Development of Taiwan Risk Score for Sarcopenia (TRSS) for Sarcopenia Screening among Community-Dwelling Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Measurements of Physical Functioning
2.3. Measures and Biomarkers
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Predictors of Sarcopenia
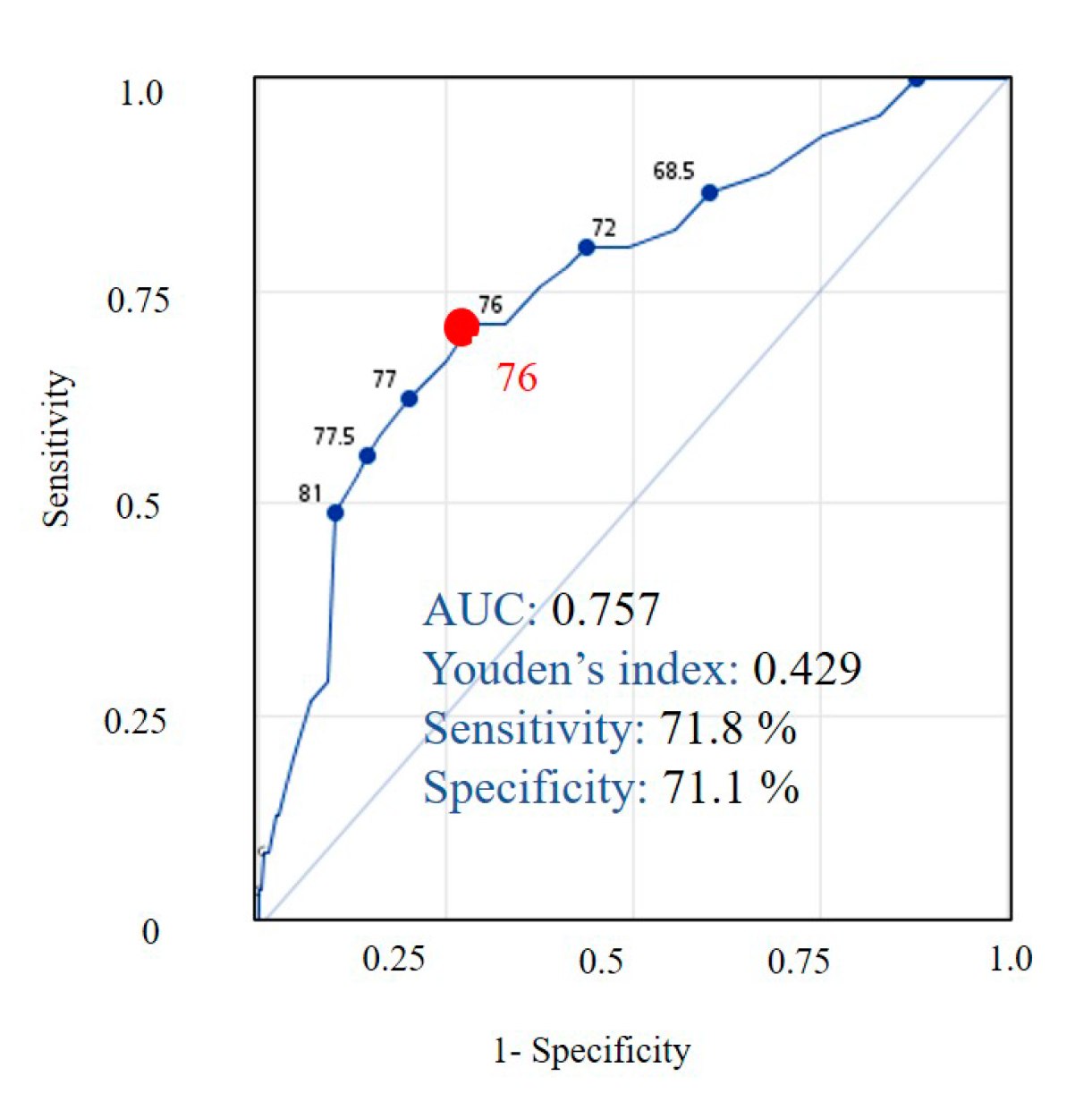
3.3. Development of Taiwan Risk Scores for Sarcopenia Model
4. Discussion
4.1. Practical Aspects of TRSS Model for Public Health Policy
4.2. The Associations between Sarcopenia and Items from TRSS Model
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.K.; Lee, W.J.; Peng, L.N.; Liu, L.K.; Arai, H.; Akishita, M. Recent Advances in Sarcopenia Research in Asia: 2016 Update from the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2016, 17, 767.e1–767.e7. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Leung, J.; Morley, J.E. Defining Sarcopenia in Terms of Incident Adverse Outcomes. J. Am. Med. Dir. Assoc. 2015, 16, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Kera, T.; Kawai, H.; Hirano, H.; Kojima, M.; Watanabe, Y.; Motokawa, K.; Fujiwara, Y.; Osuka, Y.; Kojima, N.; Kim, H.; et al. Limitations of SARC-F in the diagnosis of sarcopenia in community-dwelling older adults. Arch. Gerontol. Geriatr. 2019, 87, 103959. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, M.; Won, C.W. Validation of the Korean Version of the SARC-F Questionnaire to Assess Sarcopenia: Korean Frailty and Aging Cohort Study. J. Am. Med. Dir. Assoc. 2018, 19, 40–45. [Google Scholar] [CrossRef]
- Woo, J.; Leung, J.; Morley, J.E. Validating the SARC-F: A suitable community screening tool for sarcopenia? J. Am. Med. Dir. Assoc. 2014, 15, 630–634. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Murata, K. SARC-F for screening of sarcopenia among older adults: A meta-analysis of screening test accuracy. J. Am. Med. Dir. Assoc. 2018, 19, 685–689. [Google Scholar] [CrossRef]
- Drey, M.; Ferrari, U.; Schraml, M.; Kemmler, W.; Schoene, D.; Franke, A.; Freiberger, E.; Kob, R.; Sieber, C. German Version of SARC-F: Translation, Adaption, and Validation. J. Am. Med. Dir. Assoc. 2020. [Google Scholar] [CrossRef]
- Barbosa-Silva, T.G.; Menezes, A.M.; Bielemann, R.M.; Malmstrom, T.K.; Gonzalez, M.C.; Grupo de Estudos em Composição Corporal e Nutrição. Enhancing SARC-F: Improving Sarcopenia Screening in the Clinical Practice. J. Am. Med. Dir. Assoc. 2016, 17, 1136–1141. [Google Scholar] [CrossRef]
- Ishii, S.; Tanaka, T.; Shibasaki, K.; Ouchi, Y.; Kikutani, T.; Higashiguchi, T.; Obuchi, S.P.; Ishikawa-Takata, K.; Hirano, H.; Kawai, H.; et al. Development of a simple screening test for sarcopenia in older adults. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 93–101. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-H.; Tsai, H.-C.; Chen, H.-C.; Wang, Y.-F.; Tai, C.-J.; Hsu, Y.-N.; Lee, M.-C. Concept, Model and Pilot Project of the Integration of Health and Welfare. J. Med. Health 2017, 6, 1–16. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. Ser. Biol. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimokata, H.; Ando, F.; Yuki, A.; Otsuka, R. Age-related changes in skeletal muscle mass among community-dwelling Japanese: A 12-year longitudinal study. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 85–92. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.I.; Paik, N.J.; Kim, K.W.; Jang, H.C.; Lim, J.Y. Muscle strength: A better index of low physical performance than muscle mass in older adults. Geriatr. Gerontol. Int. 2016, 16, 577–585. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Tiedemann, A.; Shimada, H.; Sherrington, C.; Murray, S.; Lord, S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing 2008, 37, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Lemon, S.C.; Roy, J.; Clark, M.A.; Friedmann, P.D.; Rakowski, W. Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Ann. Behav. Med. 2003, 26, 172–181. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.M.; Kung, P.T.; Chiu, L.T.; Tsai, W.C. Related factors and use of free preventive health services among adults with intellectual disabilities in Taiwan. BMC Health Serv. Res. 2014, 14, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzetti, E.; Hwang, A.C.; Tosato, M.; Peng, L.N.; Calvani, R.; Picca, A.; Chen, L.K.; Landi, F. Age-related changes of skeletal muscle mass and strength among Italian and Taiwanese older people: Results from the Milan EXPO 2015 survey and the I-Lan Longitudinal Aging Study. Exp. Gerontol. 2018, 102, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Oksuzyan, A.; Crimmins, E.; Saito, Y.; O’Rand, A.; Vaupel, J.W.; Christensen, K. Cross-national comparison of sex differences in health and mortality in Denmark, Japan and the US. Eur. J. Epidemiol. 2010, 25, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Lino, V.T.; Rodrigues, N.C.; O’Dwyer, G.; Andrade, M.K.; Mattos, I.E.; Portela, M.C. Handgrip Strength and Factors Associated in Poor Elderly Assisted at a Primary Care Unit in Rio de Janeiro, Brazil. PLoS ONE 2016, 11, e0166373. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, R.J.; Simonsick, E.; Zonderman, A.; Evans, M.K. Association between Race, Household Income and Grip Strength in Middle- and Older-Aged Adults. Ethn. Dis. 2016, 26, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Green, D.; Duque, G.; Fredman, N.; Rizvi, A.; Brennan-Olsen, S.L. Is there a social gradient of sarcopenia? A meta-analysis and systematic review protocol. BMJ Open 2018, 8, e019088. [Google Scholar] [CrossRef] [Green Version]
- Fukuoka, Y.; Narita, T.; Fujita, H.; Morii, T.; Sato, T.; Sassa, M.H.; Yamada, Y. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J. Diabetes Investig. 2019, 10, 322–330. [Google Scholar] [CrossRef]
- Ishii, S.; Tanaka, T.; Akishita, M.; Ouchi, Y.; Tuji, T.; Iijima, K. Metabolic syndrome, sarcopenia and role of sex and age: Cross-sectional analysis of Kashiwa cohort study. PLoS ONE 2014, 9, e112718. [Google Scholar] [CrossRef]
- Du, Y.; Oh, C.; No, J. Associations between Sarcopenia and Metabolic Risk Factors: A Systematic Review and Meta-Analysis. J. Obes. Metab. Syndr. 2018, 27, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Poggiogalle, E.; Lubrano, C.; Sergi, G.; Coin, A.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Sarcopenic Obesity and Metabolic Syndrome in Adult Caucasian Subjects. J. Nutr. Health Aging 2016, 20, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Wells, J.C.; Smith, S.R.; Stephan, B.C.; Siervo, M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Park, H.M.; Ha, Y.C.; Yoo, J.I.; Ryu, H.J. Prevalence of Sarcopenia Adjusted Body Mass Index in the Korean Woman Based on the Korean National Health and Nutritional Examination Surveys. J. Bone Metab. 2016, 23, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.E.; Malmstrom, T.K.; Rodriguez-Mañas, L.; Sinclair, A.J. Frailty, sarcopenia and diabetes. J. Am. Med. Dir. Assoc. 2014, 15, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Abidin Ozturk, Z.A.; Turkbeyler, I.H.; Demir, Z.; Bilici, M.; Kepekci, Y. The effect of blood glucose regulation on sarcopenia parameters in obese and diabetic patients. Turk. J. Phys. Med. Rehabil. 2018, 64, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Propert, K.; Xie, D.; Hamm, L.; He, J.; Miller, E.; Ojo, A.; Shlipak, M.; Teal, V.; Townsend, R.; et al. Measured GFR does not outperform estimated GFR in predicting CKD-related complications. J. Am. Soc. Nephrol. 2011, 22, 1931–1937. [Google Scholar] [CrossRef] [Green Version]
- Hyun, Y.Y.; Lee, K.B.; Rhee, E.J.; Park, C.Y.; Chang, Y.; Ryu, S. Chronic kidney disease and high eGFR according to body composition phenotype in adults with normal BMI. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 1088–1095. [Google Scholar] [CrossRef]
- Barreto, E.F.; Poyant, J.O.; Coville, H.H.; Dierkhising, R.A.; Kennedy, C.C.; Gajic, O.; Nystrom, E.M.; Takahashi, N.; Moynagh, M.R.; Kashani, K.B. Validation of the sarcopenia index to assess muscle mass in the critically ill: A novel application of kidney function markers. Clin. Nutr. 2019, 38, 1362–1367. [Google Scholar] [CrossRef]
- Vettoretti, S.; Caldiroli, L.; Armelloni, S.; Ferrari, C.; Cesari, M.; Messa, P. Sarcopenia is Associated with Malnutrition but Not with Systemic Inflammation in Older Persons with Advanced CKD. Nutrients 2019, 11, 1378. [Google Scholar] [CrossRef] [Green Version]
- Moorthi, R.N.; Avin, K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Sarcopenia | Statistics | ||
|---|---|---|---|---|
| Yes, n = 179 | No, n = 846 | Raw p Value | Adjusted p Value | |
| Age (year), mean ± standard deviation | 76.4 ± 7.6 | 71.0 ± 6.4 | <0.001 ‡ | 0.02 |
| Female, n (%) | 86 (51.5) | 629 (54.9) | 0.23 | 0.83 |
| Education, n (%) | <0.001 † | 0.02 | ||
| Uneducated | 38 (21.2) | 82 (9.7) | ||
| Elementary school | 84 (46.9) | 297 (35.1) | ||
| Junior high school | 20 (11.2) | 131 (15.5) | ||
| Senior high school | 20 (11.2) | 191 (22.6) | ||
| College or above | 17 (9.5) | 145 (17.1) | ||
| Marital status, n (%) | 0.08 | 0.72 | ||
| Married or cohabitant | 145 (81.0) | 117 (13.8) | ||
| Single, divorced, or widowed | 34 (19.0) | 729 (86.2) | ||
| Self-reported economic status, n (%) | 0.047 † | 0.55 | ||
| High-upper income | 9 (5.0) | 15 (1.8) | ||
| Middle income | 83 (46.4) | 337 (39.8) | ||
| Low income | 87 (48.6) | 494 (58.4) | ||
| Social assistance pension, n (%) | 17 (9.5) | 22 (2.6) | <0.001 † | 0.02 |
| Smoking, n (%) | 12 (6.7) | 48 (5.7) | 0.64 | 0.83 |
| Alcohol, n (%) | <0.001 † | 0.02 | ||
| No | 169 (94.4) | 725 (85.7) | ||
| Social drinking | 6 (3.4) | 111 (13.1) | ||
| Alcoholism | 4 (2.2) | 10 (1.2) | ||
| Exercise habits, n (%) | <0.001 † | 0.02 | ||
| No | 80 (44.7) | 206 (24.4) | ||
| Domestic labor or farming | 72 (40.2) | 378 (44.7) | ||
| Exercise ≥ 2.5 h/week | 27 (15.1) | 262 (30.9) | ||
| Self-reported medical history, n (%) | ||||
| Hypertension | 85 (47.5) | 320 (37.8) | 0.02 † | 0.26 |
| Diabetes mellitus | 33 (18.4) | 120 (14.2) | 0.15 | 0.83 |
| Heart disease | 20 (11.2) | 65 (7.7) | 0.12 | 0.83 |
| Hyperlipidemia | 4 (2.2) | 49 (5.8) | 0.05 | 0.55 |
| Cerebrovascular disease | 11 (6.2) | 18 (2.1) | 0.003 † | 0.05 |
| Physical and blood examinations, mean ± standard deviation | ||||
| BMI, kg/m2 | 24.4 ± 4.0 | 24.9 ± 3.6 | 0.17 | 0.83 |
| Fasting glucose level, mg/dL | 110.6 ± 41.3 | 101.5 ± 22.6 | 0.005 ‡ | 0.08 |
| Aspartate aminotransferase, IU/L | 26.0 ± 14.3 | 25.2 ± 10.8 | 0.47 | 0.83 |
| Alanine aminotransferase, IU/L | 21.0 ± 17.0 | 23.6 ± 15.0 | 0.06 | 0.60 |
| Low-density lipoprotein cholesterol, mg/dL | 109.7 ± 33.5 | 116.5 ± 32.6 | 0.01 * | 0.14 |
| High-density lipoprotein cholesterol, mg/dL | 57.9 ± 16.8 | 57.5 ± 16.9 | 0.80 | 0.83 |
| Triglyceride, mg/dL | 118.0 ± 66.9 | 116.7 ± 73.4 | 0.83 | 0.83 |
| Creatinine, mg/dL | 1.04 ± 0.60 | 0.92 ± 0.55 | 0.01 * | 0.14 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 73.4 ± 22.8 | 82.7 ± 22.6 | < 0.001 * | 0.02 |
| Variables | Adjusted OR (95% CI) | Raw p Value | Adjusted p Value |
|---|---|---|---|
| Female | 1.41 (0.94–2.11) | 0.10 | 0.88 |
| Age (75 < age) | 3.44 (2.36–5.02) | <0.001 * | 0.02 * |
| Education (elementary school or below) | 1.36 (0.82–2.24) | 0.23 | 0.88 |
| Single, divorced or widowed | 1.15 (0.71–1.86) | 0.56 | 0.88 |
| Self-reported low income | 1.61 (0.60–4.33) | 0.35 | 0.88 |
| Social assistance pension | 3.04 (1.35–6.84) | 0.007 * | 0.13 |
| Underweight (BMI < 18.5 kg/m2) | 2.31 (1.01–5.33) | 0.05 * | 0.75 |
| Smoking | 1.85 (1.01–3.40) | 0.05 * | 0.75 |
| Alcohol | 0.61 (0.33–1.13) | 0.11 | 0.88 |
| Absence of exercise | 2.28 (1.57–3.30) | <0.001 * | 0.02 * |
| Self-reported medical history | |||
| Heart disease | 0.95 (0.51–1.78) | 0.88 | 0.88 |
| Hypertension | 1.17 (0.81–1.70) | 0.40 | 0.88 |
| Diabetes mellitus | 0.77 (0.43–1.37) | 0.37 | 0.88 |
| Cerebrovascular disease | 2.36 (0.93–5.98) | 0.07 | 0.88 |
| Hyperlipidemia | 0.48 (0.16–1.50) | 0.20 | 0.88 |
| Blood examination evaluation | |||
| Fasting glucose level (>126 mg/dL) | 2.24 (1.25–4.00) | 0.007 * | 0.13 |
| Elevated LDL-C level (>130 mg/dL) | 0.77 (0.51–1.17) | 0.23 | 0.88 |
| Low HDL-C level (<40 mg/dL) | 0.86 (0.49–1.52) | 0.61 | 0.88 |
| Elevated Triglyceride (>150 mg/dL) | 1.04 (0.67–1.63) | 0.86 | 0.88 |
| Abnormal creatinine level † | 1.97 (0.99–3.89) | 0.05 * | 0.75 |
| eGFR (<60 mL/min/1.73 m2) | 1.10 (0.80–1.52) | 0.55 | 0.88 |
| Variables | Adjusted OR (95% CI) | Risk Score Weights |
|---|---|---|
| Age | 1.11 (1.08–1.15) | 1 |
| Female | 1.53 (1.01–2.35) | 1 |
| Social assistance pension | 3.90 (1.66–9.15) | 4 |
| Absence of exercise | 2.30 (1.50–3.50) | 2 |
| Underweight † | 3.07 (1.06–8.87) | 3 |
| Abnormal fasting glucose level ‡ | 2.17 (1.22–3.88) | 2 |
| Abnormal creatinine level § | 2.26 (1.15–4.47) | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, T.-G.; Lu, C.-K.; Hsiao, Y.-H.; Pan, S.-C.; Tai, C.-J.; Lee, M.-C. Development of Taiwan Risk Score for Sarcopenia (TRSS) for Sarcopenia Screening among Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2020, 17, 2859. https://doi.org/10.3390/ijerph17082859
Tseng T-G, Lu C-K, Hsiao Y-H, Pan S-C, Tai C-J, Lee M-C. Development of Taiwan Risk Score for Sarcopenia (TRSS) for Sarcopenia Screening among Community-Dwelling Older Adults. International Journal of Environmental Research and Public Health. 2020; 17(8):2859. https://doi.org/10.3390/ijerph17082859
Chicago/Turabian StyleTseng, Tzyy-Guey, Chun-Kuan Lu, Yu-Han Hsiao, Shu-Chuan Pan, Chi-Jung Tai, and Meng-Chih Lee. 2020. "Development of Taiwan Risk Score for Sarcopenia (TRSS) for Sarcopenia Screening among Community-Dwelling Older Adults" International Journal of Environmental Research and Public Health 17, no. 8: 2859. https://doi.org/10.3390/ijerph17082859






