First Trimester Uterine Rupture: A Case Report and Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Case Report
2.3. Systematic Review of the Literature
3. Discussion
3.1. First-Trimester Uterine Rupture in Scarred Uterus
3.2. First-Trimester Uterine Rupture in Unscarred Uterus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fogelberg, M.; Baranov, A.; Herbst, A.; Osser, O.V. Underreporting of complete uterine rupture and uterine dehiscence in women with previous cesarean section. J. Matern. Neonatal Med. 2016, 30, 2058–2061. [Google Scholar] [CrossRef] [PubMed]
- Guise, J.-M.; Denman, M.A.; Emeis, C.; Marshall, N.; Walker, M.; Fu, R.; Janik, R.; Nygren, P.; Eden, K.B.; McDonagh, M. Vaginal Birth After Cesarean. Obstet. Gynecol. 2010, 115, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.S. The CARE guidelines: Consensus-based clinical case report guideline development. J. Clin. Epidemiol. 2014, 67, 46–51. [Google Scholar] [CrossRef]
- Olivola, S.; Xodo, S.; Olivola, E.; Cecchini, F.; Londero, A.P.; Driul, L. Parkinson’s Disease in Pregnancy: A Case Report and Review of the Literature. Front. Neurol. 2020, 10, 1349. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.M.; Ali, S.S.; Michael, A.; Badran, S.A. Caesarean Scar Ectopic Pregnancy Complicated by Uterine Rupture at 10 Weeks Gestation. J. Gynecol. Surg. 2017, 33, 261–263. [Google Scholar] [CrossRef]
- Abbas, A.M.; Hussein, R.S.; Ali, M.N.; Shahat, M.A.; Mahmoud, A.-R. Spontaneous first trimester posterior uterine rupture in a multiparous woman with scarred uterus: A case report. Middle East Fertil. Soc. J. 2018, 23, 81–83. [Google Scholar] [CrossRef]
- Abbas, A.M.; Michael, A.; Ali, S.S.; Abdalmageed, O.S. Placenta percreta presenting with marked hemoperitoneum in the first trimester of pregnancy: A case report. Middle East Fertil. Soc. J. 2018, 23, 251–253. [Google Scholar] [CrossRef]
- Akbaş, M.; Ömeroğlu, I.; Birge, Ö. Spontaneous Uterine Rupture with Retroperitoneal Hematoma in the First Trimester: Case Report. Turk. Klin. J. Gynecol. Obstet. 2015, 25, 205–208. [Google Scholar] [CrossRef]
- Alloub AH, M. Spontaneous rupture of a 13 week gravid scarred uterus. J. Obstet. Gynaecol. 1999, 19, 316–317. [Google Scholar] [CrossRef]
- Ambrogi, G.; Ambrogi, G.; Marchi, A.A. Placenta Percreta and Uterine Rupture in the First Trimester of Pregnancy. Case Rep. Obstet. Gynecol. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amro, B.; Lotfi, G. Spontaneous rupture of an unscarred uterus in early pregnancy: A rare but life-threatening emergency. BMJ Case Rep. 2019, 12, e228493. [Google Scholar] [CrossRef] [PubMed]
- Arbab, F.; Bied, V.; Payan, F.; Lornage, J.; Boulieu, D.; Guérin, J. Uterine rupture in first or second trimester of pregnancy after in-vitro fertilization and embryo transfer. Hum. Reprod. 1996, 11, 1120–1122. [Google Scholar] [CrossRef]
- Bandarian, M.; Bandarian, F. Spontaneous rupture of the uterus during the 1st trimester of pregnancy. J. Obstet. Gynaecol. 2014, 35, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Bechem, E.; Leopold, D.; Ako, T.W. Small bowel exteriorisation after uterine perforation from manual vacuum aspiration for abortion in a young cameroonian: A case report. Pan. Afr. med. j. 2016, 25. [Google Scholar] [CrossRef]
- Biljan, M.M.; Cushing, K.; McDicken, I.W.; Garden, A.S. Spontaneous uterine rupture in the first trimester of pregnancy. J. Obstet. Gynaecol. 1996, 16, 174–175. [Google Scholar] [CrossRef]
- Bruand, M.; Thubert, T.; Winer, N.; Gueudry, P.; Dochez, V. Rupture of Non-communicating Rudimentary Horn of Uterus at 12 Weeks’ Gestation. Cureus 2020, 12, e7191. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.K.; Ryu, H.K.; Kim, C.H. Placenta Percreta–Induced Uterine Rupture at 7th Week of Pregnancy After In Vitro Fertilization in a Primigravida Woman: Case Report. J. Emerg. Med. 2017, 53, 126–129. [Google Scholar] [CrossRef]
- Dabulis, S.A.; McGuirk, T.D. An Unusual Case of Hemoperitoneum: Uterine Rupture at 9 Weeks Gestational Age. J. Emerg. Med. 2007, 33, 285–287. [Google Scholar] [CrossRef]
- Dandawate, B.; Carpenter, T. Caesarean scar pregnancy presenting as anaemia. J. Obstet. Gynaecol. 2009, 29, 772–773. [Google Scholar] [CrossRef]
- Deroux, S.J.; Prendergast, N.C.; Adsay, N.V. Spontaneous uterine rupture with fatal hemoperitoneum due to placenta accreta percreta: A case report and review of the literature. Int. J. Gynecol. Pathol. 1999, 18, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Dibbs, K.; Ball, R.; Huettner, P. Spontaneous Uterine Rupture and Hemoperitoneum in the First Trimester. Am. J. Perinatol. 1995, 12, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Esmans, A.; Gerris, J.; Corthout, E.; Verdonk, P.; Declercq, S. Placenta percreta causing rupture of an unscarred uterus at the end of the first trimester of pregnancy: Case report. Hum. Reprod. 2004, 19, 2401–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galinski, M.; Pétrovic, T.; Rodrigues, A.; Hermann, M.; Catineau, J.; Adnet, F.; Lapostolle, F. Out-of-Hospital Diagnosis of a Ruptured Ectopic Pregnancy: Myometrial Embryo Implantation, an Exceptional Diagnosis. Prehospital Emerg. Care 2010, 14, 496–498. [Google Scholar] [CrossRef]
- Hefny, A.F.; Kunhivalappil, F.T.; Nambiar, R.; Bashir, M.O. A rare case of first-trimester ruptured bicornuate uterus in a primigravida. Int. J. Surg. Case Rep. 2015, 14, 98–100. [Google Scholar] [CrossRef] [Green Version]
- Iddenden, D.; Nuttall, I. Early spontaneous rupture of the gravid uterus. Am. J. Obstet. Gynecol. 1983, 147, 971–972. [Google Scholar] [CrossRef]
- Ijaz, S.; Mahendru, A.; Sanderson, D. Spontaneous uterine rupture during the 1st trimester: A rare but life-threatening emergency. J. Obstet. Gynaecol. 2011, 31, 772. [Google Scholar] [CrossRef]
- Ismail, S.I.M.F.; Toon, P.G. First trimester rupture of previous caesarean section scar. J. Obstet. Gynaecol. 2007, 27, 202–204. [Google Scholar] [CrossRef]
- Ito, M.; Nawa, T.; Mikamo, H.; Tamaya, T. Lower segment uterine rupture related to early pregnancy by in vitro fertilization and embryo transfer after a previous cesarean delivery. J. Med. 1998, 29, 85–91. [Google Scholar]
- Jain, S.; Chaudhary, S.; Jain, N.; Ranjan, R. Ruptured caesarean scar ectopic pregnancy: A rare case report. Int. J. Reprod. Contraception Obstet. Gynecol. 2015, 3011–3012, 3011–3012. [Google Scholar] [CrossRef] [Green Version]
- Jang, D.G. Placenta Percreta-Induced Uterine Rupture Diagnosed By Laparoscopy in the First Trimester. Int. J. Med Sci. 2011, 8, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerve, F.; Fylling, P.; Stenby, S. Rupture of the uterus following treatment with 16-16-dimethyl E 2 prostaglandin vagitories. Prostaglandins 1979, 17, 121–123. [Google Scholar] [CrossRef]
- Jwarah, E.; Greenhalf, J.O. Rupture of the uterus after 800 micrograms misoprostol given vaginally for termination of pregnancy. BJOG: Int. J. Obstet. Gynaecol. 2000, 107, 807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabra, S.L.; Laul, P.; Godha, Z.; Kadam, V.K. Case Series: Spontaneous Rupture of Uterus in Early Pregnancy. J. Obstet. Gynecol. India 2016, 66, 710–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, S.; Gardner, F.J.E.; De Chazal, R.; Brown, L.J.R. Ruptured rudimentary horn and TRAP syndrome. J. Obstet. Gynaecol. 2008, 28, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Alison, G. Uterine rupture at 8 weeks’ gestation following 600 Mg of oral misoprostol for management of delayed miscarriage. J. Obstet. Gynaecol 2007, 27, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Han, J.Y.; Choi, J.S.; Ahn, H.K.; Yang, J.H.; Kang, I.S.; Song, M.J.; Nava-Ocampo, A.A. Oral misoprostol and uterine rupture in the first trimester of pregnancy: A case report. Reprod. Toxicol. 2005, 20, 575–577. [Google Scholar] [CrossRef]
- Lazarus, E.J. Early rupture of the gravid uterus. Am. J. Obstet. Gynecol. 1978, 132, 224. [Google Scholar] [CrossRef]
- Lee, F.; Zahn, K.; Knittel, A.K.; Morse, J.; Louie, M. Laparoscopic hysterectomy to manage uterine rupture due to placenta percreta in the first trimester: A case report. Case Rep. Women’s Heal. 2019, 25, e00165. [Google Scholar] [CrossRef]
- Liang, H.-S.; Jeng, C.-J.; Sheen, T.-C.; Lee, F.-K.; Yang, Y.-C.; Tzeng, C.-R. First-trimester uterine rupture from a placenta percreta. A case report. J Reprod. Med. 2003, 48, 474–478. [Google Scholar]
- Liao, C.-Y.; Ding, D. Repair of Uterine Rupture in Twin Gestation after Laparoscopic Cornual Resection. J. Minim. Invasive Gynecol. 2009, 16, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Lincenberg, K.R.; Behrman, E.R.; Bembry, J.S.; Kovac, C.M. Uterine Rupture with Cesarean Scar Heterotopic Pregnancy with Survival of the Intrauterine Twin. Case Rep. Obstet. Gynecol. 2016, 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, N.; Moretti, M.L.; Lakhi, N.A. Spontaneous early first and second trimester uterine rupture following robotic-assisted myomectomy. J. Obstet. Gynaecol. 2018, 39, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Marcellus, M.; Jenkins, D.M.; Keohane, C. Intra abdominal rupture of first trimester cervical pregnancy. Ir. J. Med Sci. 1989, 158, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.; Cheng, E.; Goff, B. Extrauterine pregnancy resulting from early uterine rupture. J. Obstet. Gynaecol. 1999, 94, 804–805. [Google Scholar]
- Masia, F.; Zoric, L.; Ripart-Neveu, S.; Mares, P.; Ripart, J. Spontaneous uterine rupture at 14 weeks gestation during a pregnancy consecutive to an oocyte donation in a woman with Turner’s syndrome. Anaesth. Crit. Care Pain Med. 2015, 34, 101–103. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimoya, K.; Shinkai, T.; Ohashi, H.; Koyama, M.; Yamasaki, M.; Murata, Y. Uterine rupture of cesarean scar related to spontaneous abortion in the first trimester. J. Obstet. Gynaecol. Res. 2004, 30, 34–36. [Google Scholar] [CrossRef]
- Miranda, A.; Castro, L.; Rocha, M.J.; Cardoso, L.; Reis, I. Uterine Rupture in Early Pregnancy. Int. J. Pregnancy Child Birth 2017, 2, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Mosad, A.; Altraigey, A. Scar pregnancy and spontaneous rupture uterus — a case report. Ginekol. Polska 2017, 88, 698–699. [Google Scholar] [CrossRef] [Green Version]
- Nassar, A.H.; Charara, I.; Nawfal, A.K.; Ghulmiyyah, L.; Usta, I.M. Ectopic pregnancy in a uterine perforation site. Am. J. Obstet. Gynecol. 2009, 201, e15–e16. [Google Scholar] [CrossRef]
- Okada, Y.; Hasegawa, J.; Mimura, T.; Arakaki, T.; Yoshikawa, S.; Yamashita, Y.; Oba, T.; Nakamura, M.; Matsuoka, R.; Sekizawa, A. Uterine rupture at 10 weeks of gestation after laparoscopic myomectomy. J. Med. Ultrason. 2015, 43, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Ozeren, M.; Ulusoy, M.; Uyanik, E. First-trimester spontaneous uterine rupture after traditional myomectomy: Case report. Isr. J. Med Sci. 1997, 33, 752–753. [Google Scholar] [PubMed]
- Panayotidis, C.; Abdel-Fattah, M.; Leggott, M. Rupture of rudimentary uterine horn of a unicornuate uterus at 15 weeks’ gestation. J. Obstet. Gynaecol. 2004, 24, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Tripathi, B.N.; Mishra, P. Rudimentary Horn Pregnancy: A Rare First Trimester Acute Presentation. Int. J. Women’s Heal. Reprod. Sci. 2015, 3, 115–117. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-J.; Ryu, K.-Y.; Lee, J.-I.; Park, M.-I. Spontaneous Uterine Rupture in the First Trimester: A Case Report. J. Korean Med Sci. 2005, 20, 1079–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyser, M.R.; Toaff, R. Rupture of uterus in the first trimester caused by high-conentration oxytocin drip. Obstet. Gynecol. 1972, 40, 371–372. [Google Scholar]
- Porcu, G.; Courbière, B.; Sakr, R.; Carcopino, X.; Gamerre, M. Spontaneous rupture of a first-trimester gravid uterus in a woman exposed to diethylstilbestrol in utero. A case report. J. Reprod. Med. 2003, 48, 744–746. [Google Scholar]
- Pridjian, G.; Rich, N.E.; Montag, A.G. Pregnancy hemoperitoneum and placenta percreta in a patientwith previous pelvic irradiation and ovarian failure. Am. J. Obstet. Gynecol. 1990, 162, 1205–1206. [Google Scholar] [CrossRef]
- A Rouzi, A.; AlMarzouki, A.; Tallab, F.; Ashkar, L. Medical management of early pregnancy failure with misoprostol with rupture of the cesarean section scar pregnancy. Clin. Exp. Obstet. Gynecol. 2017, 44, 477–479. [Google Scholar]
- Saghafi, N.; Maleki, A.; Ayati, S.; Shirinzadeh, L. First Trimester Uterine Rupture, a Rare but Life-Threatening Event: A Case Report. Iran. J. Med. Sci. 2019, 44, 422–426. [Google Scholar]
- Shah, P.; Manandhar, R.; Thapa, M.; Saha, R. Ruptured Cesarean Scar Pregnancy: A Case Report. J. Nepal. Med. Assoc. 2019, 57, 209–212. [Google Scholar] [CrossRef]
- Shaikh, S.; Shaikh, N.B.; Channa, S.; Ghori, A. First trimester uterine rupture due to scar ectopic pregnancy. Med. Channel 2012, 19, 68–70. [Google Scholar]
- Sherer, D.M.; Dalloul, M.; Cho, Y.; Mylvaganam, S.R.; Adeyemo, I.; Zinn, H.L.; Abulafia, O. Spontaneous First-Trimester Perforation of the Uterus following Cesarean Scar Pregnancy Choriocarcinoma. Ultrasound Obstet. Gynecol. 2016, 47, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jain, S. Spontaneous rupture of unscarred uterus in early pregnancy: - a rare entity. Acta Obstet. et Gynecol. Scand. 2000, 79, 431–432. [Google Scholar] [CrossRef]
- Singh, K.; Soni, A.; Rana, S. Ruptured Ectopic Pregnancy in Caesarean Section Scar: A Case Report. Case Rep. Obstet. Gynecol. 2012, 2012, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Singh, U.; Verma, M.L. Ruptured bicornuate uterus mimicking ectopic pregnancy: A case report. J. Obstet. Gynaecol. Res. 2012, 39, 364–366. [Google Scholar] [CrossRef]
- Sinha, P.; Agrawal, N.R. Spontaneous uterine rupture in first trimester of pregnancy. Int. J. Reprod. Contraception Obstet. Gynecol. 2014, 3, 831–832. [Google Scholar] [CrossRef]
- Smith, L.; Mueller, P. Abdominal pain and hemoperitoneum in the gravid patient: A case report of placenta percreta. Am. J. Emerg. Med. 1996, 14, 45–47. [Google Scholar] [CrossRef]
- Sujatha, B.; Bharatnur, S.; Virmani, S.; Hebbar, S.; Bishnu, A. Ruptured CaesarianScar Ectopic Pregnancy. Online J. Health. Allied Scs 2017, 16, 14. [Google Scholar]
- Sultana, R.; Islam, S. Nurjahan, - Caesarean Scar Pregnancy - A Rare Case Report. Bangladesh J. Obstet. Gynaecol. 2016, 27, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Surve, M.; Pawar, S.; Panigrahi, P.P. A Case Report ofFirst In mester Spontaneous Uterine Scar Rupture. MMJ-A J. by MIMER Med Coll. Pune., India 2017, 1, 26–28. [Google Scholar] [CrossRef]
- Takashima, A.; Takeshita, N.; Kinoshita, T. A case of scarred uterine rupture at 11 weeks of gestation having a uterine scar places induced by in vitro fertilization-embryo transfer. Clin. Pr. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanyi, J.L.; Coleman, N.M.; Johnston, N.D.; Ayensu-Coker, L.; Rajkovic, A. Placenta percreta at 7th week of pregnancy in a woman with previous caesarean section. J. Obstet. Gynaecol. 2008, 28, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Taskin, M.I.; Adali, E. Spontaneous uterine rupture in the first trimester with missed fetus. J. Cases Obstet. Gynecol. 2015, 2, 97–99. [Google Scholar]
- Tola, E.N. First Trimester Spontaneous Uterine Rupture in a Young Woman with Uterine Anomaly. Case Rep. Obstet. Gynecol. 2014, 2014, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufail, A.; A Hashmi, H. Ruptured ectopic pregnancy in rudimentary horn of the uterus. J. Coll. Physicians Surg. Pak. 2007, 17, 105–106. [Google Scholar]
- Vaezi, M. Unexpected Rupture of Unscarred Uterus at 12 Weeks of Pregnancy: A Case Report and Literature Review. Int. J. Women’s Heal. Reprod. Sci. 2017, 5, 339–341. [Google Scholar] [CrossRef] [Green Version]
- Visariya, N.; Purandare, C.N.; Bandukwalla, V.; Purandare, N. First trimester uterine rupture previous lower segment cesarean scar. J. Obstet. Gynecol. India 2011, 61, 88–89. [Google Scholar] [CrossRef] [Green Version]
- Willmott, F.; Scherf, C.; Ford, S.; Lim, K. Rupture of uterus in the first trimester during medical termination of pregnancy for exomphalos using mifepristone/misoprostol. BJOG: Int. J. Obstet. Gynaecol. 2008, 115, 1575–1577. [Google Scholar] [CrossRef]
- Jauniaux, E.; Jurkovic, D. Long-term complications after cesarean section. In Textbook of Caesarean Section; Jauniaux, E., Grobman, W.A., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 129–144. [Google Scholar]
- Jauniaux, E.; Collins, S.L.; Burton, G.J. Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am. J. Obstet. Gynecol. 2017, 218, 75–87. [Google Scholar] [CrossRef]
- Timor-Tritsch, I.E.; Monteagudo, A.; Calì, G.; D’Antonio, F.; Agten, A.K. Cesarean Scar Pregnancy: Diagnosis and Pathogenesis. Obstet. Gynecol. Clin. North Am. 2019, 46, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Downes, K.L.; Hinkle, S.N.; Sjaarda, L.A.; Albert, P.S.; Grantz, K.L. Previous prelabor or intrapartum cesarean delivery and risk of placenta previa. Am. J. Obstet. Gynecol 2015, 212, 669.e1–669.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calì, G.; Timor-Tritsch, I.E.; Palacios-Jaraquemada, J.; Monteaugudo, A.; Buca, D.; Forlani, F.; Familiari, A.; Scambia, G.; Acharya, G.; D’Antonio, F. Outcome of Cesarean scar pregnancy managed expectantly: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zosmer, N.; Fuller, J.; Shaikh, H.; Johns, J.; Grossman, K.B. Natural history of early first-trimester pregnancies implanted in Cesarean scars. Ultrasound Obstet. Gynecol. 2015, 46, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Grimbizis, G.F.; Camus, M.; Tarlatzis, B.C.; Bontis, J.N.; Devroey, P. Clinical implications of uterine malformations and hysteroscopic treatment results. Hum. Reprod. Updat. 2001, 7, 161–174. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Jayaprakasan, K.; Tan, A.; Thornton, J.G.; Coomarasamy, A.; Raine-Fenning, N.J. Reproductive outcomes in women with congenital uterine anomalies: A systematic review. Ultrasound Obstet. Gynecol. 2011, 38, 371–382. [Google Scholar] [CrossRef]
- Żyła, M.M.; Wilczyński, J.; Nowakowska-Głąb, A.; Maniecka-Bryła, I.; Nowakowska, D. Pregnancy and Delivery in Women with Uterine Malformations. Adv. Clin. Exp. Med. 2015, 24, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, G.; Locci, M.; Marilena, M.; Salzano, E.; Palmieri, T.; De Placido, G. Differentiating Between Septate and Bicornuate Uterus: Bi-dimensional and 3-Dimensional power Doppler Findings. J. Minim. Invasive Gynecol. 2014, 21, 870–876. [Google Scholar] [CrossRef]


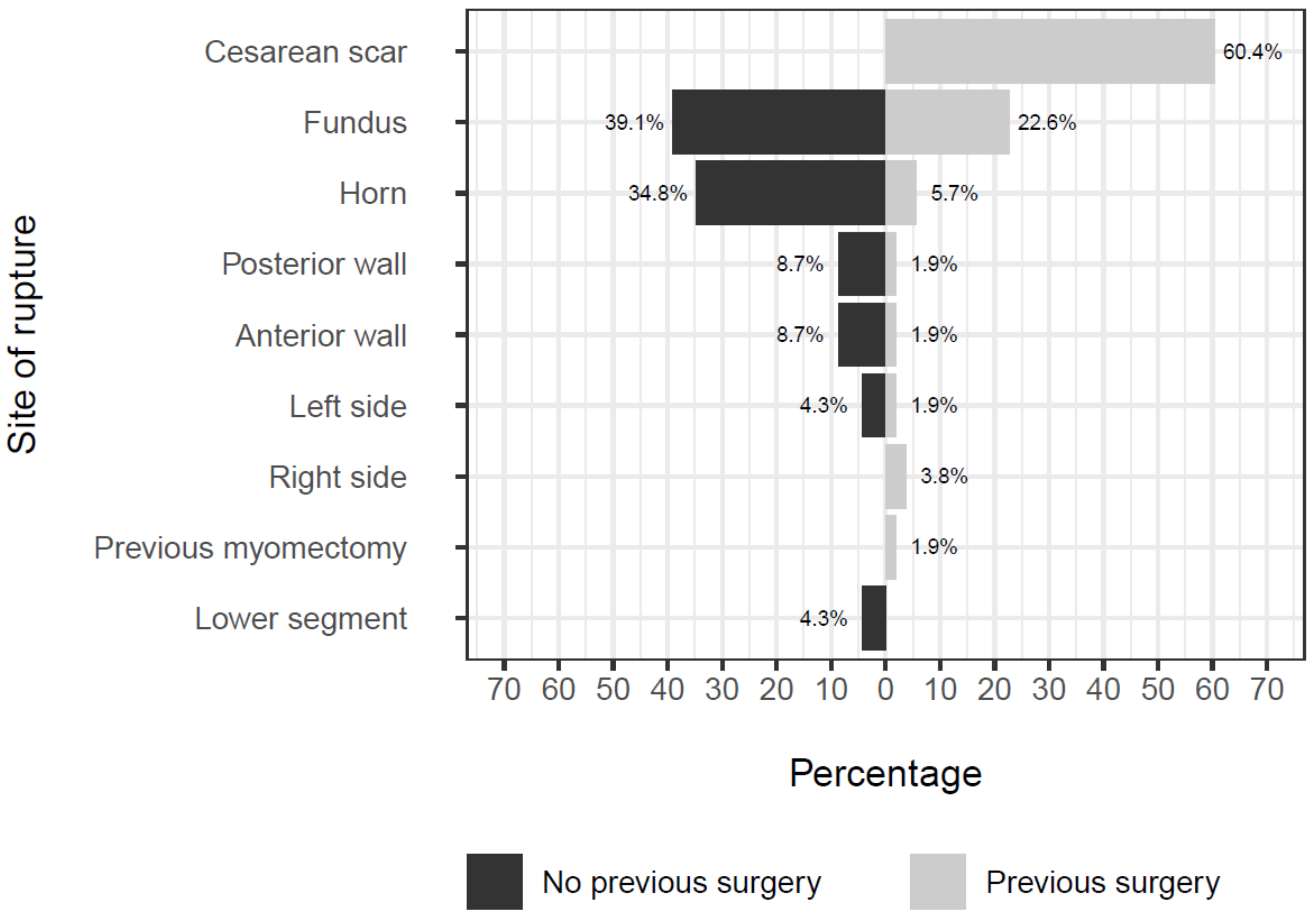
| Variables | All (76) | No Previous Surgery (23) | Previous Surgery (53) |
|---|---|---|---|
| Age (years) | 32 (27–34) | 29 (24–33) | 33 (27–36) |
| Parity | 13.16% (10/76) | 30.43% (7/23) | 5.66% (3/53) |
| Para 0 | 26.32% (20/76) | 43.48% (10/23) | 18.87% (10/53) |
| Para 1–2 | 53.95% (41/76) | 39.13% (9/23) | 60.38% (32/53) |
| Para ≥3 | 19.74% (15/76) | 17.39% (4/23) | 20.75% (11/53) |
| Comorbidities | 21.05% (16/76) | 8.70% (2/23) | 26.42% (14/53) |
| Leiomyoma | 5.26% (4/76) | 0.00% (0/23) | 7.55% (4/53) |
| Adenomyosis | 3.95% (3/76) | 4.35% (1/23) | 3.77% (2/53) |
| Twins | 6.58% (5/76) | 8.70% (2/23) | 5.66% (3/53) |
| Type of previous surgery | |||
| CS | 67.92% (36/53) | ||
| D&C | 28.3% (15/53) | ||
| Myomectomy | 9.43% (5/53) | ||
| Cornual resection | 3.77% (2/53) | ||
| Months since previous surgery | 24 (10–36) | ||
| Treatment | |||
| Diagnostic intervention | 39.47% (30/76) | 47.83% (11/23) | 35.85% (19/53) |
| Lifesaving surgery | 59.21% (45/76) | 52.17% (12/23) | 62.26% (33/53) |
| LPS | 9.21% (7/76) | 13.04% (3/23) | 7.55% (4/53) |
| LPT | 75% (57/76) | 78.26% (18/23) | 73.58% (39/53) |
| Conversion from LPS to LPT | 14.47% (11/76) | 8.70% (2/23) | 16.98% (9/53) |
| Defect repair | 63.16% (48/76) | 73.91% (17/23) | 58.49% (31/53) |
| Hysterectomy | 30.26% (23/76) | 17.39% (4/23) | 35.85% (19/53) |
| Total blood loss (mL) | 1800 (1000–2500) | 2250 (1500–2625) | 1500 (1000–2000) |
| Variables | All (76) | No Previous Surgery (23) | Previous Surgery (53) |
|---|---|---|---|
| ART induced | 11.84% (9/76) | 8.70% (2/23) | 13.21% (7/53) |
| Drugs | 11.84% (9/76) | 8.70% (2/23) | 13.21% (7/53) |
| Retroverted uterus | 7.69% (1/13) | 0.00% (0/10) | 33.33% (1/3) |
| Uterine anomalies | 15.79% (12/76) | 43.48% (10/23) | 3.77% (2/53) |
| Type of uterine anomalies | |||
| Bicornuate uterus | 23.08% (3/13) | 30.00% (3/10) | 0.00% (0/3) |
| Rudimentary horn | 61.54% (8/13) | 60.00% (6/10) | 66.67% (2/3) |
| Case of T-shaped uterus | 7.69% (1/13) | 10.00% (1/10) | 0.00% (0/3) |
| CSP | 31.58% (24/76) | 45.28% (24/53) | |
| PAS | 26.32% (20/76) | 13.04% (3/23) | 32.08% (17/53) |
| Histology | |||
| Accretism (CSP included) or abnormal intermediate trophoblast | 19.74% (15/76) | 13.04% (3/23) | 22.64% (12/53) |
| Other | 2.63% (2/76) | 4.35% (1/23) | 1.89% (1/53) |
| No histological anomalies | 18.42% (14/76) | 21.74% (5/23) | 16.98% (9/53) |
| Unknown | 59.21% (45/76) | 60.87% (14/23) | 58.49% (31/53) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecchini, F.; Tassi, A.; Londero, A.P.; Baccarini, G.; Driul, L.; Xodo, S. First Trimester Uterine Rupture: A Case Report and Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 2976. https://doi.org/10.3390/ijerph17082976
Cecchini F, Tassi A, Londero AP, Baccarini G, Driul L, Xodo S. First Trimester Uterine Rupture: A Case Report and Literature Review. International Journal of Environmental Research and Public Health. 2020; 17(8):2976. https://doi.org/10.3390/ijerph17082976
Chicago/Turabian StyleCecchini, Fabiana, Alice Tassi, Ambrogio P. Londero, Giovanni Baccarini, Lorenza Driul, and Serena Xodo. 2020. "First Trimester Uterine Rupture: A Case Report and Literature Review" International Journal of Environmental Research and Public Health 17, no. 8: 2976. https://doi.org/10.3390/ijerph17082976
APA StyleCecchini, F., Tassi, A., Londero, A. P., Baccarini, G., Driul, L., & Xodo, S. (2020). First Trimester Uterine Rupture: A Case Report and Literature Review. International Journal of Environmental Research and Public Health, 17(8), 2976. https://doi.org/10.3390/ijerph17082976






