacarb on pests and toxicological risk to Daphnia magna. Ecotoxicol. Environ. Saf. 2018, 149, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Sobrino-Figueroa, A. Toxic effect of commercial detergents on organisms from different trophic levels. Environ. Sci. Pollut. Res. 2018, 25, 13283–13291. [Google Scholar] [CrossRef] [PubMed]
- Verge, C.; Moreno, A.; Bravo, J.; Berna, J.L. Influence of water hardness on the bioavailability and toxicity of linear alkylbenzene sulphonate (LAS). Chemosphere 2001, 44, 1749–1757. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. Available online: https://www.jbc.org/content/249/22/7130.full.pdf (accessed on 26 February 2020). [PubMed]
- Badawy, M.E.I.; Nasr, H.M.; Rabea, E.I. Toxicity and biochemical changes in the honey bee Apis mellifera exposed to four insecticides under laboratory conditions. Apidologie 2015, 46, 177–193. [Google Scholar] [CrossRef] [Green Version]
- Saggioro, E.M.; do Espírito Santo, D.G.; Sales Júnior, S.F.; Hauser-Davis, R.A.; Correia, F.V. Lethal and sublethal effects of acetamiprid on Eisenia andrei: Behavior, reproduction, cytotoxicity and oxidative stress. Ecotoxicol. Environ. Saf. 2019, 183, 109572. [Google Scholar] [CrossRef]
- Jemec, A.; Tišler, T.; Drobne, D.; Sepcič, K.; Fournier, D.; Trebše, P. Comparative toxicity of imidacloprid, of its commercial liquid formulation and of diazinon to a non-target arthropod, the microcrustacean Daphnia magna. Chemosphere 2007, 68, 1408–1418. [Google Scholar] [CrossRef]
- Song, Y.; Chen, M.; Zhou, J. Effects of three pesticides on superoxide dismutase and glutathione-S-transferase activities and reproduction of Daphnia magna. Arch. Environ. Prot. 2017, 43, 80–86. [Google Scholar] [CrossRef]
- Sur, R.; Stork, A. Uptake, translocation and metabolism of imidacloprid in plants. Bull. Insectol. 2003, 56, 35–40. Available online: http://www.bulletinofinsectology.org/pdfarticles/vol56-2003-035-040sur.pdf (accessed on 26 February 2020).
- Beketov, M.A.; Liess, M. Acute and delayed effects of the neonicotinoid insecticide thiacloprid on seven freshwater arthropods. Environ. Toxicol. Chem. 2008, 27, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Dubetz, C.; Palace, V.P. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. 2015, 22, 68–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dondero, F.; Negri, A.; Boatti, L.; Marsano, F.; Mignone, F.; Viarengo, A. Transcriptomic and proteomic effects of a neonicotinoid insecticide mixture in the marine mussel (Mytilus galloprovincialis, Lam.). Sci. Total Environ. 2010, 408, 3775–3786. [Google Scholar] [CrossRef]
- Rico, A.; Van den Brink, P.J. Evaluating aquatic invertebrate vulnerability to insecticides based on intrinsic sensitivity, biological traits, and toxic mode of action. Environ. Toxicol. Chem. 2015, 34, 1907–1917. [Google Scholar] [CrossRef]
- Prosser, R.S.; de Solla, S.R.; Holman, E.A.M.; Osborne, R.; Robinson, S.A.; Bartlett, A.J.; Maisonneuve, F.J.; Gillis, P.L. Sensitivity of the early-life stages of freshwater mollusks to neonicotinoid and butenolide insecticides. Environ. Pollut. 2016, 218, 428–435. [Google Scholar] [CrossRef]
- Tufi, S.; Stel, J.M.; de Boer, J.; Lamoree, M.H.; Leonards, P.E. Metabolomics to explore imidacloprid-induced toxicity in the central nervous system of the freshwater snail Lymnaea stagnalis. Environ. Sci. Technol. 2015, 49, 14529–14536. [Google Scholar] [CrossRef]
- Vehovszky, Á.; Farkas, A.; Csikós, V.; Székács, A.; Mörtl, M.; Győri, J. Neonicotinoid insecticides are potential substrates of the multixenobiotic resistance (MXR) mechanism in the non-target invertebrate, Dreissena sp. Aquatic Toxicol. 2018, 205, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Kiss, T.; Györi, J.; Osipenko, O.N.; Maginyan, S.B. Copper-induced non-selective permeability changes in intracellularly perfused snail neurons. J. Appl. Toxicol. 1991, 11, 349–354. [Google Scholar] [CrossRef]
- Salanki, J.; Farkas, A.; Kamardina, T.; Rozsa, K.S. Molluscs in biological monitoring of water quality. Toxicol. Lett. 2003, 140–141, 403–410. [Google Scholar] [CrossRef]
- Gyori, J.; Varro, P.; Zielinska, E.; Banczerowski-Pelyhe, I.; Vilagi, I. Bensultap decreases neuronal excitability in molluscan and mammalian central nervous system. Toxicol. In Vitro 2007, 21, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nierop, P.; Bertrand, S.; Munno, D.W.; Gouwenberg, Y.; van Minnen, J.; Spafford, D.; Syed, N.I.; Bertrand, D.; Smit, A.B. Identification and functional expression of a family of nicotinic acetylcholine receptor subunits in the central nervous system of the mollusc Lymnaea stagnalis. J. Biol. Chem. 2006, 281, 1680–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slater, N.T.; Filbert, M.; Carpenter, D.O. Multiple interactions of anticholinesterases with aplysia acetylcholine responses. Brain Res. 1986, 375, 407–412. [Google Scholar] [CrossRef]
- Belan, P.V.; Kiss, T.; Snitsarev, V.; Storozhuk, M.V.; Osipenko, O.N. The effects of acetylcholine and serotonin on calcium transients and calcium currents in identified Helix pomatia L. neurons. Cell. Sign. 1994, 6, 551–559. [Google Scholar] [CrossRef]
- Krajcs, N.; Pirger, Z.; Hernadi, L.; Kiss, T. Nicotinic acetylcholine receptors containing the alpha 7-like subunit mediate contractions of muscles responsible for space positioning of the snail tentacle. Acta Physiol. 2014, 211, 81. [Google Scholar] [CrossRef] [Green Version]
- Vehovszky, Á.; Kovács, W.A.; Farkas, A.; Győri, J.; Szabó, H.; Vasas, G. Pharmacological studies confirm neurotoxic metabolite(s) produced by the bloom-forming Cylindrospermopsis raciborskii in Hungary. Environ. Toxicol. 2015, 30, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Tomizawa, M.; Casida, J.E. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 2003, 48, 339–364. [Google Scholar] [CrossRef]
- Morakchi, S.; Maiza, A.; Farine, P.; Aribi, N.; Soltani, N. Effects of a neonicotinoid insecticide (acetamiprid) on acetylcholinesterase activity and cuticular hydrocarbons profil in German cockroaches. Comm. Agric. Appl. Biol. Sci. 2005, 70, 843–848. [Google Scholar]
- Azevedo-Pereira, H.M.; Lemos, M.F.; Soares, A.M. Effects of imidacloprid exposure on Chironomus riparius Meigen larvae: Linking acetylcholinesterase activity to behaviour. Ecotoxicol. Environ. Saf. 2011, 74, 1210–1215. [Google Scholar] [CrossRef]
- Boily, M.; Sarrasin, B.; DeBlois, C.; Aras, P.; Chagnon, M. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: Laboratory and field experiments. Environ. Sci. Pollut. Res. 2013, 20, 5603–5614. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qi, S.; Mu, X.; Chai, T.; Yang, Y.; Wang, D.; Li, D.; Che, W.; Wang, C. Evaluation of the toxicity, AChE activity and DNA damage caused by imidacloprid on earthworms, Eisenia fetida. Bull. Environ. Contam. Toxicol. 2015, 95, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.A.; Mohamed, M.S. Imidacloprid induced alterations in enzyme activities and energy reserves of the land snail, Helix aspersa. Ecotoxicol. Environ. Saf. 2013, 95, 91–97. [Google Scholar] [CrossRef]
- Shao, X.; Xia, S.; Durkin, K.A.; Casida, J.E. Insect nicotinic receptor interactions in vivo with neonicotinoid, organophosphorus, and methylcarbamate insecticides and a synergist. Proc. Natl. Acad. Sci. USA 2013, 110, 17273–17277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smulders, C.J.; Bueters, T.J.; Vailati, S.; van Kleef, R.G.; Vijverberg, H.P. Block of neuronal nicotinic acetylcholine receptors by organophosphate insecticides. Toxicol. Sci. 2004, 82, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Győri, J.; Farkas, A.; Stolyar, O.; Székács, A.; Mörtl, M.; Vehovszky, Á. Inhibitory effects of four neonicotinoid active ingredients on acetylcholine esterase activity. Acta Biol. Hung. 2017, 68, 345–357. [Google Scholar] [CrossRef]
- ISO. ISO 11269-2:2012 Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
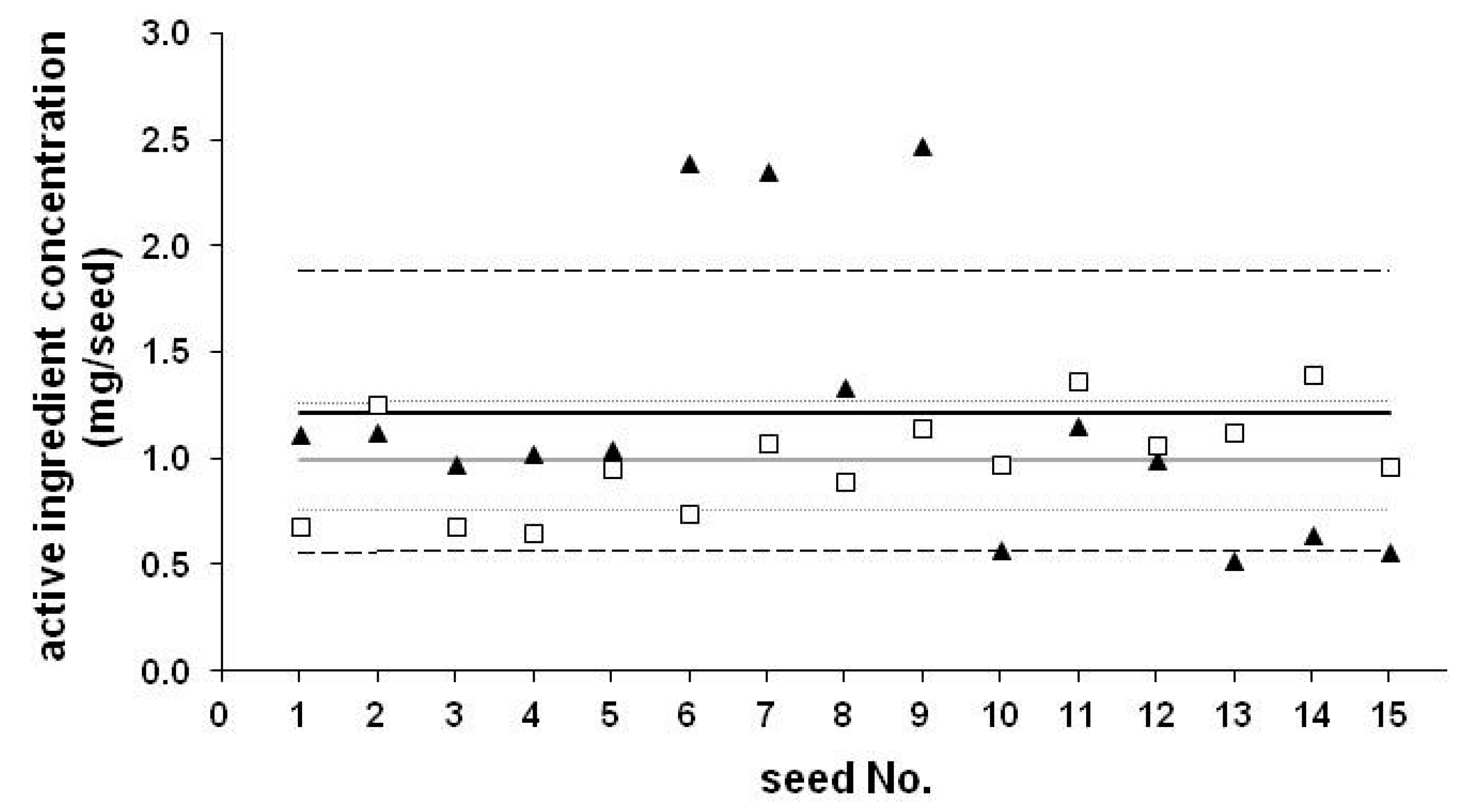









| Code | AI | Concentration (mg/seed) | RSD (%) | Number of Seeds | Year | Recommended Dose (mg/seed) | Typical Doses (mg/seed) |
|---|---|---|---|---|---|---|---|
| CS-1 | TMX | 0.29 | 18.6 | 10 | 2007 | 0.125–1.25 | 1.26 or 0.63 |
| CS-2 | TMX | 0.26 | 15.3 | 10 | 2007 | 0.125–1.25 | 1.26 or 0.63 |
| CS-3 | CLO | 0.997 | 24.2 | 15 | 2009 | 0.25–1.25 | 1.25 |
| HC-1 | CLO | 0.61 | 16.8 | 10 | 2014 | 0.25–1.25 | 1.25 |
| HC-2 | TMX | 0.15 | 20.5 | 10 | 2014 | 0.125–1.25 | 1.26 or 0.63 |
| HC-3 | TCL | 0.054 | 9.7 | 10 | 2015 | 1.00 | 1.00 |
| CS-4 | CLO | 1.217 | 54.3 | 15 | 2013 | 0.25–1.25 | 1.25 |
| CS-5 | TMX | 0.605 | 12.3 | 10 | 2013 | 0.125–1.25 | 1.26 or 0.63 |
| CS-6 | TCL | 1.18 | 11.2 | 10 | 2015 | 1.00 | 1.00 |
| Code | Sampling Date | Concentration (ng/L) | |
|---|---|---|---|
| TMX | CLO | ||
| V1265 | May 4 | <LOD | <LOD |
| V1266 | May 11 | <LOD | <LOD |
| V1267 | May 18 | <LOD | 4.22 |
| V1268 | May 25 | <LOD | <LOD |
| V1269 | June 1 | <LOD | <LOD |
| V1270 | June 8 | <LOD | <LOD |
| V1277 | June 14 | 16.83 | 11.43 |
| V1278 | June 21 | 4.65 | 4.10 |
| V1279 | June 28 | 5.20 | 3.54 |
| V1280 | July 5 | 15.80 | <LOD |
| V1281 | July 11 | <LOD | <LOD |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mörtl, M.; Vehovszky, Á.; Klátyik, S.; Takács, E.; Győri, J.; Székács, A. Neonicotinoids: Spreading, Translocation and Aquatic Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 2006. https://doi.org/10.3390/ijerph17062006
Mörtl M, Vehovszky Á, Klátyik S, Takács E, Győri J, Székács A. Neonicotinoids: Spreading, Translocation and Aquatic Toxicity. International Journal of Environmental Research and Public Health. 2020; 17(6):2006. https://doi.org/10.3390/ijerph17062006
Chicago/Turabian StyleMörtl, Mária, Ágnes Vehovszky, Szandra Klátyik, Eszter Takács, János Győri, and András Székács. 2020. "Neonicotinoids: Spreading, Translocation and Aquatic Toxicity" International Journal of Environmental Research and Public Health 17, no. 6: 2006. https://doi.org/10.3390/ijerph17062006
APA StyleMörtl, M., Vehovszky, Á., Klátyik, S., Takács, E., Győri, J., & Székács, A. (2020). Neonicotinoids: Spreading, Translocation and Aquatic Toxicity. International Journal of Environmental Research and Public Health, 17(6), 2006. https://doi.org/10.3390/ijerph17062006








