Prevalence and Indicators of Vitamin B12 Insufficiency among Young Women of Childbearing Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria
2.3. Sample Size
2.4. Assessment of Vitamin B12 Status
2.5. Anthropometric Assessment
2.6. Socioeconomic Status and Lifestyle Assessment
2.7. Dietary Data
2.8. Physical Activity Estimates
2.9. Statistical Analysis
3. Results
3.1. Prevalence of Vitamin B12 Insufficiency (≤220 pmol/L)
3.2. Baseline Characteristics by Vitamin B12 Status
3.3. Indicators of Vitamin B12 Insufficiency (≤220 pmol/L)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Herrmann, W.; Schorr, H.; Obeid, R.; Geisel, J. Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. Am. J. Clin. Nutr. 2003, 78, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Rolfes, S.R.; Pinna, K.; Whitney, E.N.; Rolfes, S.R. Normal and Clinical Nutrition; Brooks/Cole: Monterey, CA, USA, 2011. [Google Scholar]
- Morris, M.S.; Jacques, P.F.; Selhub, J. Relation between homocysteine and B-vitamin status indicators and bone mineral density in older Americans. Bone 2005, 37, 234–242. [Google Scholar] [CrossRef]
- Knight, B.A.; Shields, B.M.; Brook, A.; Hill, A.; Bhat, D.S.; Hattersley, A.T.; Yajnik, C.S. Lower circulating B12 is associated with higher obesity and insulin resistance during pregnancy in a non-diabetic white British population. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Adaikalakoteswari, A.; Jayashri, R.; Sukumar, N.; Venkataraman, H.; Pradeepa, R.; Gokulakrishnan, K.; Anjana, R.M.; McTernan, P.G.; Tripathi, G.; Patel, V. Vitamin B12 deficiency is associated with adverse lipid profile in Europeans and Indians with type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 129. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; González-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. (CCLM) 2015, 53, 1149–1159. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Schleicher, R.L.; Rybak, M.E. Dietary supplement use and smoking are important correlates of biomarkers of water-soluble vitamin status after adjusting for sociodemographic and lifestyle variables in a representative sample of US adults. J. Nutr. 2013, 143, 957S–965S. [Google Scholar] [CrossRef]
- Battat, R.; Kopylov, U.; Szilagyi, A.; Saxena, A.; Rosenblatt, D.S.; Warner, M.; Bessissow, T.; Seidman, E.; Bitton, A. Vitamin B12 deficiency in inflammatory bowel disease: Prevalence, risk factors, evaluation, and management. Inflamm. Bowel Dis. 2014, 20, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Via, M.A.; Mechanick, J.I. Nutritional and Micronutrient Care of Bariatric Surgery Patients: Current Evidence Update. Curr. Obes. Rep. 2017, 6, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Quay, T.A.; Schroder, T.H.; Jeruszka-Bielak, M.; Li, W.; Devlin, A.M.; Barr, S.I.; Lamers, Y. High prevalence of suboptimal vitamin B12 status in young adult women of South Asian and European ethnicity. Appl. Physiol. Nutr. Metab. 2015, 40, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148, 1995S–2027S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulvik, A.; Vollset, S.E.; Hoff, G.; Ueland, P.M. Coffee consumption and circulating B-vitamins in healthy middle-aged men and women. Clin. Chem. 2008, 54, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Rjater, R.G.; Kale-Pradhan, P.B. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev. Clin. Pharmacol. 2013, 6, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Wong, C. Vitamin B12 deficiency in the elderly: Is it worth screening. Hong Kong Med. J. 2015, 21, 155–164. [Google Scholar] [CrossRef]
- Palacios, G.; Sola, R.; Barrios, L.; Pietrzik, K.; Castillo, M.J.; González-Gross, M. Algorithm for the early diagnosis of vitamin B12 deficiency in elderly people. Nutr. Hosp. 2013, 28, 1447–1452. [Google Scholar] [CrossRef]
- Bailey, R.L.; Carmel, R.; Green, R.; Pfeiffer, C.M.; Cogswell, M.E.; Osterloh, J.D.; Sempos, C.T.; Yetley, E.A. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am. J. Clin. Nutr. 2011, 94, 552–561. [Google Scholar] [CrossRef]
- El-Khateeb, M.; Khader, Y.; Batieha, A.; Jaddou, H.; Hyassat, D.; Belbisi, A.; Ajlouni, K. Vitamin B12 deficiency in Jordan: A population-based study. Ann. Nutr. Metab. 2014, 64, 101–105. [Google Scholar] [CrossRef]
- Devi, A.; Rush, E.; Harper, M.; Venn, B. Vitamin B12 Status of Various Ethnic Groups Living in New Zealand: An Analysis of the Adult Nutrition Survey 2008/2009. Nutrients 2018, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- De Benoist, B. Conclusions of a WHO Technical Consultation on Folate and Vitamin B12 Deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef]
- Sukumar, N.; Venkataraman, H.; Wilson, S.; Goljan, I.; Selvamoni, S.; Patel, V.; Saravanan, P. Vitamin B12 status among pregnant women in the UK and its association with obesity and gestational diabetes. Nutrients 2016, 8, 768. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Kurpad, A.V.; Duggan, C.P.; Bosch, R.J.; Dwarkanath, P.; Mhaskar, A.; Mhaskar, R.; Thomas, A.; Vaz, M.; Bhat, S.; et al. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur. J. Clin. Nutr. 2006, 60, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.P.; Christian, P.; Schulze, K.J.; Arguello, M.; LeClerq, S.C.; Khatry, S.K.; West, K.P., Jr. Low Maternal Vitamin B-12 Status Is Associated with Offspring Insulin Resistance Regardless of Antenatal Micronutrient Supplementation in Rural Nepal. J. Nutr. 2011, 141, 1912–1917. [Google Scholar] [CrossRef]
- Molloy, A.M.; Kirke, P.N.; Troendle, J.F.; Burke, H.; Sutton, M.; Brody, L.C.; Scott, J.M.; Mills, J.L. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 2009, 123, 917–923. [Google Scholar] [CrossRef]
- Fatima, W.; Ahmad, L.M. Prevalence of disordered eating attitudes among adolescent girls in Arar City, Kingdom of Saudi Arabia. Health Psychol. Res. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Alyousefi, N.A.; Alharbi, A.A.; Almugheerah, B.A.; Alajmi, N.A.; Alaiyashi, S.M.; Alharbi, S.S.; Alnoumasi, Z.K. Factors influencing Saudi mothers’ success in exclusive breastfeeding for the first six months of infant life: A cross-sectional observational study. Int. J. Med. Res. Health Sci. 2017, 6, 68–78. [Google Scholar] [CrossRef]
- Jahlan, I.; Plummer, V.; McIntyre, M.; Moawed, S. What Women Have to Say about Giving Birth in Saudi Arabia. Middle East J. 2016, 10. [Google Scholar] [CrossRef]
- Sukumar, N.; Rafnsson, S.B.; Kandala, N.-B.; Bhopal, R.; Yajnik, C.S.; Saravanan, P. Prevalence of vitamin B-12 insufficiency during pregnancy and its effect on offspring birth weight: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 1232–1251. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, T.J.; Tourkmani, A.M.; Abdelhay, O.; Alkhashan, H.I.; Al-Asmari, A.K.; Rsheed, A.M.B.; Abuhaimed, S.N.; Mohammed, N.; AlRasheed, A.N.; AlHarbi, N.G. The association of metformin use with vitamin B12 deficiency and peripheral neuropathy in Saudi individuals with type 2 diabetes mellitus. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Finer, L.B.; Zolna, M.R. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception 2011, 84, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Fayed, A.A.; Wahabi, H.; Mamdouh, H.; Kotb, R.; Esmaeil, S. Demographic profile and pregnancy outcomes of adolescents and older mothers in Saudi Arabia: Analysis from Riyadh Mother (RAHMA) and Baby cohort study. BMJ Open 2017, 7, e016501. [Google Scholar] [CrossRef] [Green Version]
- General Authority for Statistics. Population by Gender, Age Groups and Nationality (Saudi/Non-Saudi). In Saudi Census; 2019. Available online: https://www.stats.gov.sa/en/5680 (accessed on 5 March 2020).
- Abdollahi, Z.; Elmadfa, I.; Djazayeri, A.; Sadeghian, S.; Freisling, H.; Mazandarani, F.S.; Mohamed, K. Folate, vitamin B12 and homocysteine status in women of childbearing age: Baseline data of folic acid wheat flour fortification in Iran. Ann. Nutr. Metab. 2008, 53, 143–150. [Google Scholar] [CrossRef]
- General Authority of Statistics. Population in Riyadh region by Gender, Age Groups and Nationality 2015. Available online: https://www.stats.gov.sa/en/3134 (accessed on 22 September 2020).
- Ankar, A.; Kumar, A. Vitamin B12 Deficiency (Cobalamin). In StatPearls; 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441923/ (accessed on 3 March 2020).
- World Health Organizaion. Obesity and Overweight. 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 January 2020).
- Al-Musharaf, S.; Fouda, M.A.; Turkestani, I.Z.; Al-Ajlan, A.; Sabico, S.; Alnaami, A.M.; Wani, K.; Hussain, S.D.; Alraqebah, B.; Al-Serehi, A. Vitamin D deficiency prevalence and predictors in early pregnancy among Arab women. Nutrients 2018, 10, 489. [Google Scholar] [CrossRef] [Green Version]
- Alkhalaf, M.; Edwards, C.; Combet, E. Validation of a food frequency questionnaire specific for salt intake in Saudi Arabian adults using urinary biomarker and repeated multiple pass 24-hour dietary recall. Proc. Nutr. Soc. 2015, 74. [Google Scholar] [CrossRef] [Green Version]
- Roe, M.; Pinchen, H.; Church, S.; Finglas, P. McC ance and Widdowson’s The Composition of Foods Seventh Summary Edition and updated Composition of Foods Integrated Dataset. Nutr. Bull. 2015, 40, 36–39. [Google Scholar] [CrossRef]
- Mearns, G.J.; Rush, E.C. Screening for inadequate dietary vitamin B-12 intake in South Asian women using a nutrient-specific, semi-quantitative food frequency questionnaire. Asia Pac. J. Clin. Nutr. 2017, 26, 1119. [Google Scholar] [CrossRef]
- Sukumar, N.; Adaikalakoteswari, A.; Venkataraman, H.; Maheswaran, H.; Saravanan, P. Vitamin B12 status in women of childbearing age in the UK and its relationship with national nutrient intake guidelines: Results from two National Diet and Nutrition Surveys. BMJ Open 2016, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkahtani, S.A. Convergent validity: Agreement between accelerometry and the Global Physical Activity Questionnaire in college-age Saudi men. BMC Res. Notes 2016, 9, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohrn, I.-M.; Sjöström, M.; Kwak, L.; Oja, P.; Hagströmer, M. Accelerometer-measured sedentary time and physical activity—A 15-year follow-up of mortality in a Swedish population-based cohort. J. Sci. Med. Sport 2018, 21, 702–707. [Google Scholar] [CrossRef]
- Ku, P.-W.; Steptoe, A.; Liao, Y.; Hsueh, M.-C.; Chen, L.-J. A cut-off of daily sedentary time and all-cause mortality in adults: A meta-regression analysis involving more than 1 million participants. BMC Med. 2018, 16, 1–9. [Google Scholar] [CrossRef]
- Abdel-Fattah, M.; Hifnawy, T.; El Said, T.I.; Moharam, M.M.; Mahmoud, M.A. Determinants of birth spacing among saudi women. J. Fam. Community Med. 2007, 14, 103–111. [Google Scholar]
- Karabulut, A.; Güler, Ö.T.; Karahan, H.T.; Özkan, S.; Koyuncu, H.; Demirciler, I. Premarital screening of 466 Mediterranean women for serum ferritin, vitamin B12, and folate concentrations. Turk. J. Med. Sci. 2015, 45, 358–363. [Google Scholar] [CrossRef]
- Bor, M.V.; Lydeking-Olsen, E.; Møller, J.; Nexø, E. A daily intake of approximately 6 μg vitamin B-12 appears to saturate all the vitamin B-12–related variables in Danish postmenopausal women. Am. J. Clin. Nutr. 2006, 83, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Berstad, P.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087. [Google Scholar] [CrossRef]
- Alwosaifer, A.M.; Alawadh, S.A.; Wahab, M.M.A.; Boubshait, L.A.; Almutairi, B.A. Eating disorders and associated risk factors among Imam Abdulrahman bin Faisal university preparatory year female students in Kingdom of Saudi Arabia. Saudi Med. J. 2018, 39, 910. [Google Scholar] [CrossRef]
- Wiebe, N.; Field, C.; Tonelli, M. A systematic review of the vitamin B12, folate and homocysteine triad across body mass index. Obes. Rev. 2018, 19, 1608–1618. [Google Scholar] [CrossRef]
- Kim, Y.N.; Hwang, J.H.; Cho, Y.-O. The effects of exercise training and acute exercise duration on plasma folate and vitamin B12. Nutr. Res. Pract. 2016, 10, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Woolf, K.; Hahn, N.L.; Christensen, M.M.; Carlson-Phillips, A.; Hansen, C.M. Nutrition assessment of B-vitamins in highly active and sedentary women. Nutrients 2017, 9, 329. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Gamaldo, A.A.; Canas, J.A.; Beydoun, H.A.; Shah, M.T.; McNeely, J.M.; Zonderman, A.B. Serum nutritional biomarkers and their associations with sleep among US adults in recent national surveys. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Dankner, R.; Chetrit, A.; Dror, G.K.; Sela, B.-A. Physical activity is inversely associated with total homocysteine levels, independent of C677T MTHFR genotype and plasma B vitamins. Age 2007, 29, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Clarke, R.; Bennett, D.A.; Parish, S.; Verhoef, P.; Dötsch-Klerk, M.; Lathrop, M.; Xu, P.; Nordestgaard, B.G.; Holm, H.; Hopewell, J.C. Homocysteine and coronary heart disease: Meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012, 9. [Google Scholar] [CrossRef] [Green Version]
- Shahab-Ferdows, S.; Engle-Stone, R.; Hampel, D.; Ndjebayi, A.O.; Nankap, M.; Brown, K.H.; Allen, L.H. Regional, socioeconomic, and dietary risk factors for vitamin B-12 deficiency differ from those for folate deficiency in Cameroonian women and children. J. Nutr. 2015, 145, 2587–2595. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, K. Saudi Arabia’s Riches Conceal a Growing Problem of Poverty. The Guardian. 1 January 2013. Available online: https://www.theguardian.com/world/2013/jan/01/saudi-arabia-riyadh-poverty-inequality (accessed on 2 October 2019).
- Murphy, M.M.; Molloy, A.M.; Ueland, P.M.; Fernandez-Ballart, J.D.; Schneede, J.R.; Arija, V.; Scott, J.M. Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. J. Nutr. 2007, 137, 1863–1867. [Google Scholar] [CrossRef]
- Hvas, A.-M.; Nexo, E. Diagnosis and treatment of vitamin B12 deficiency—An update. Haematologica 2006, 91, 1506–1512. [Google Scholar]
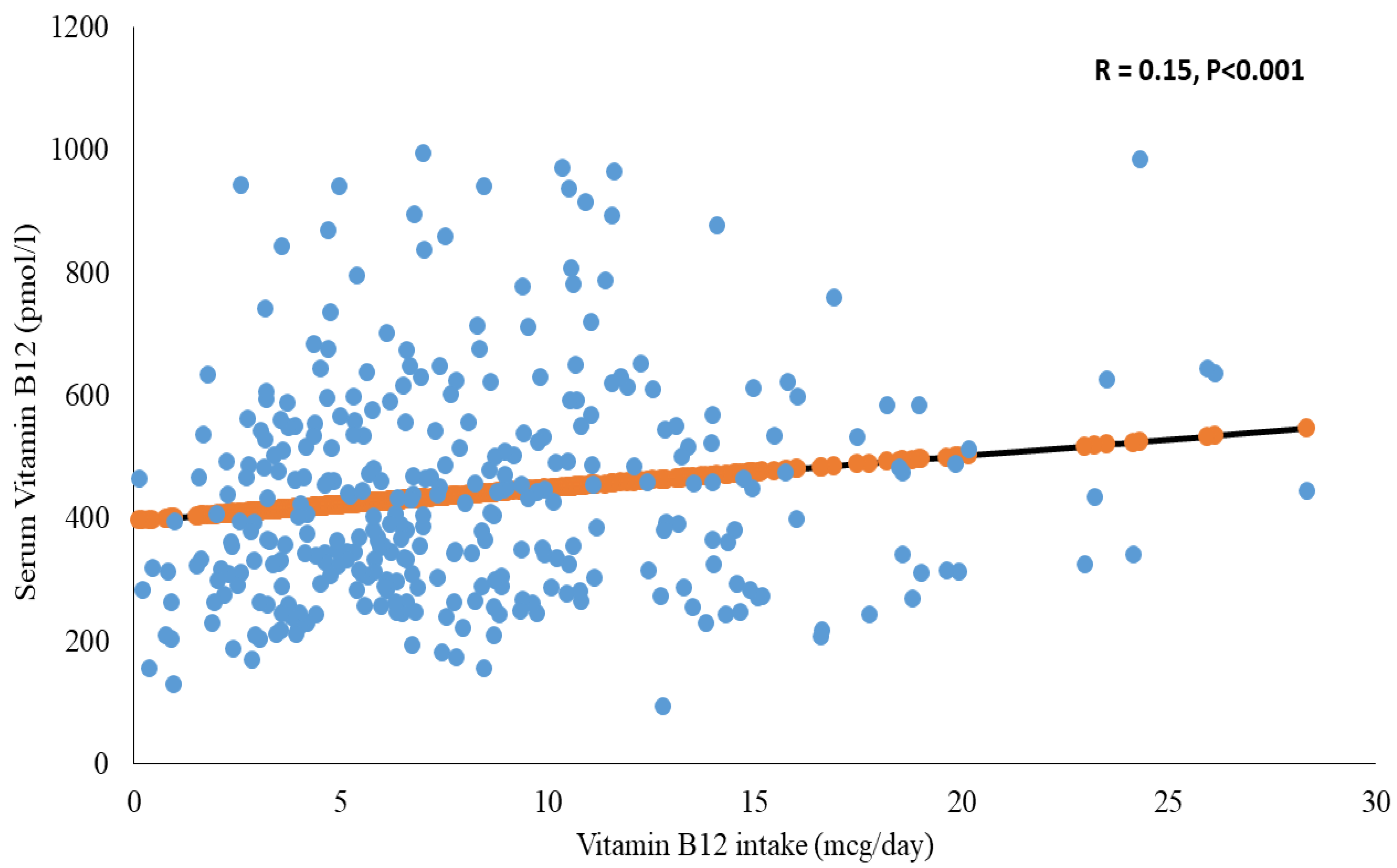
| Overall | Insufficient (≤220 pmol/L) | Sufficient (>220 pmol/L) | p | |
|---|---|---|---|---|
| N | 346 | 21 | 325 | |
| Anthropometrics | ||||
| Age (y) | 20.7 ± 1.5 | 21.2 ± 2.2 | 20.7 ± 1.5 | 0.14 |
| BMI (kg/m2) | 23.6 ± 5.2 | 23.8 ± 5.4 | 23.6 ± 5.2 | 0.92 |
| Waist-to-hip ratio | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.4 | 0.96 |
| Fat (%) | 36.9 ± 8.2 | 37.4 ± 9.0 | 37.0 ± 8.0 | 0.80 |
| Socio-demographics | ||||
| Income (<10K SAR/m) | 74 (20.8) | 7 (33.3) | 63 (19.4) | 0.16 |
| Residence | ||||
| North Riyadh | 136 (39.0) | 6 (30.0) | 127 (39.7) | 0.23 |
| West Riyadh | 62 (17.8) | 1 (5.0) | 59 (18.4) | |
| East Riyadh | 81 (23.2) | 7 (35.0) | 70 (21.9) | |
| South Riyadh | 52 (14.9) | 5 (25.0) | 47 (14.7) | |
| Central Riyadh | 18 (5.2) | 1 (5.0) | 17 (5.3) | |
| Dietary | ||||
| Fat (g/d) | 125.1 (82.7–178.5) | 107.4 (88.6–162.2) | 125.5 (82.7–175.5) | 0.61 |
| Protein (g/d) | 105.3 (78.0–141.8) | 93.3 (79.7–143.4) | 107.1 (79.3–140.0) | 0.41 |
| Carbohydrate (g/d) | 367.6 (267.7–478.7) | 356.7 (260.5–518.4) | 368.5 (268.4–481.5) | 0.91 |
| Fiber (g/d) | 29.8 (20.2–41.7) | 35.0 (23.4–52.0) | 29.5 (20.2–41.2) | 0.24 |
| Energy (kcal/d) | 2918.6 (2133–3810) | 2467.8 (2294.9–3820.3) | 2941.0 (2132.5–3774.5) | 0.83 |
| Tea | 103.2 (33.6–240.0) | 103.2 (33.6–240.0) | 103.2 (33.6–240.0) | 0.60 |
| Caffeine | 105.0 (38.7–210.0) | 135.0 (64.4–285.0) | 103.2 (38.7–210.0) | 0.21 |
| VB12 (mcg/d) | 6.9 (4.4–10.8) | 3.9 (2.8–7.9) | 7.0 (4.7–10.9) | 0.01 |
| VB12 intake ≥ 2.4 mcg/d | 328 (92.4) | 17 (81.0) | 303 (93.2) | 0.04 |
| Protein supplement | 12 (3.5) | 1 (4.8) | 11 (3.4) | 0.53 |
| Multivitamin supplement | 2 (0.6) | 1 (4.8) | 1 (0.3) | 0.12 |
| Physical Activity | ||||
| Vigorous (min/w) # | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.75 |
| MET vigorous (min/w) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.75 |
| Vigorous (≥60 min/w) | 62 (18.0) | 3 (14.3) | 59 (18.2) | 1.00 |
| Moderate (min/w) # | 90.0 (30.0–215.0) | 135.0 (60.0–175.0) | 90.0 (30.0–212.0) | 0.35 |
| MET moderate (min/w) | 360.0 (120.0–860.0) | 540.0 (240.0–700.0) | 360.0 (120.0–848.0) | 0.35 |
| Sitting (min/d) # | 420.0 (240.0–600.0) | 480.0 (360.0–600.0) | 360.0 (240.0–600.0) | 0.33 |
| Sitting (≥7 h/d) | 174 (50.3) | 14 (66.7) | 160 (49.2) | 0.12 |
| GPAQ (MET-min/w) # | 504.0 (160.0–1240.0) | 660.0 (428.0–960.0) | 480.0 (160.0–1280.0) | 0.72 |
| Biochemical Data | ||||
| Glucose (mmol/L) | 4.6 ± 1.0 | 4.7 ± 1.1 | 4.6 ± 1.0 | 0.87 |
| Parameter | OR (95%CI) | p Value |
|---|---|---|
| Age (y) | 1.38 (1.04–1.83) | 0.02 |
| BMI (kg/m2) | 1.01 (0.92–1.10) | 0.85 |
| Glucose level (>5.6 mmol/L) | 0.86 (0.17–4.33) | 0.85 |
| Vitamin B12 intake (RDA > 2.4 mcg/d) | 0.10 (0.02–0.63) | 0.01 |
| Using protein supplement | 2.94 (0.27–32.29) | 0.38 |
| Protein intake (>46 g/d) | 3.03 (0.20–46.63) | 0.42 |
| Coffee intake (>750 mL/d) | 2.04 (0.17–24.68) | 0.58 |
| Income (<10,000 Saudi riyal) | 4.13 (1.25–13.64) | 0.02 |
| Sitting time (≥7 h/d) | 4.60 (1.31–16.21) | 0.02 |
| Vigorous physical activity (≥60 min/w) | 0.52 (0.10–2.72) | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Musharaf, S.; McTernan, P.G.; Hussain, S.D.; Aleisa, K.A.; Alnaami, A.M.; Wani, K.; Saravanan, P.; Al-Daghri, N. Prevalence and Indicators of Vitamin B12 Insufficiency among Young Women of Childbearing Age. Int. J. Environ. Res. Public Health 2021, 18, 1. https://doi.org/10.3390/ijerph18010001
Al-Musharaf S, McTernan PG, Hussain SD, Aleisa KA, Alnaami AM, Wani K, Saravanan P, Al-Daghri N. Prevalence and Indicators of Vitamin B12 Insufficiency among Young Women of Childbearing Age. International Journal of Environmental Research and Public Health. 2021; 18(1):1. https://doi.org/10.3390/ijerph18010001
Chicago/Turabian StyleAl-Musharaf, Sara, Philip G. McTernan, Syed Danish Hussain, Khalid Abdullah Aleisa, Abdullah M. Alnaami, Kaiser Wani, Ponnusamy Saravanan, and Nasser Al-Daghri. 2021. "Prevalence and Indicators of Vitamin B12 Insufficiency among Young Women of Childbearing Age" International Journal of Environmental Research and Public Health 18, no. 1: 1. https://doi.org/10.3390/ijerph18010001
APA StyleAl-Musharaf, S., McTernan, P. G., Hussain, S. D., Aleisa, K. A., Alnaami, A. M., Wani, K., Saravanan, P., & Al-Daghri, N. (2021). Prevalence and Indicators of Vitamin B12 Insufficiency among Young Women of Childbearing Age. International Journal of Environmental Research and Public Health, 18(1), 1. https://doi.org/10.3390/ijerph18010001








