Dynamic Transmission of Staphylococcus Aureus in the Intensive Care Unit
Abstract
1. Introduction
2. Methods
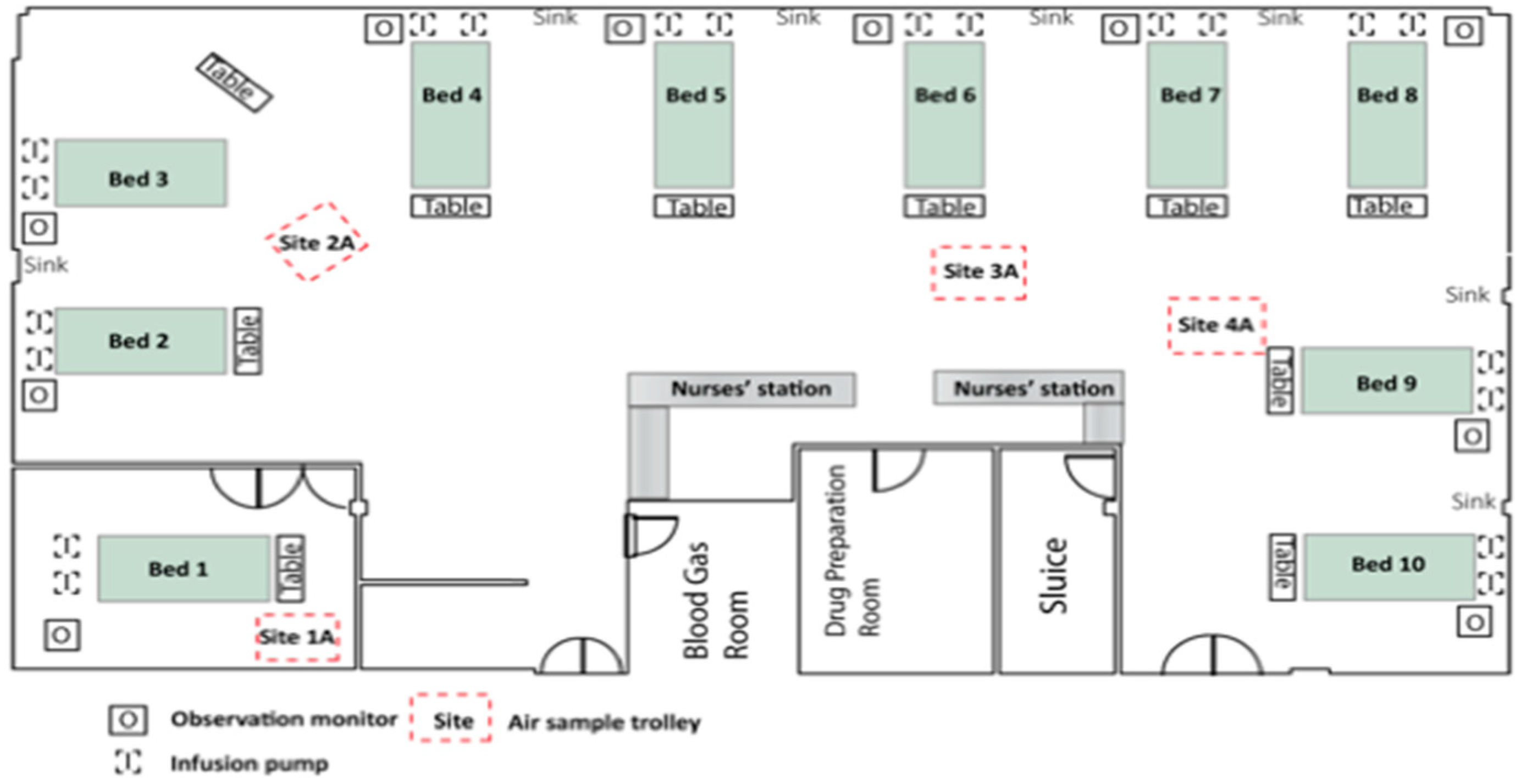
2.1. Setting
2.2. Environmental Screening
2.3. Patients, Visitors and Staff
2.4. Staphylococcal Genotyping
3. Results
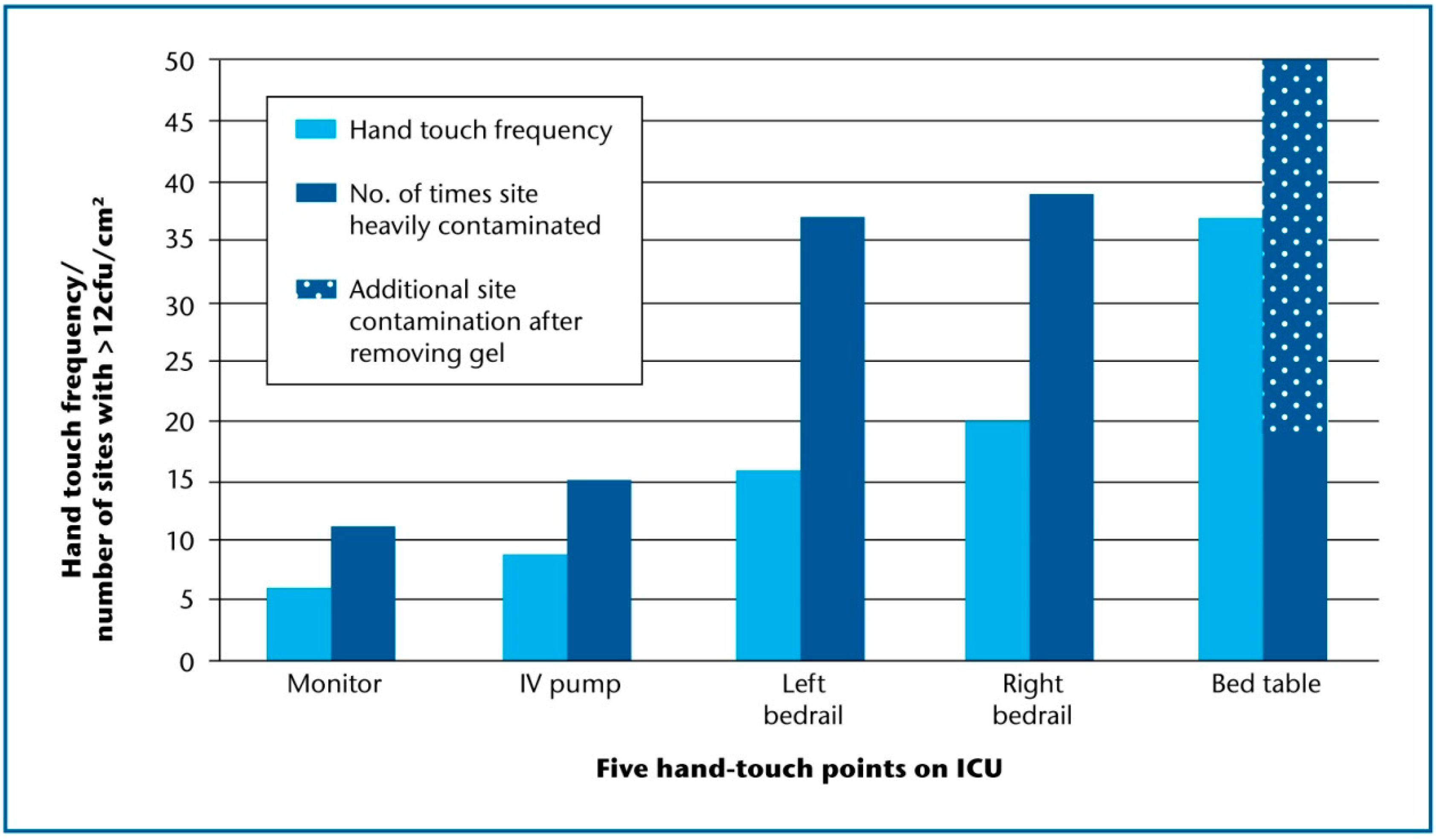
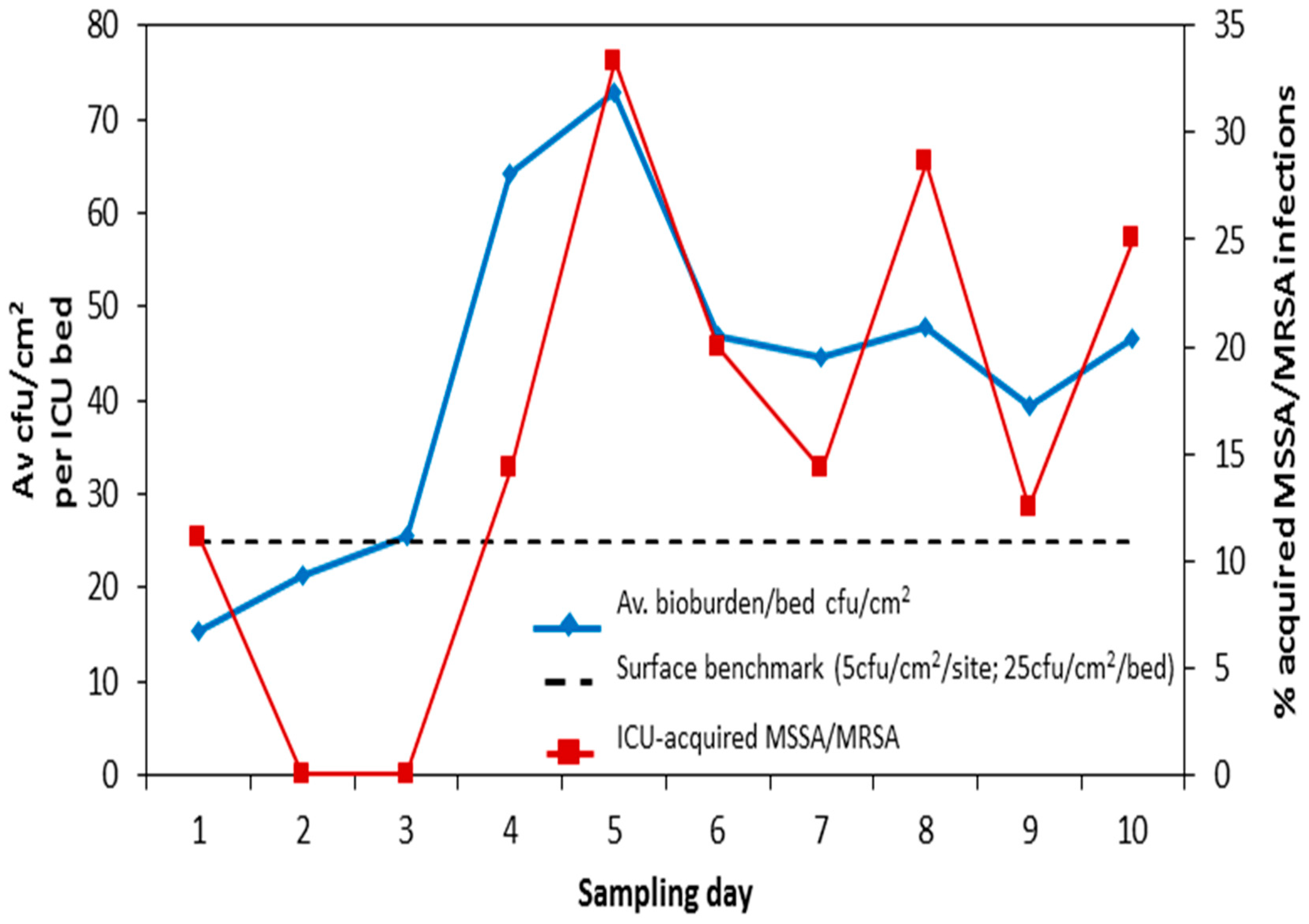
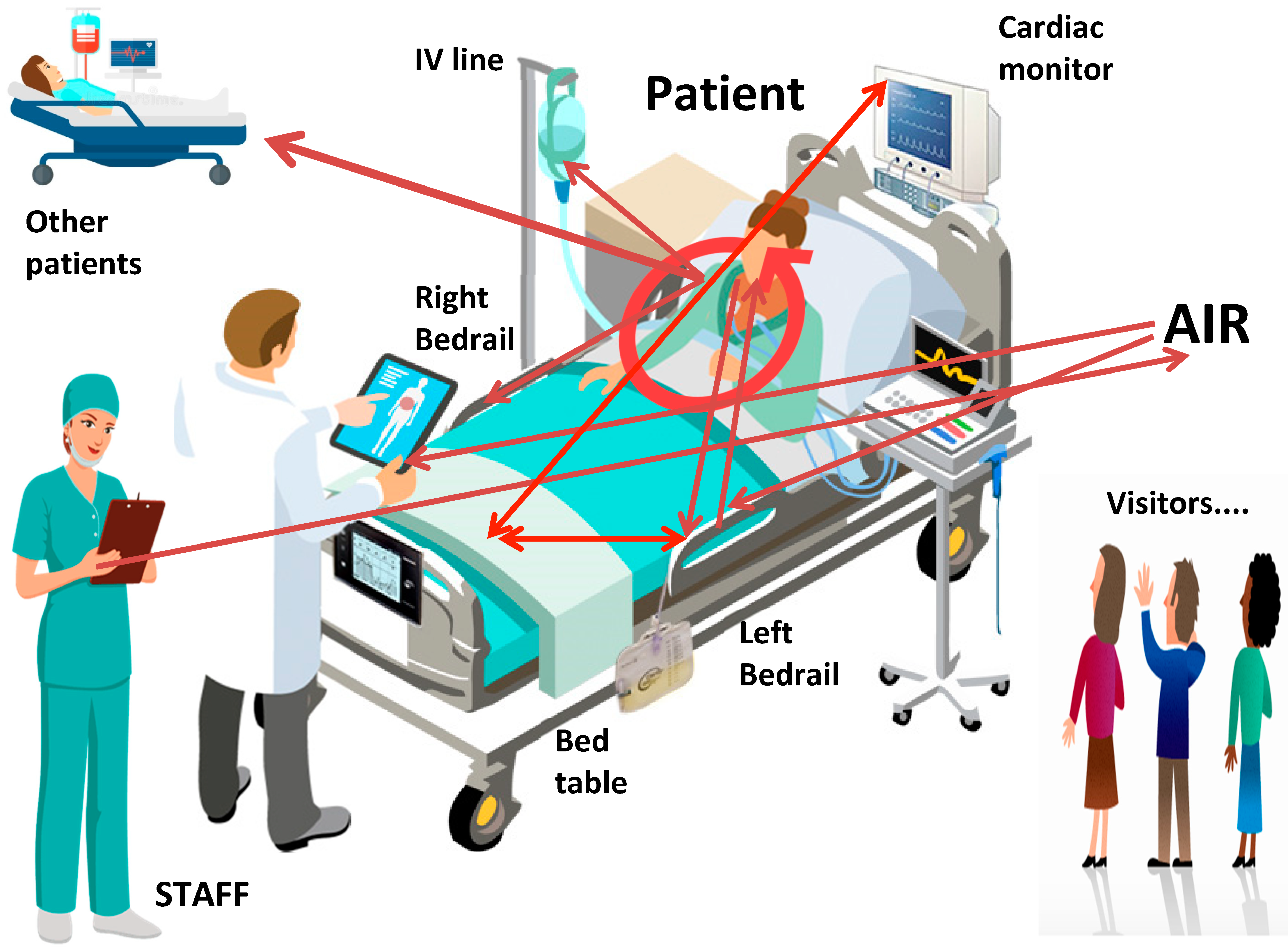
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dancer, S.J. Importance of the environment in MRSA acquisition: The case for hospital cleaning. Lancet Infect. Dis. 2008, 8, 101–113. [Google Scholar] [CrossRef]
- Adams, C.E.; Smith, J.; Robertson, C.; Watson, V.; Dancer, S.J. Examining the relationship between surface bioburden and frequently touched sites in Intensive Care. J. Hosp. Infect. 2017, 95, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Adams, C.E.; King, M.-F.; Robertson, C.; Noakes, C.; Dancer, S.J. Is there a relationship between airborne and surface microbes in the critical care environment? J. Hosp. Infect. 2018, 100, e123–e129. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J.; Adams, C.E.; Smith, J.; Pichon, B.; Kearns, A.; Morrison, D. Tracking Staphylococcus aureus in ICU using Whole-Genome Sequencing. J. Hosp. Infect. 2019, 103, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, A.; Stewart, M.; Hunter, J.; Yip, B.; Reid, D.; Robertson, C. How quickly do hospital surfaces become contaminated after detergent cleaning? Healthc. Infect. 2013, 18, 3–9. [Google Scholar] [CrossRef]
- Pasquarella, C.; Pitzurra, O.; Svino, A. The index of microbial air contamination. J. Hosp. Infect. 2000, 46, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Pasquarella, C.; Vitali, P.; Saccani, E.; Manotti, P.; Boccuni, C.; Ugolotti, M. Microbial air monitoring in operating theatres: Experience at the University Hospital of Parma. J. Hosp. Infect. 2012, 81, 50–57. [Google Scholar] [CrossRef] [PubMed]
- NHS Scotland National Infection Prevention and Control Manual (NIPCM). 2012. Available online: http://www.nipcm.scot.nhs.uk (accessed on 18 March 2020).
- Harmsen, D.; Claus, H.; Witte, W.; Rothganger, J.; Claus, H.; Turnwald, D. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Lahuerta-Marin, A.; Guelbenzu-Gonzalo, M.; Pichon, B.; Allen, A.; Doumith, M.; Lavery, J.F. First report of lukM-positive livestock-associated methicillin-resistant Staphylococcus aureus CC30 from fattening pigs in Northern Ireland. Vet. Microbiol. 2016, 182, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Coll, F.; Harrison, E.M.; Toleman, M.S.; Reuter, S.; Raven, K.E.; Blane, B. Longitudinal genomic surveillance of MRSA in the UK reveals transmission patterns in hospitals and the community. Sci. Transl. Med. 2017, 9, 413. [Google Scholar] [CrossRef]
- White, L.; Dancer, S.J.; Robertson, C.; MacDonald, J. Are hygiene standards useful in assessing infection risk? Am. J. Infect. Control 2008, 36, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.A.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.M.; Ludden, C.; Brodrick, H.J.; Blane, B.; Brennan, G.; Morris, D. Transmission of methicillin-resistant Staphylococcus aureus in long-term care facilities and their related healthcare networks. Genome Med. 2016, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J.; Kramer, A. Four steps to clean hospitals: Look; Plan; Clean; and Dry. J. Hosp. Infect. 2019, 103, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Birnbach, D.J.; Rosen, L.F.; Fitzpatrick, M.; Arheart, K.L.; Munoz-Price, L.S. An evaluation of hand hygiene in an intensive care unit: Are visitors a potential vector for pathogens? J. Infect. Public Health 2015, 8, 570–574. [Google Scholar] [CrossRef] [PubMed]

| Site | No Growth | Scanty Growth < 2.5 cfu/cm2 | Light Growth > 2.5–12 cfu/cm2 | Moderate Growth > 12–40 cfu/cm2 | Heavy Growth > 40 cfu/cm2 | No. of Hygiene Fails (>2.5 cfu/cm2) |
|---|---|---|---|---|---|---|
| Infusion Pump | 16 | 47 MSSA | 22 | 13 MSSA | 2 | 37/100: 37% |
| Cardiac Monitor | 45 | 28 | 16 MSSA | 9 | 2 | 27/100: 27% |
| Right Bedrail | 6 | 38 | 17 | 27 | 12 MSSA | 56/100: 56% |
| Over-bed Table | 13 | 35 | 33 MSSA | 16 MSSA | 3 | 52/100: 52% |
| Left Bedrail | 6 | 31 | 26 | 25 MSSA × 2 | 12 MSSA & MRSA | 63/100: 63% |
| Passive Air Sampling n = 40 | No Growth | Scanty Growth 0–2 cfu/plate | Light Growth > 2–10 cfu/plate | Moderate Growth > 10–40 cfu/plate | Heavy Growth > 40 cfu/plate | No. of Hygiene Fails > 2 cfu/plate/h |
| Air settle cfu/plate/h | 1 | 19 MSSA | 18 | 2 | 0 | 20/40 = 50% |
| Active Air Sampling n = 40 | No Growth | Scanty Growth 0–2 cfu/m3 | L. Growth > 2–10 cfu/m3 | Mod. Growth > 10–40 cfu/m3 | Heavy Growth > 40 cfu/m3 | No. of Hygiene Fails > 10 cfu/m3 |
| Air sampler cfu/m3 | 1 | 6 | 18 MSSA × 2 | 15 MSSA | 0 | 15/40 = 37.5% |
| WGS Category | Transmission Pathway | Lineage (MLST-CC) | Patients and Sites Involved | Days between Clusters | No. SNP Differences |
|---|---|---|---|---|---|
| Highly likely [10] | 1. Autogenous | 8 | Nose & Resp | 2 | <5 |
| 2. Pt ↔ fomite (touch site) | 5 | Pt. 2 Resp, bed 3 → IVP, bed 3 | 3 | <5 | |
| 3. Pt ↔ fomite (touch site) | 5 | Pt. 2 Resp, bed 3 ↔ R/Rail, bed 3 | 3 | <5 | |
| 4. Autogenous | 15 | Nose & Resp | 5 | <5 | |
| 5. Autogenous | 15 | Nose ↔ CLT | 5 | <25 | |
| 6. Autogenous | 22 (MRSA) | Pt. 4 Per & Pt. 4 DRF | 2 | <5 | |
| 7. Autogenous | 22 (MRSA) | Nose & Resp | 2 | 0 | |
| 8. Autogenous | 22 | Nose & Resp | 1 | <5 | |
| 9. Pt ↔ fomite (touch site) | 22 (MRSA) | L/Rail ↔ Pt. 4 Per & Pt. 4 DRF | 1 | <5 | |
| 10. Autogenous | 30 | Resp & Nose | 4 | <5 | |
| 11. Autogenous | 30 | Nose & Resp | 2 | <5 | |
| 12. Autogenous | 30 | Pt. 7 Nose & Pt. 7 Per/Wound | 5 | <5 | |
| 13. Autogenous | 30 | Nose & Wound | 1 | <5 | |
| 14. Autogenous | 45 | Nose ↔ Resp | 1 | <25 | |
| 15. Autogenous | 45 | Nose ↔ Resp | 2 | <5 | |
| 16. Autogenous | 45 | Resp ↔ Nose | 2 | <25 | |
| 17. Autogenous | 45 | Pt. 3 Per ↔ Pt. 3 Wound | 3 | <5 | |
| 18. Air ↔ fomite | 45 | Air, beds 5–7 ↔ L/Rail, bed 7 | 0 | <5 | |
| 19. Fomite ↔ fomite | 45 | Table ↔ CM | 0 | 0 | |
| 20. Autogenous | 7 | Pt. 6 nose ↔ Pt. 6 CLT | 8 | <10 | |
| 21. Autogenous | 34 | Nose ↔ Resp ↔ Thr | 2 | <25 | |
| 22. Autogenous | 59 | Nose ↔ Resp | 5 | <25 | |
| 23. Autogenous | 59 | Nose ↔ Resp | 0 | <25 | |
| 24. Autogenous | 188 | Resp ↔ Nose | 0 | <10 | |
| 25. Autogenous | 121 | Abscess ↔ Nose | 2 | <10 | |
| 26.Staff hand ↔ air | 25 | Hand ↔ Air, beds 5–7 | 43 | <5 | |
| 27. Staff hand ↔ air | 25 | Hand ↔ Air, beds 8–10 | 43 | <5 | |
| Possible | 28. Pt ↔ Pt Cross-infection | 59 | Wound ↔ Nose & Resp | 2 | <25 |
| 29. Pt ↔ Pt Cross-infection | 1 | Nose ↔ Nose | 4 | <25 | |
| Uncertain [14] | 30. Pt ↔ fomite (touch site) | 5 | Resp, bed 2 ↔ L/Rail, bed 2 | 4 | <50 |
| 31. Staff hand ↔ air | 5 | Hand ↔ Settle plate | 50 | <25 | |
| 32. Pt ↔ Pt Cross-infection | 22 (MRSA) | Per ↔ Nose | 161 | <25 | |
| 33. Pt ↔ Pt Cross-infection | 22 (MRSA) | Nose ↔ Nose | 3 | <25 | |
| 34. Fomite ↔ fomite | 30 | L/Rail, bed 4 ↔ Table, bed 7 | 0 | <25 | |
| Presumed (Phenotypic and epidemiologic relationships only) | 1. Autogenous * | 30 | Pt. 5 Nose → Pt. 5 Resp Matching antibiograms | 1 | N/A |
| 2. Autogenous * | 45 | Pt. 8 Nose→ Pt. 8 Wound Matching antibiograms | 4 | N/A | |
| 3. Autogenous * | 1 | Nose → Wound Matching antibiograms | 0 | N/A | |
| 4. Pt ↔ Pt Cross-infection *# | 7 | Pt. 6 Nose/CLT → Pt. 9 Resp | 48 | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, C.E.; Dancer, S.J. Dynamic Transmission of Staphylococcus Aureus in the Intensive Care Unit. Int. J. Environ. Res. Public Health 2020, 17, 2109. https://doi.org/10.3390/ijerph17062109
Adams CE, Dancer SJ. Dynamic Transmission of Staphylococcus Aureus in the Intensive Care Unit. International Journal of Environmental Research and Public Health. 2020; 17(6):2109. https://doi.org/10.3390/ijerph17062109
Chicago/Turabian StyleAdams, Claire E., and Stephanie J. Dancer. 2020. "Dynamic Transmission of Staphylococcus Aureus in the Intensive Care Unit" International Journal of Environmental Research and Public Health 17, no. 6: 2109. https://doi.org/10.3390/ijerph17062109
APA StyleAdams, C. E., & Dancer, S. J. (2020). Dynamic Transmission of Staphylococcus Aureus in the Intensive Care Unit. International Journal of Environmental Research and Public Health, 17(6), 2109. https://doi.org/10.3390/ijerph17062109





