The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder
Abstract
1. Introduction
2. Autism Spectrum Disorder
2.1. Etiological Factors Leading to Autism
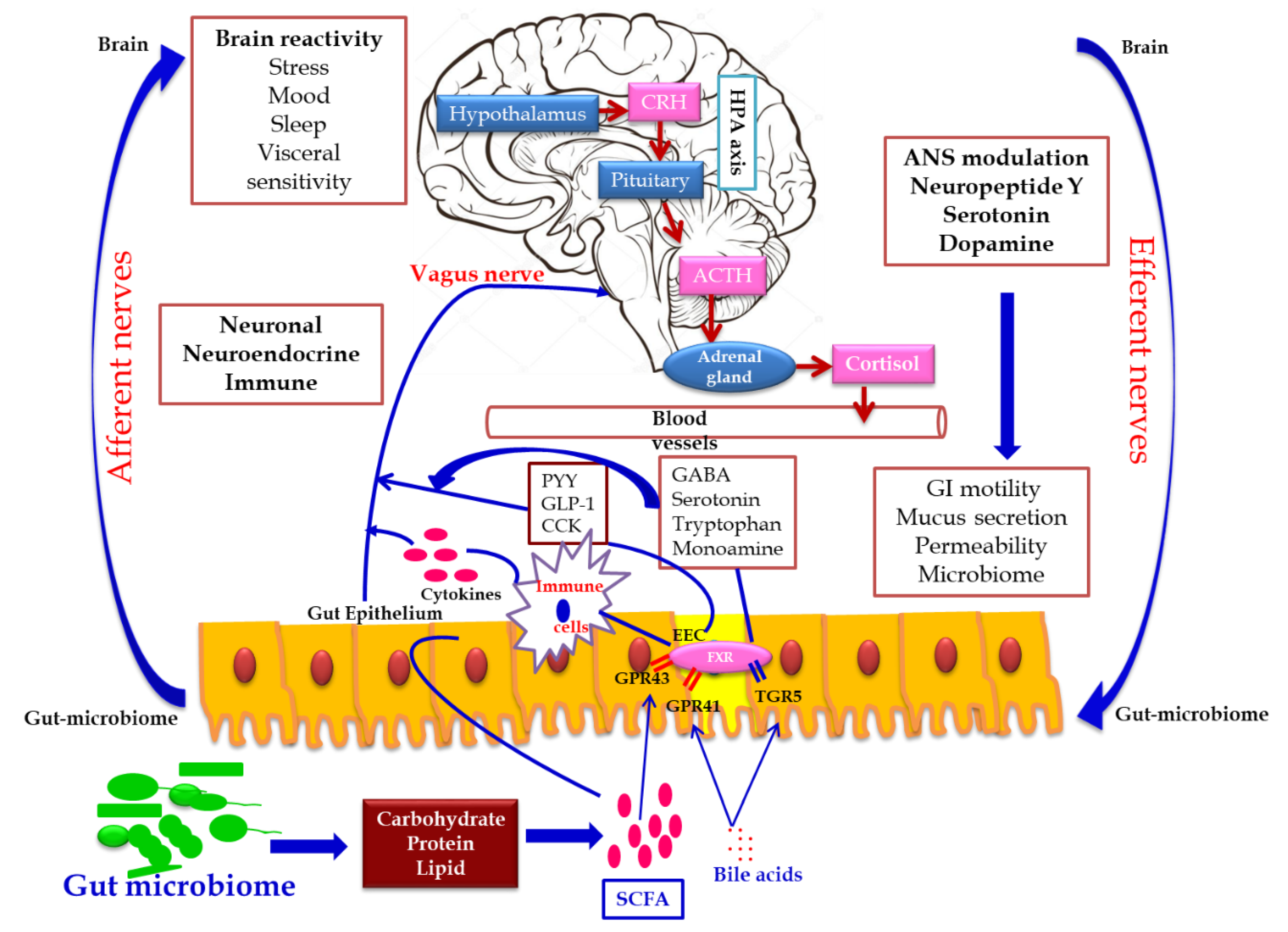
2.2. Link between Gut Microbiome and ASD
2.2.1. Role of Gut Microbiota in Human Nutrition and Health
2.2.2. Link between Gut Microbiome and ASD
2.2.3. Microbial Metabolites Interrelated with ASD
2.2.4. Gut Microbiome-Associated Immune Deregulation
2.2.5. Maternal Risk Factors Regulating Gut Microbiome
3. Diet and ASD
3.1. Recommended Food Supplements for ASD Children
3.2. Elimination Foods of ASD
4. Influence of Probiotic Supplementation on the Health Status of Individuals with ASD
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soke, G.N.; Rosenberg, S.A.; Hamman, R.F.; Fingerlin, T.; Robinson, C.; Carpenter, L.; Giarelli, E.; Lee, L.-C.; Wiggins, L.D.; Durkin, M.S.; et al. Brief report: Prevalence of self-injurious behaviors among children with autism spectrum disorder-a population-based study. J. Autism Dev. Disord. 2016, 46, 3607–3614. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Hsiao, E.Y. Review emerging roles for the gut microbiome in autism spectrum disorder. Biol. Psychiatry 2016, 81, 411–423. [Google Scholar] [CrossRef] [PubMed]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, D.H.; State, M.W. Gene hunting in autism spectrum disorder: On the path to precision medicine. Lancet Neurol. 2015, 14, 1109–1120. [Google Scholar] [CrossRef]
- Amaral, D.G. Examining the Causes of Autism. Cerebrum 2017, 2017, cer-01-17. [Google Scholar] [PubMed]
- Yang, Y.; Tian, J.; Yang, B. Targeting gut microbiome: A novel and potential therapy for autism. Life Sci. 2018, 194, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Cekici, H.; Sanlier, N. Current nutritional approaches in managing autism spectrum disorder: A review. Nutr. Neurosci. 2019, 22, 145–155. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- Mayer, E.A.; Padua, D.; Tillisch, K. Altered brain-gut axis in autism: Comorbidity or causative mechanisms? BioEssays 2014, 36, 933–939. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel. Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism-comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Peretti, S.; Mariano, M.; Mazzocchetti, C.; Mazza, M.; Pino, M.C.; Verrotti Di Pianella, A.; Valenti, M. Diet: The keystone of autism spectrum disorder? Nutr. Neurosci. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tas, A.A. Dietary strategies in Autism Spectrum Disorder (ASD). Prog. Nutr. 2018, 20, 554–562. [Google Scholar]
- Sivamaruthi, B.S.; Prasanth, M.I.; Kesika, P.; Chaiyasut, C. Role of probiotics in human mental health and diseases-A mini review. Trop. J. Pharm. Res. 2019, 18, 889–895. [Google Scholar]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Influence of probiotic supplementation on climacteric symptoms in menopausal women—A mini review. Int. J. App. Pharm. 2018, 10, 43–46. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Probiotic based therapy for atopic dermatitis: Outcomes of clinical studies—A mini review. Asian Pac. J. Trop. Biomed. 2018, 8, 328–332. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S. A comprehensive review on clinical outcome of probiotic and synbiotic therapy for inflammatory bowel diseases. Asian Pac. J. Trop. Biomed. 2018, 8, 179–186. [Google Scholar] [CrossRef]
- Fakhoury, M. Autistic spectrum disorders: A review of clinical features, theories and diagnosis. Int. J. Dev. Neurosci. 2015, 43, 70–77. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef]
- De la Torre-Ubieta, L.; Won, H.; Stein, J.L.; Geschwind, D.H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 2016, 22, 345–361. [Google Scholar] [CrossRef]
- Sanders, S.J.; He, X.; Willsey, A.J.; Ercan-Sencicek, A.G.; Samocha, K.E.; Cicek, A.E.; Murtha, M.T.; Bal, V.H.; Bishop, S.L.; Dong, S.; et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 2015, 87, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Lin, K.; Zhu, L.; Werling, D.M.; Dong, S.; Brand, H.; Wang, H.Z.; Zhao, X.; Schwartz, G.B.; Collins, R.L.; et al. Genome-wide de novo risk score implicates promoter variation in autism spectrum disorder. Science 2018, 362, eaat6576. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Zhang, P.; Hadjimichael, E.; Walker, R.L.; Chen, C.; Liu, S.; Won, H.; van Bakel, H.; Varghese, M.; Wang, Y.; et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018, 362, eaat8127. [Google Scholar] [CrossRef] [PubMed]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef]
- Takano, T. Interneuron dysfunction in syndromic autism: Recent advances. Dev. Neurosci. 2015, 37, 467–475. [Google Scholar] [CrossRef]
- Rais, M.; Binder, D.K.; Razak, K.A.; Ethell, I.M. Sensory Processing Phenotypes in Fragile X Syndrome. ASN Neuro. 2018, 10, 1759091418801092. [Google Scholar] [CrossRef]
- Wiśniowiecka-Kowalnik, B.; Nowakowska, B.A. Genetics and epigenetics of autism spectrum disorder-current evidence in the field. J. Appl. Genet. 2019, 60, 37–47. [Google Scholar] [CrossRef]
- Campistol, J.; Díez-Juan, M.; Callejón, L.; Fernandez-De Miguel, A.; Casado, M.; Garcia Cazorla, A.; Lozano, R.; Artuch, R. Inborn error metabolic screening in individuals with nonsyndromic autism spectrum disorders. Dev. Med. Child Neurol. 2016, 58, 842–847. [Google Scholar] [CrossRef]
- Mazina, V.; Gerdts, J.; Trinh, S.; Ankenman, K.; Ward, T.; Dennis, M.Y.; Girirajan, S.; Eichler, E.E.; Bernier, R. Epigenetics of autism-related impairment: Copy number variation and maternal infection. J. Dev. Behav. Pediatrics 2015, 36, 61–67. [Google Scholar] [CrossRef]
- Schaafsma, S.M.; Gagnidze, K.; Reyes, A.; Norstedt, N.; Mansson, K.; Francis, K.; Pfaff, D.W. Sex-specific gene-environment interactions underlying ASD-like behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, 1383–1388. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef]
- Hsuchou, H.; Pan, W.; Kastin, A.J. Fibroblast growth factor 19 entry into brain. Fluids Barriers CNS 2013, 10, 32. [Google Scholar] [CrossRef]
- Marcelin, G.; Jo, Y.H.; Li, X.; Schwartz, G.J.; Zhang, Y.; Dun, N.J.; Lyu, R.M.; Blouet, C.; Chang, J.K.; Chua, S., Jr. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol. Metab. 2014, 3, 19–28. [Google Scholar] [CrossRef]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Kim, D.Y.; Camilleri, M. Serotonin: A mediator of the brain-gut connection. Am. J. Gastroenterol. 2000, 95, 2698–2709. [Google Scholar]
- Nohr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Moller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The central nervous system and the gut microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, A.J.; Puleston, J.M.; Montgomery, S.M.; Anthony, A.; O’leary, J.J.; Murch, S.H. The concept of entero-colonic encephalopathy, autism and opioid receptor ligands. Aliment Pharmacol. Ther. 2002, 16, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.G.; Jaquess, D.L.; Lukens, C.T. Multi-method assessment of feeding problems among children with autism spectrum disorders. Autism Spectr. Disord. 2013, 7, 56–65. [Google Scholar] [CrossRef]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L.; Hornig, M.; Parekh, T.; Ian Lipkin, W. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio 2012, 3, e00261-11. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.M.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xu, X.; Li, J.; Li, F. Association between gut microbiota and autism spectrum disorder: A systematic review and meta-analysis. Front. Psychiatry 2019, 10, 473. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- De Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A.; Gobbetti, M. Autism spectrum disorders and intestinal microbiota. Gut Microbes 2015, 6, 207–213. [Google Scholar] [CrossRef]
- Macfabe, D.F. Short-chain fatty acid fermentation products of the gut microbiome: Implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2012, 23, 1–24. [Google Scholar] [CrossRef]
- Wang, L.; Conlon, M.A.; Christophersen, C.T.; Sorich, M.J.; Angley, M.T. Gastrointestinal microbiota and metabolite biomarkers in children with autism spectrum disorders. Biomark Med. 2014, 8, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, L.S.; Samsam, A.; Naser, S.A. Propionic acid induces gliosis and neuro-inflammation through modulation of PTEN/AKT pathway in autism spectrum disorder. Sci. Rep. 2019, 9, 8824. [Google Scholar] [CrossRef] [PubMed]
- Altieri, L.; Neri, C.; Sacco, R.; Curatolo, P.; Benvenuto, A.; Muratori, F.; Santocchi, E.; Bravaccio, C.; Lenti, C.; Saccani, M.; et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers 2011, 16, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Yap, I.K.; Angley, M.; Veselkov, K.A.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J. Proteome Res. 2010, 9, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr. Neurosci. 2010, 13, 135–143. [Google Scholar] [CrossRef]
- Wang, H.; Liang, S.; Wang, M.; Gao, J.; Sun, C.; Wang, J.; Xia, W.; Wu, S.; Sumner, S.J.; Zhang, F.; et al. Potential serum biomarkers from a metabolomics study of autism. J. Psychiatry Neurosci. 2016, 41, 27–37. [Google Scholar] [CrossRef]
- Hsiao, E.Y. Immune dysregulation in autism spectrum disorder. Int. Rev. Neurobiol. 2013, 113, 269–302. [Google Scholar]
- Bjorklund, G.; Saad, K.; Chirumbolo, S.; Kern, J.K.; Geier, D.A.; Geier, M.R.; Urbina, M.A. Immune dysfunction and neuroinflammation in autism spectrum disorder. Acta Neurobiol. Exp. 2016, 76, 257–268. [Google Scholar] [CrossRef]
- Machado, C.J.; Whitaker, A.M.; Smith, S.E.; Patterson, P.H.; Bauman, M.D. Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biol. Psychiatry 2015, 77, 823–832. [Google Scholar] [CrossRef]
- Kim, J.W.; Hong, J.Y.; Bae, S.M. Microglia and autism spectrum disorder: Overview of current evidence and novel immunomodulatory treatment options. Clin. Psychopharmacol. Neurosci. 2018, 16, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010, 32, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Patterson, P.H. Maternal infection and immune involvement in autism. Trends Mol. Med. 2011, 17, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Magnusson, C.; Gardner, R.M.; Blomström, A.; Newschaffer, C.J.; Burstyn, I.; Karlsson, H.; Dalman, C. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav. Immun. 2015, 44, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Nousen, E.K.; Chamlou, K.A.; Grove, K.L. The impact of maternal high-fat diet consumption on neural development and behavior of offspring. Int. J. Obes. Suppl. 2012, 2 (Suppl. 2), S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Donnelly, R.; Elkabes, S.; Zhang, P.; Davini, D.; David, B.T.; Ponzio, N.M. Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav. Immun. 2013, 33, 33–45. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Nardone, S.; Elliott, E. The interaction between the immune system and epigenetics in the etiology of autism spectrum disorders. Front. Neurosci. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Sullivan, E.L.; Nousen, L.; Chamlou, K. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol. Behav. 2014, 123, 236–242. [Google Scholar] [CrossRef]
- Lyall, K.; Schmidt, R.J.; Hertz-Picciotto, I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int. J. Epidemiol. 2014, 43, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tancredi, D.J.; Tassone, F.; Hertz-Picciotto, I. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 2011, 22, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Suren, P.; Roth, C.; Bresnahan, M.; Haugen, M.; Hornig, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Magnus, P.; Reichborn-Kjennerud, T.; et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013, 309, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, J.; Cierebiej, M. Evaluation of the nutrition manner and nutritional status of children with autism. Pediatr. Współcz. Gastroenterol. Hepatol. Żywienie Dziecka 2011, 13, 155–160. [Google Scholar]
- Ho, H.H.; Eaves, L.C. Nutrient intake and obesity in children with autism. Focus Autism Dev. Disabil. 1997, 12, 187–193. [Google Scholar] [CrossRef]
- Levy, S.; Souders, M.C.; Ittenbach, R.F.; Giarelli, E.; Mulberg, A.E.; Pinto-Martin, J.A. Relationship of dietary intake to gastrointestinal symptoms in children with autistic spectrum disorders. Biol. Psych. 2007, 61, 492–497. [Google Scholar] [CrossRef]
- Evangeliou, A.; Vlachonikolis, I.; Mihailidou, H.; Spilioti, M.; Skarpalezou, A.; Makaronas, N.; Prokopiou, A.; Christodoulou, P.; Liapi-Adamidou, G.; Helidonis, E.; et al. Application of a ketogenic diet in children with autistic behavior: Pilot study. J. Child Neurol. 2003, 18, 113–118. [Google Scholar] [CrossRef]
- Castro, K.; Baronio, D.; Perry, I.S.; Riesgo R dos, S.; Gottfried, C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutr. Neurosci. 2016, 20, 343–350. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Murphy, M.I.; Slade, S.L.; Masino, S.A. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS ONE 2017, 12, e0171643. [Google Scholar] [CrossRef]
- Napoli, E.; Duenas, N.; Giulivi, C. Potential therapeutic use of the ketogenic diet in autism spectrum disorders. Front. Pediatrics 2014, 2, 69. [Google Scholar] [CrossRef]
- Castro, K.; Faccioli, L.S.; Baronio, D.; Gottfried, C.; Perry, I.S.; dos Santos Riesgo, R. Effect of a ketogenic diet on autism spectrum disorder: A systematic review. Res. Autism Spectr. Disord. 2015, 20, 31–38. [Google Scholar] [CrossRef]
- Bent, S.; Bertoglio, K.; Hendren, R.L. Omega-3 fatty acids for autistic spectrum disorder: A systematic review. J. Autism Dev. Disord. 2009, 39, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.M. Omega-3 DHA and EPA for cognition, behavior, and mood: Clinical findings and structural-functional synergies with cell membrane phospholipids. Altern. Med. Rev. 2007, 12, 207–227. [Google Scholar] [PubMed]
- Das, U.N. Autism as a disorder of deficiency of brain-derived neurotrophic factor and altered metabolism of polyunsaturated fatty acids. Nutrition 2013, 29, 1175–1185. [Google Scholar] [CrossRef]
- Parletta, N.; Niyonsenga, T.; Duff, J. Omega-3 and Omega-6 Polyunsaturated Fatty Acid Levels and Correlations with symptoms in children with attention deficit hyperactivity disorder, autistic spectrum disorder and typically developing controls. PLoS ONE 2016, 11, e0156432. [Google Scholar] [CrossRef]
- Ooi, Y.P.; Weng, S.-J.; Jang, L.Y.; Low, L.; Seah, J.; Teo, S.; Ang, R.P.; Lim, C.G.; Liew, A.; Fung, D.S.; et al. Omega-3 fatty acids in the management of autism spectrum disorders: Findings from an open-label pilot study in Singapore. Eur. J. Clin. Nutr. 2015, 69, 969–971. [Google Scholar] [CrossRef]
- Mankad, D.; Dupuis, A.; Smile, S.; Roberts, W.; Brian, J.; Lui, T.; Genore, L.; Zaghloul, D.; Iaboni, A.; Marcon, P.M.; et al. A randomised, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Mol. Autism 2015, 6, 18. [Google Scholar] [CrossRef]
- Bent, S.; Hendren, R.L.; Zandi, T.; Law, K.; Choi, J.E.; Widjaja, F.; Kalb, L.; Nestle, J.; Law, P. Internet-based, randomised, controlled trial of omega-3 fatty acids for hyperactivity in autism. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 658–666. [Google Scholar] [CrossRef]
- Mazahery, H.; Stonehouse, W.; Delshad, M.; Kruger, M.; Conlon, C.; Beck, K.; von Hurst, P.R. Relationship between long chain n-3 polyunsaturated fatty acids and autism spectrum disorder: Systematic review and meta-analysis of case-control and randomized controlled trials. Nutrients 2017, 9, 155. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Oxidative stress in autism. Pathophysiology 2006, 13, 171–181. [Google Scholar] [CrossRef]
- Essa, M.M.; Braidy, N.; Waly, M.I.; Al-Farsi, Y.M.; Al-Sharbati, M.; Subash, S.; Amanat, A.; Al-Shaffaee, M.A.; Guillemin, G.J. Impaired antioxidant status and reduced energy metabolism in autistic children. Res. Autism Spectr. Disord. 2013, 7, 557–565. [Google Scholar] [CrossRef]
- Smaga, I.; Niedzielska, E.; Gawlik, M.; Moniczewski, A.; Krzek, J.; Przegaliński, E.; Pera, J.; Filip, M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol. Rep. 2015, 67, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Dolske, M.C.; Spollen, J.; McKay, S.; Lancashire, E.; Tolbert, L. A preliminary trial of ascorbic acid as supplemental therapy for autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 1993, 17, 765–774. [Google Scholar] [CrossRef]
- Nye, C.; Brice, A. Combined vitamin B6-magnesium treatment in autism spectrum disorder. Cochrane Database Syst. Rev. 2005, 4, CD003497. [Google Scholar] [CrossRef]
- Kaluzna-Czaplinska, J.; Socha, E.; Rynkowski, J. B vitamin supplementation reduces excretion of urinary dicarboxylic acids in autistic children. Nutr. Res. 2011, 31, 497–502. [Google Scholar] [CrossRef]
- Goodarzi, M.; Hemayattalab, R. Bone mineral density accrual in students with autism spectrum disorders: Effects of calcium intake and physical training. Res. Autism. Spectr. Disord. 2012, 6, 690–695. [Google Scholar] [CrossRef]
- Saad, K.; Abdel-Rahman, A.A.; Elserogy, Y.M.; Al-Atram, A.A.; El-Houfey, A.A.; Othman, H.A.-K.; Bjørklund, G.; Jia, F.; Urbina, M.A.; Abo-Elela, M.G.M.; et al. Randomised controlled trial of vitamin D supplementation in children with autism spectrum disorder. J. Child Psychol. Psychiatry 2018, 59, 20–29. [Google Scholar] [CrossRef]
- Alanazi, A.S. The role of nutraceuticals in the management of autism. Saudi Pharm. J. 2013, 21, 233–243. [Google Scholar] [CrossRef]
- Pennesi, C.M.; Klein, L.C. Effectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: Based on parental report. Nutr. Neurosci. 2012, 15, 85–91. [Google Scholar] [CrossRef]
- Marí-Bauset, S.; Zazpe, I.; Mari-Sanchis, A.; Llopis-González, A.; Morales-Suárez-Varela, M. Evidence of the gluten-free and casein-free diet in autism spectrum disorders: A systematic review. J. Child Neurol. 2014, 29, 1718–1727. [Google Scholar] [CrossRef]
- Knivsberg, A.M.; Reichelt, K.L.; Hoien, T.; Nodland, M. A randomised, controlled study of dietary intervention in autistic syndromes. Nutr. Neurosci. 2002, 5, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, P.; Haracopos, D.; Knivsberg, A.M.; Reichelt, K.L.; Parlar, S.; Jacobsen, J.; Seim, A.; Pedersen, L.; Schondel, M.; Shattock, P. The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr. Neurosci. 2010, 13, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.H.; Shankar, M.; Shuster, J.; Teriaque, D.; Burns, S.; Sherrill, L. Te gluten-free, casein-free diet in autism: Results of a preliminary double blind clinical trial. J. Autism Dev. Disord. 2006, 36, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Handen, B.L.; Zimmer, M.; Sacco, K.; Turner, K. Effects of gluten free / casein free diet in young children with autism: A pilot study. J. Dev. Phys. Disabil. 2011, 23, 213–225. [Google Scholar] [CrossRef]
- Hyman, S.L.; Stewart, P.A.; Foley, J.; Cain, U.; Peck, R.; Morris, D.D.; Wang, H.; Smith, T. Te Gluten-Free/Casein-Free Diet: A double-blind challenge trial in children with autism. J. Autism Dev. Disord. 2016, 46, 205–220. [Google Scholar] [CrossRef]
- Ghalichi, F.; Ghaemmaghami, J.; Malek, A.; Ostadrahimi, A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: A randomised clinical trial. World J. Pediatrics 2016, 12, 436–442. [Google Scholar] [CrossRef]
- Marcason, W. What is the current status of research concerning use of a gluten-free, casein-free diet for children diagnosed with autism? J. Am. Diet. Assoc. 2009, 109, 572. [Google Scholar] [CrossRef]
- Neumeyer, A.M.; Gates, A.; Ferrone, C.; Lee, H.; Misra, M. Bone density in peripubertal boys with autism spectrum disorders. J. Autism Dev. Disord. 2013, 43, 1623–1629. [Google Scholar] [CrossRef]
- Carlin, J.; Hill-Smith, T.E.; Lucki, I.; Reyes, T.M. Reversal of dopamine system dysfunction in response to high-fat diet. Obesity 2013, 21, 2513–2521. [Google Scholar] [CrossRef]
- Sharma, S.; Fernandes, M.F.; Fulton, S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int. J. Obes. 2013, 37, 1183–1191. [Google Scholar] [CrossRef]
- Lane-Donovan, C.; Herz, J. High-fat diet changes hippocampal apolipoprotein E (ApoE) in a genotype- and carbohydrate dependent manner in mice. PLoS ONE 2016, 11, e0148099. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.M.R.T.; Gibson, G.R.; Knott, F.; Bosscher, D.; Kleerebezem, M.; McCartney, A.L. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int. J. Probiotics Prebiotics 2010, 5, 69–74. [Google Scholar]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kałużna-Czaplińska, J.; Błaszczyk, S. The level of arabinitol in autistic children after probiotic therapy. Nutrition 2012, 28, 124–126. [Google Scholar] [CrossRef]
- Grossi, E.; Melli, S.; Dunca, D.; Terruzzi, V. Unexpected improvement in core autism spectrum disorder symptoms after long-term treatment with probiotics. SAGE Open Med. Case Rep. 2016, 4, 2050313X16666231. [Google Scholar] [CrossRef]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef]
- Pärtty, A.; Kalliomäki, M.; Wacklin, P.; Salminen, S.; Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatric Res. 2015, 77, 823–828. [Google Scholar] [CrossRef]

| Subjects | Probiotics | Dose and Duration | Key Findings | Ref. |
|---|---|---|---|---|
| ASD-Children (2.5 to 18 years old) | Any type of probiotic | Daily usage (33%) | Low level of short chain fatty acids | [11] |
| ASD-Children (4 to 16 years old) | Lactobacillus plantarum WCSF1 | 4.5 × 1010 CFU per capsule per day for 3 weeks in the 12 week study duration | ↑ Enterococci and Lactobacilli group. ↓ Clostridium cluster XIVa Improved the stool consistency compared to placebo, and behavioral scores compared to baseline | [112] |
| ASD-Children (2 to 9 years old); Their siblings (5 to 7 years old); Chidren in control group (2 to 11 years old) | 3 Lactobacillus strains, 2 Bifidobacterium strains, and a Streptococcus strain (60:25:15 ratio) | 3 capsules per day (1 capsule thrice a day) for 4 months | In ASD children, Probiotic supplementation normalized Bacteroidetes/Firmicutes ratio ↓ Desulfovibrio spp. ↓ TNFα level in feces | [113] |
| Autistic children (4 to 10 years old) | L. acidophilus Rosell-11 | 5 × 109 CFU per gram; twice a day for 2 months | ↓ d-arabinitol, and d-arabinitol/l-arabinitol ratio in urine | [114] |
| ASD-Child (12 years old boy) | VSL#3 (a mixture of live cells of Lactobacillus delbrueckii subsp. Bulgaricus, L. acidophilus, B. breve, B. longum, B. infantis, L. paracasei, L. plantarum, S. thermophiles) | 5 months of treatment period (4 weeks of initial treatment + 4 months of follow up treatment); 10 months of follow up period | ↓ Severity of abdominal symptoms Improvement in autistic core symptoms | [115] |
| Autistic children (5 to 9 years old) | B. longum, L. rhamnosus, L. acidophilus | 1 × 108 CFU per gram; 5 g per day for 3 months | ↑ Bifidobacteria and Lactobacilli level ↓ Severity of the ASD and GI symptoms | [116] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivamaruthi, B.S.; Suganthy, N.; Kesika, P.; Chaiyasut, C. The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2020, 17, 2647. https://doi.org/10.3390/ijerph17082647
Sivamaruthi BS, Suganthy N, Kesika P, Chaiyasut C. The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder. International Journal of Environmental Research and Public Health. 2020; 17(8):2647. https://doi.org/10.3390/ijerph17082647
Chicago/Turabian StyleSivamaruthi, Bhagavathi Sundaram, Natarajan Suganthy, Periyanaina Kesika, and Chaiyavat Chaiyasut. 2020. "The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder" International Journal of Environmental Research and Public Health 17, no. 8: 2647. https://doi.org/10.3390/ijerph17082647
APA StyleSivamaruthi, B. S., Suganthy, N., Kesika, P., & Chaiyasut, C. (2020). The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder. International Journal of Environmental Research and Public Health, 17(8), 2647. https://doi.org/10.3390/ijerph17082647








