A Metabolically Healthy Profile Is a Transient Stage When Exercise and Diet Are Not Supervised: Long-Term Effects in the EXERDIET-HTA Study
Abstract
1. Introduction
2. Methods
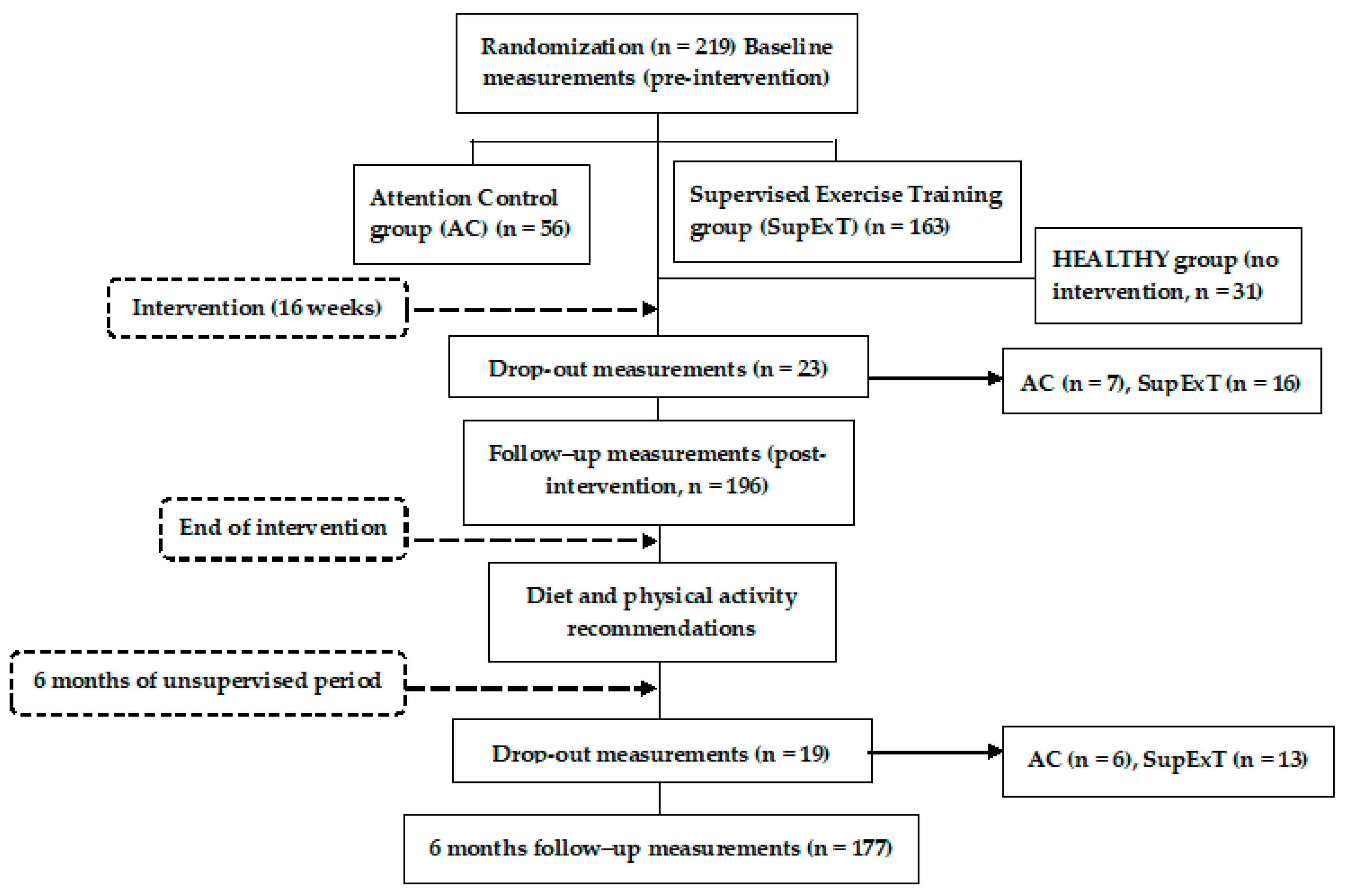
2.1. Research Design
2.2. Participants
2.3. Measurements
2.4. Intervention and 6-Month Post-Intervention Follow-up
2.5. Statistical Analysis
3. Results
3.1. PRE versus POST Changes
3.2. POST versus 6-Month Follow-up Changes
3.3. PRE versus 6-Month Follow-up Changes
3.4. Medication Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Phillips, C.M. Metabolically healthy obesity across the life course: Epidemiology, determinants, and implications. Ann. N. Y. Acad. Sci. 2017, 1391, 85–100. [Google Scholar] [CrossRef]
- Landsberg, L.; Aronne, L.J.; Beilin, L.J.; Burke, V.; Igel, L.I.; Lloyd-Jones, D.; Sowers, J. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment: A position paper of The Obesity Society and the American Society of Hypertension. J. Clin. Hypertens. (Greenwich) 2013, 15, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Jackson, K.L.; Sata, Y.; Head, G.A. Factors Responsible for Obesity-Related Hypertension. Curr. Hypertens. Rep. 2017, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Larrad, M.T.; Corbaton Anchuelo, A.; Del Prado, N.; Ibarra Rueda, J.M.; Gabriel, R.; Serrano-Rios, M. Profile of individuals who are metabolically healthy obese using different definition criteria. A population-based analysis in the Spanish population. PLoS ONE 2014, 9, e106641. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Cadenas-Sanchez, C.; Migueles, J.H.; Labayen, I.; Ruiz, J.R.; Sui, X.; Blair, S.N.; Martinez-Vizcaino, V.; Lavie, C.J. Role of Physical Activity and Fitness in the Characterization and Prognosis of the Metabolically Healthy Obesity Phenotype: A Systematic Review and Meta-analysis. Prog. Cardiovasc. Dis. 2018, 61, 190–205. [Google Scholar] [CrossRef]
- Magkos, F. Metabolically healthy obesity: What’s in a name? Am. J. Clin. Nutr. 2019, 110, 533–539. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Tousoulis, D. The molecular mechanisms of obesity paradox. Cardiovasc. Res. 2017, 113, 1074–1086. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Gorostegi-Anduaga, I.; Corres, P.; MartinezAguirre-Betolaza, A.; Perez-Asenjo, J.; Aispuru, G.R.; Fryer, S.M.; Maldonado-Martin, S. Effects of different aerobic exercise programmes with nutritional intervention in sedentary adults with overweight/obesity and hypertension: EXERDIET-HTA study. Eur. J. Prev. Cardiol. 2018, 25, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Babyak, M.A.; Sherwood, A.; Craighead, L.; Lin, P.H.; Johnson, J.; Watkins, L.L.; Wang, J.T.; Kuhn, C.; Feinglos, M.; et al. Effects of the dietary approaches to stop hypertension diet alone and in combination with exercise and caloric restriction on insulin sensitivity and lipids. Hypertension 2010, 55, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Corres, P.; MartinezAguirre-Betolaza, A.; Fryer, S.M.; Gorostegi-Anduaga, I.; Arratibel-Imaz, I.; Aispuru, G.R.; Maldonado-Martin, S. Long-term Effects in the EXERDIET-HTA Study: Supervised Exercise Training vs. Physical Activity Advice. Res. Q. Exerc. Sport 2019. [Google Scholar] [CrossRef]
- Mora-Rodriguez, R.; Ortega, J.F.; Hamouti, N.; Fernandez-Elias, V.E.; Canete Garcia-Prieto, J.; Guadalupe-Grau, A.; Saborido, A.; Martin-Garcia, M.; Guio de Prada, V.; Ara, I.; et al. Time-course effects of aerobic interval training and detraining in patients with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 792–798. [Google Scholar] [CrossRef]
- Volaklis, K.A.; Douda, H.T.; Kokkinos, P.F.; Tokmakidis, S.P. Physiological alterations to detraining following prolonged combined strength and aerobic training in cardiac patients. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 375–380. [Google Scholar] [CrossRef]
- Moker, E.A.; Bateman, L.A.; Kraus, W.E.; Pescatello, L.S. The relationship between the blood pressure responses to exercise following training and detraining periods. PLoS ONE 2014, 9, e105755. [Google Scholar] [CrossRef]
- Maldonado-Martín, S.; Gorostegi-Anduaga, I.; Aispuru, G.; Illera-Villas, M.; Jurio-Iriarte, B.; Francisco-Terreros, S.; Pérez-Asenjo, J. Effects of different aerobic exercise programs with nutritional intervention in primary hypertensive and overweight/obese adults: EXERDIET-HTA controlled trial. J. Clin. Trials 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- WHO. Global Recommendations on Physical Activity for Health; World Health Organization (WHO): Geneva, Switzerland, 2010. [Google Scholar]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Stamatakis, E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J. Clin. Endocrinol. Metab. 2012, 97, 2482–2488. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Sasaki, H.; Kumagai, S. Association of cardiorespiratory fitness with elevated hepatic enzyme and liver fat in Japanese patients with impaired glucose tolerance and type 2 diabetes mellitus. J. Sports Sci. Med. 2010, 9, 405–410. [Google Scholar] [PubMed]
- Grundy, S.; Becker, D.; Clark, L.; Cooper, R.; Denke, M.; Howard, J.; Hunninghake, D.; Illingworth, D.; Luepker, R.; McBride, P. Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Circulation 2002, 106, 3143–3421. [Google Scholar]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar] [CrossRef]
- Ascaso, J.F.; Romero, P.; Real, J.T.; Priego, A.; Valdecabres, C.; Carmena, R. Insulin resistance quantification by fasting insulin plasma values and HOMA index in a non-diabetic population. Med. Clin. (Barc.) 2001, 117, 530–533. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; Imboden, M.T.; Arena, R.; Myers, J. Reference Standards for Cardiorespiratory Fitness Measured With Cardiopulmonary Exercise Testing Using Cycle Ergometry: Data From the Fitness Registry and the Importance of Exercise National Database (FRIEND) Registry. Mayo Clin. Proc. 2017, 92, 228–233. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Guan, S.; Liu, H.; Wu, X.; Zhang, Z.; Gu, X.; Zhang, Y.; Zhao, Y.; Tse, L.A.; et al. The Prevalence of Metabolically Healthy and Unhealthy Obesity according to Different Criteria. Obes. Facts 2019, 12, 78–90. [Google Scholar] [CrossRef]
- Gorostegi-Anduaga, I.; Corres, P.; Jurio-Iriarte, B.; Martinez-Aguirre, A.; Perez-Asenjo, J.; Aispuru, G.R.; Arenaza, L.; Romaratezabala, E.; Arratibel-Imaz, I.; Mujika, I.; et al. Clinical, physical, physiological, and dietary patterns of obese and sedentary adults with primary hypertension characterized by sex and cardiorespiratory fitness: EXERDIET-HTA study. Clin. Exp. Hypertens. 2018, 40, 141–149. [Google Scholar] [CrossRef]
- Naja, F.; Itani, L.; Nasrallah, M.P.; Chami, H.; Tamim, H.; Nasreddine, L. A healthy lifestyle pattern is associated with a metabolically healthy phenotype in overweight and obese adults: A cross-sectional study. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.; Chen, S.; Durstine, J.L. The effects of exercise training on the traditional lipid profile and beyond. Curr. Sports Med. Rep. 2014, 13, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Kasapis, C.; Thompson, P.D. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S.; Keeffe, E.B. Elevated AST or ALT to nonalcoholic fatty liver disease: Accurate predictor of disease prevalence? Am. J. Gastroenterol. 2003, 98, 955–956. [Google Scholar] [CrossRef]
- Pugh, C.J.; Sprung, V.S.; Jones, H.; Richardson, P.; Shojaee-Moradie, F.; Umpleby, A.M.; Green, D.J.; Cable, N.T.; Trenell, M.I.; Kemp, G.J.; et al. Exercise-induced improvements in liver fat and endothelial function are not sustained 12 months following cessation of exercise supervision in nonalcoholic fatty liver disease. Int. J. Obes. (Lond.) 2016, 40, 1927–1930. [Google Scholar] [CrossRef]
- Johnson, N.A.; George, J. Fitness versus fatness: Moving beyond weight loss in nonalcoholic fatty liver disease. Hepatology 2010, 52, 370–381. [Google Scholar] [CrossRef]
- Haus, J.M.; Solomon, T.P.; Kelly, K.R.; Fealy, C.E.; Kullman, E.L.; Scelsi, A.R.; Lu, L.; Pagadala, M.R.; McCullough, A.J.; Flask, C.A.; et al. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2013, 98, E1181–E1188. [Google Scholar] [CrossRef]
- Borghouts, L.B.; Keizer, H.A. Exercise and insulin sensitivity: A review. Int. J. Sports Med. 2000, 21, 1–12. [Google Scholar] [CrossRef]
- Fletcher, B.; Berra, K.; Ades, P.; Braun, L.T.; Burke, L.E.; Durstine, J.L.; Fair, J.M.; Fletcher, G.F.; Goff, D.; Hayman, L.L.; et al. Managing abnormal blood lipids: A collaborative approach. Circulation 2005, 112, 3184–3209. [Google Scholar] [CrossRef]
- Theodorou, A.A.; Panayiotou, G.; Volaklis, K.A.; Douda, H.T.; Paschalis, V.; Nikolaidis, M.G.; Smilios, I.; Toubekis, A.; Kyprianou, D.; Papadopoulos, I.; et al. Aerobic, resistance and combined training and detraining on body composition, muscle strength, lipid profile and inflammation in coronary artery disease patients. Res. Sports Med. 2016, 24, 171–184. [Google Scholar] [CrossRef]
- Orio, F.; Giallauria, F.; Palomba, S.; Manguso, F.; Orio, M.; Tafuri, D.; Lombardi, G.; Carmina, E.; Colao, A.; Vigorito, C. Metabolic and cardiopulmonary effects of detraining after a structured exercise training programme in young PCOS women. Clin. Endocrinol. (Oxf.) 2008, 68, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Obarzanek, E.; Sacks, F.M.; Vollmer, W.M.; Bray, G.A.; Miller, E.R., 3rd; Lin, P.H.; Karanja, N.M.; Most-Windhauser, M.M.; Moore, T.J.; Swain, J.F.; et al. Effects on blood lipids of a blood pressure-lowering diet: The Dietary Approaches to Stop Hypertension (DASH) Trial. Am. J. Clin. Nutr. 2001, 74, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Bergeron, N.; Williams, P.T.; Bray, G.A.; Sutherland, B.; Krauss, R.M. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Razavi Zade, M.; Telkabadi, M.H.; Bahmani, F.; Salehi, B.; Farshbaf, S.; Asemi, Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: A randomized clinical trial. Liver Int. 2016, 36, 563–571. [Google Scholar] [CrossRef]
- Azadbakht, L.; Surkan, P.J.; Esmaillzadeh, A.; Willett, W.C. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J. Nutr. 2011, 141, 1083–1088. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Sabihi, S.S.; Esmaillzadeh, A. A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition 2013, 29, 619–624. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef]
- Leitao, L.; Pereira, A.; Mazini, M.; Venturini, G.; Campos, Y.; Vieira, J.; Novaes, J.; Vianna, J.; da Silva, S.; Louro, H. Effects of Three Months of Detraining on the Health Profile of Older Women after a Multicomponent Exercise Program. Int. J. Environ. Res. Public. Health. 2019, 16, 3881. [Google Scholar] [CrossRef]
- Kouvari, M.; Panagiotakos, D.B.; Yannakoulia, M.; Georgousopoulou, E.; Critselis, E.; Chrysohoou, C.; Tousoulis, D.; Pitsavos, C.; ATTICA Study Investigators. Transition from metabolically benign to metabolically unhealthy obesity and 10-year cardiovascular disease incidence: The ATTICA cohort study. Metabolism 2019, 93, 18–24. [Google Scholar] [CrossRef]
- Gilardini, L.; Zambon, A.; Soranna, D.; Croci, M.; Invitti, C. Predictors of the transition from metabolically healthy obesity to unhealthy obesity. Eat. Weight Disord. 2018, 23, 739–744. [Google Scholar] [CrossRef] [PubMed]

| Variables | HEALTHY | EXERDIET-HTA | P PHEALTHY-EXERDIET-HTA |
|---|---|---|---|
| (n = 31) | (n = 219) | ||
| Age (years) | 40.0 ± 9.0 | 53.3 ± 7.6 | <0.001 |
| Body mass (kg) | 66.1 ± 10.5 | 92.4 ± 15.1 | <0.001 |
| BMI (kg/m2) | 22.7 ± 2.2 | 32.4 ± 4.2 | <0.001 |
| Waist circumference (cm) | 74.7 ± 8.0 | 103.5 ± 11.2 | <0.001 |
| FFM (%) | 79.1 ± 6.2 | 65.0 ± 7.7 | <0.001 |
| SBP (mmHg) | 114.0 ± 6.6 | 136.0 ± 11.8 | <0.001 |
| DBP (mmHg) | 68.1 ± 7.2 | 78.0 ± 8.3 | <0.001 |
| MBP (mmHg) | 83.4 ± 5.9 | 97.4 ± 8.5 | <0.001 |
| O2peak (mL·kg−1·min−1) | 48.0 ± 8.2 | 22.4 ± 5.4 | <0.001 |
| CRP (mg/L) | 0.8 ± 0.7 | 4.1 ± 3.9 | <0.001 |
| AST (U/L) | 21.9 ± 3.6 | 25.1 ± 9.4 | 0.001 |
| ALT (U/L) | 18.1 ± 5.9 | 33.3 ± 21.2 | <0.001 |
| GGT (U/L) | 16.7 ± 8.3 | 40.1 ± 41.9 | <0.001 |
| TC (mg/dL) | 180.9 ± 35.8 | 206.5 ± 38.3 | 0.001 |
| HDL-C (mg/dL) | 63.0 ± 10.8 | 47.2 ± 11.0 | <0.001 |
| LDL-C (mg/dL) | 104.8 ± 30.3 | 132.0 ± 34.1 | <0.001 |
| TG (mg/dL) | 70.6 ± 21.3 | 139.6 ± 78.9 | <0.001 |
| TC/HDL-C | 2.9 ± 0.5 | 4.6 ± 1.5 | <0.001 |
| Glucose (mg/dL) | 83.1 ± 9.3 | 101.9 ± 24.4 | <0.001 |
| Insulin (mU/L) | 3.9 ± 1.3 | 12.3 ± 7.3 | <0.001 |
| HOMA-IR | 0.8 ± 0.4 | 3.3 ± 2.4 | <0.001 |
| HbA1c (%) | 5.5 ± 0.3 | 5.9 ± 0.8 | 0.019 |
| Medication Intake and Smoking Status | |||
| Statin (%) | 0.0 | 14.2 | 0.024 |
| Hypoglycaemic (%) | 0.0 | 7.8 | 0.105 |
| ACEI (%) | 0.0 | 38.4 | <0.001 |
| ARB (%) | 0.0 | 39.3 | <0.001 |
| Diuretic (%) | 0.0 | 38.8 | <0.001 |
| CCB (%) | 0.0 | 14.2 | 0.024 |
| BB (%) | 0.0 | 6.8 | 0.129 |
| Antiplatelet (%) | 0.0 | 3.7 | 0.275 |
| Smokers (%) | 15.6 | 11.0 | 0.533 |
| Variables | All | Effect | AC | ExT | Time × Group | p Groups PRE-POST | p Groups POST-6 M | p Groups PRE-6 M |
|---|---|---|---|---|---|---|---|---|
| (n = 177) | Size (ηp2) | (n = 43) | (n = 134) | |||||
| CRP (mg/L) | ||||||||
| PRE | 4.0 ± 3.9 * | 0.113 | 3.2 ± 3.2 * | 4.1 ± 4.0 * | 0.595 | 0.677 | 0.937 | 0.361 |
| POST | 2.6 ± 2.6 | 2.2 ± 2.1 | 2.7 ± 2.7 | |||||
| 6 M | 2.9 ± 3.0 φ | 2.7 ± 2.5 | 3.0 ± 3.1 φ | |||||
| AST (U/L) | ||||||||
| PRE | 24.7 ± 8.6 * | 0.060 | 25.2 ± 5.8 | 24.6 ± 9.2 | 0.465 | 0.684 | 0.380 | 0.657 |
| POST | 22.6 ± 8.5 $ | 21.3 ± 5.0 | 22.9 ± 9.2 $ | |||||
| 6 M | 25.0 ± 11.3 | 23.9 ± 6.2 | 25.3 ± 12.3 | |||||
| ALT (U/L) | ||||||||
| PRE | 33.4 ± 20.8 * | 0.112 | 29.4 ± 12.8 | 34.4 ± 22.3 * | 0.529 | 0.090 | 0.103 | 0.923 |
| POST | 25.1 ± 15.4 | 24.2 ± 14.7 | 25.3 ± 15.7 | |||||
| 6 M | 27.9 ± 20.4 | 27.3 ± 13.6 | 28.1 ± 21.9 φ | |||||
| GGT (U/L) | ||||||||
| PRE | 36.1 ± 24.1 * | 0.155 | 36.2 ± 24.8 | 36.1 ± 24.1 * | 0.230 | 0.914 | 0.152 | 0.459 |
| POST | 27.4 ± 18.4 $ | 28.9 ± 27.6 $ | 27.0 ± 15.5 $ | |||||
| 6 M | 31.8 ± 20.6 | 37.1 ± 33.5 | 30.4 ± 15.9 φ | |||||
| TC (mg/dL) | ||||||||
| PRE | 209.2 ± 36.3 * | 0.103 | 207.3 ± 35.7 | 209.7 ± 36.6 * | 0.281 | 0.102 | 0.330 | 0.195 |
| POST | 197.1 ± 35.4 $ | 202.1 ± 35.9 $ | 195.8 ± 35.2 $ | |||||
| 6 M | 207.8 ± 36.1 | 211.0 ± 39.7 | 206.9 ± 35.2 | |||||
| HDL-C (mg/dL) | ||||||||
| PRE | 48.6 ± 11.0 | 0.090 | 47.7 ± 8.0 | 48.8 ± 11.7 | 0.419 | 0.177 | 0.421 | 0.615 |
| POST | 48.5 ± 11.2 $ | 47.1 ± 7.9 | 48.9 ± 12.0 $ | |||||
| 6 M | 51.0 ± 12.7 φ | 48.8 ± 8.6 | 51.6 ± 13.6 φ | |||||
| LDL-C (mg/dL) | ||||||||
| PRE | 135.2 ± 33.5 * | 0.056 | 133.8 ± 35.4 | 135.6 ± 33.1 * | 0.228 | 0.566 | 0.889 | 0.121 |
| POST | 127.3 ± 31.6 $ | 131.2 ± 33.4 $ | 126.2 ± 31.1 $ | |||||
| 6 M | 134.8 ± 31.5 | 140.4 ± 36.0 | 133.3 ± 30.1 | |||||
| TG (mg/dL) | ||||||||
| PRE | 125.2 ± 49.8 * | 0.092 | 121.1 ± 38.2 | 126.3 ± 52.6 * | 0.081 | 0.790 | 0.111 | 0.420 |
| POST | 108.2 ± 44.3 | 118.5 ± 45.4 | 105.4 ± 43.8 | |||||
| 6 M | 109.8 ± 43.2 φ | 108.9 ± 41.8 | 110.0 ± 43.7 φ | |||||
| TC/HDL-C | ||||||||
| PRE | 4.6 ± 1.6 * | 0.050 | 4.5 ± 1.1 | 4.5 ± 1.2 * | 0.130 | 0.723 | 0.588 | 0.609 |
| POST | 4.3 ± 1.2 | 4.4 ± 1.2 | 4.2 ± 1.2 | |||||
| 6 M | 4.4 ± 1.2 φ | 4.5 ± 1.1 | 4.2 ± 1.0 φ | |||||
| Glucose (mg/dL) | ||||||||
| PRE | 102.3 ± 25.7 * | 0.043 | 96.9 ± 12.6 | 104.0 ± 28.3 * | 0.392 | 0.098 | 0.137 | 0.765 |
| POST | 96.8 ± 22.5 $ | 95.2 ± 11.4 | 97.2 ± 24.9 $ | |||||
| 6 M | 101.1 ± 29.7 | 97.1 ± 16.0 | 102.3 ± 32.7 | |||||
| Insulin (mU/L) | ||||||||
| PRE | 11.5 ± 6.1 * | 0.077 | 10.6 ± 5.5 | 11.8 ± 6.3 * | 0.492 | 0.576 | 0.679 | 0.827 |
| POST | 9.6 ± 6.0 | 9.5 ± 5.0 | 9.6 ± 6.3 | |||||
| 6 M | 10.3 ± 5.9 | 9.3 ± 5.3 | 10.6 ± 6.1 | |||||
| HOMA-IR | ||||||||
| PRE | 3.1 ± 2.3 * | 0.094 | 2.5 ± 1.3 | 3.3 ± 2.5 * | 0.250 | 0.183 | 0.882 | 0.836 |
| POST | 2.3 ± 1.7 $ | 2.2 ± 1.1 | 2.4 ± 1.8 $ | |||||
| 6 M | 2.8 ± 2.3 | 2.2 ± 1.5 | 3.0 ± 2.5 | |||||
| HbA1c (%) | ||||||||
| PRE | 5.9 ± 0.9 * | 0.056 | 5.7 ± 0.3 | 6.0 ± 1.0 * | 0.279 | 0.207 | 0.225 | 0.890 |
| POST | 5.7 ± 0.7 $ | 5.6 ± 0.3 | 5.8 ± 0.7 $ | |||||
| 6 M | 5.9 ± 1.0 | 5.6 ± 0.4 | 5.9 ± 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corres, P.; Fryer, S.M.; Aguirre-Betolaza, A.M.; Gorostegi-Anduaga, I.; Arratibel-Imaz, I.; Pérez-Asenjo, J.; Francisco-Terreros, S.; Saracho, R.; Maldonado-Martín, S. A Metabolically Healthy Profile Is a Transient Stage When Exercise and Diet Are Not Supervised: Long-Term Effects in the EXERDIET-HTA Study. Int. J. Environ. Res. Public Health 2020, 17, 2830. https://doi.org/10.3390/ijerph17082830
Corres P, Fryer SM, Aguirre-Betolaza AM, Gorostegi-Anduaga I, Arratibel-Imaz I, Pérez-Asenjo J, Francisco-Terreros S, Saracho R, Maldonado-Martín S. A Metabolically Healthy Profile Is a Transient Stage When Exercise and Diet Are Not Supervised: Long-Term Effects in the EXERDIET-HTA Study. International Journal of Environmental Research and Public Health. 2020; 17(8):2830. https://doi.org/10.3390/ijerph17082830
Chicago/Turabian StyleCorres, Pablo, Simon M. Fryer, Aitor Martínez Aguirre-Betolaza, Ilargi Gorostegi-Anduaga, Iñaki Arratibel-Imaz, Javier Pérez-Asenjo, Silvia Francisco-Terreros, Ramón Saracho, and Sara Maldonado-Martín. 2020. "A Metabolically Healthy Profile Is a Transient Stage When Exercise and Diet Are Not Supervised: Long-Term Effects in the EXERDIET-HTA Study" International Journal of Environmental Research and Public Health 17, no. 8: 2830. https://doi.org/10.3390/ijerph17082830
APA StyleCorres, P., Fryer, S. M., Aguirre-Betolaza, A. M., Gorostegi-Anduaga, I., Arratibel-Imaz, I., Pérez-Asenjo, J., Francisco-Terreros, S., Saracho, R., & Maldonado-Martín, S. (2020). A Metabolically Healthy Profile Is a Transient Stage When Exercise and Diet Are Not Supervised: Long-Term Effects in the EXERDIET-HTA Study. International Journal of Environmental Research and Public Health, 17(8), 2830. https://doi.org/10.3390/ijerph17082830






