Investigation of the Impact of Endodontic Therapy on Survival among Dialysis Patients in Taiwan: A Nationwide Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Definition of Dialysis Patients
2.3. Definition of Dialysis Patients with RCT
2.4. Primary Outcome
2.5. Definition of Death
2.6. Definition of Other Variables
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Predictors of Survival Rates
3.3. Causes of Death
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2019 USRDS Annual Data Report. Volume 2: ESRD in the United States. Chapter 11: International Comparisons. Available online: https://www.usrds.org/annual-data-report/previous-adrs/ (accessed on 31 December 2020).
- Tsai, S.Y.; Tseng, H.F.; Tan, H.F.; Chien, Y.S.; Chang, C.C. End-stage renal disease in Taiwan: A case-control study. J. Epidemiol. 2009, 19, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Alrukhaimi, M.; Ashuntantang, G.E.; Basnet, S.; Rotter, R.C.; Douthat, W.G.; Kazancioglu, R.; Köttgen, A.; Nangaku, M.; Powe, N.R.; et al. Complications of chronic kidney disease: Current state, knowledge gaps, and strategy for action. Kidney Int. Suppl. 2017, 7, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, J.L.; van Stralen, K.J.; Noordzij, M.; Diez, J.A.; Carrero, J.J.; Couchoud, C.; Dekker, F.W.; Finne, P.; Fouque, D.; Heaf, J.G.; et al. Mortality from infections and malignancies in patients treated with renal replacement therapy: Data from the ERA-EDTA registry. Nephrol. Dial. Transpl. 2015, 30, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Al Wakeel, J.S.; Mitwalli, A.H.; Al Mohaya, S.; Abu-Aisha, H.; Tarif, N.; Malik, G.H.; Hammad, D. Morbidity and mortality in ESRD patients on dialysis. Saudi J. Kidney Dis. Transpl. 2002, 13, 473–477. [Google Scholar]
- Aoki, J.; Ikari, Y. Cardiovascular disease in patients with end-stage renal disease on hemodialysis. Ann. Vasc. Dis. 2017, 10, 327–337. [Google Scholar] [CrossRef]
- Katarzynska-Konwa, M.; Obersztyn, I.; Trzcionka, A.; Mocny-Pachonska, K.; Mosler, B.; Tanasiewicz, M. Oral Status in Pregnant Women from Post-Industrial Areas of Upper Silesia in Reference to Occurrence of: Preterm Labors, Low Birth Weight and Type of Labor. Healthcare 2020, 8, 528. [Google Scholar] [CrossRef]
- Ruospo, M.; Palmer, S.C.; Craig, J.C.; Gentile, G.; Johnson, D.W.; Ford, P.J.; Tonelli, M.; Petruzzi, M.; De Benedittis, M.; Strippoli, G.F. Prevalence and severity of oral disease in adults with chronic kidney disease: A systematic review of observational studies. Nephrol. Dial. Transpl. 2014, 29, 364–375. [Google Scholar] [CrossRef]
- Akar, H.; Akar, G.C.; Carrero, J.J.; Stenvinkel, P.; Lindholm, B. Systemic consequences of poor oral health in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 218–226. [Google Scholar] [CrossRef]
- Borawski, J.; Wilczyńska-Borawska, M.; Stokowska, W.; Myśliwiec, M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol. Dial. Transpl. 2007, 22, 457–464. [Google Scholar] [CrossRef]
- Thorman, R.; Neovius, M.; Hylander, B. Clinical findings in oral health during progression of chronic kidney disease to end-stage renal disease in a Swedish population. Scand. J. Urol. Nephrol. 2009, 43, 154–159. [Google Scholar] [CrossRef]
- Palmer, S.C.; Ruospo, M.; Wong, G.; Craig, J.C.; Petruzzi, M.; De Benedittis, M.; Ford, P.; Johnson, D.W.; Tonelli, M.; Natale, P.; et al. Dental health and mortality in people with end-stage kidney disease treated with hemodialysis: A multinational cohort study. Am. J. Kidney Dis. 2015, 66, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.L.; Lyu, J. A study of dental services in Taiwan before and after global budgeting. Int. J. Electron. Bus. Manag. 2010, 8, 139–151. [Google Scholar]
- Trzcionka, A.; Twardawa, H.; Mocny-Pachońska, K.; Tanasiewicz, M. Oral cavity status of long-term hemodialized patients vs. their socio-economic status. Med. Pr. 2020, 71, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Cantos, A. Oral inflammation and infection, and chronic medical diseases: Implications for the elderly. Periodontology 2000 2016, 72, 153–175. [Google Scholar] [CrossRef] [PubMed]
- López-Pintor, R.M.; López-Pintor, L.; Casañas, E.; de Arriba, L.; Hernández, G. Risk factors associated with xerostomia in haemodialysis patients. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e185–e192. [Google Scholar] [CrossRef]
- Cheng, C.L.; Kao, Y.H.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef]
- Tsai, Y.D.; Chien, W.C.; Tsai, S.H.; Chung, C.H.; Chu, S.J.; Chen, S.J.; Liao, W.I.; Yang, C.J.; Liao, M.T.; Wang, J.C. Increased risk of aortic aneurysm and dissection in patients with Sjögren’s syndrome: A nationwide population-based cohort study in Taiwan. BMJ Open 2018, 8, e022326. [Google Scholar] [CrossRef]
- Shih, C.P.; Lin, H.C.; Chung, C.H.; Hsiao, P.J.; Wang, C.H.; Lee, J.C.; Chien, W.C. Increased risk of tinnitus in patients with chronic kidney disease: A nationwide, population-based cohort study. PLoS ONE 2017, 12, e0183192. [Google Scholar] [CrossRef]
- Hsiao, P.J.; Wu, K.L.; Chiu, S.H.; Chan, J.S.; Lin, Y.F.; Wu, C.Z.; Wu, C.C.; Kao, S.; Fang, T.C.; Lin, S.H.; et al. Impact of the use of anti-diabetic drugs on survival of diabetic dialysis patients: A 5-year retrospective cohort study in Taiwan. Clin. Exp. Nephrol. 2017, 21, 694–704. [Google Scholar] [CrossRef]
- Honarmand, M.; Farhad-Mollashahi, L.; Nakhaee, A.; Sargolzaie, F. Oral manifestation and salivary changes in renal patients undergoing hemodialysis. J. Clin. Exp. Dent. 2017, 9, e207–e210. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, A.V.; Craig, R.G.; Beck, J.D.; Moss, K.; Offenbacher, S.; Kotanko, P.; Yoshino, M.; Levin, N.W.; Yip, J.K.; Almas, K.; et al. Severe periodontitis is associated with low serum albumin among patients on maintenance hemodialysis therapy. Clin. J. Am. Soc. Nephrol. 2007, 2, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33, iii35–iii40. [Google Scholar] [CrossRef]
- Ariyamuthu, V.K.; Nolph, K.D.; Ringdahl, B.E. Periodontal disease in chronic kidney disease and end-stage renal disease patients: A review. Cardiorenal. Med. 2013, 3, 71–78. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Tuomisto, K.; Jousilahti, P.; Havulinna, A.S.; Sundvall, J.; Salomaa, V. Endotoxemia, immune response to periodontal pathogens, and systemic inflammation associate with incident cardiovascular disease events. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1433–1439. [Google Scholar] [CrossRef]
- Wang, H.E.; Gamboa, C.; Warnock, D.G.; Muntner, P. Chronic kidney disease and risk of death from infection. Am. J. Nephrol. 2011, 34, 330–336. [Google Scholar] [CrossRef]
- Costantinides, F.; Castronovo, G.; Vettori, E.; Frattini, C.; Artero, M.L.; Bevilacqua, L.; Berton, F.; Nicolin, V.; Di Lenarda, R. Dental care for patients with end-stage renal disease and undergoing hemodialysis. Int. J. Dent. 2018, 2018, 9610892. [Google Scholar] [CrossRef]
- Huang, R.Y.; Lin, Y.F.; Kao, S.Y.; Shieh, Y.S.; Chen, J.S. A retrospective case-control analysis of the outpatient expenditures for western medicine and dental treatment modalities in CKD patients in Taiwan. PLoS ONE 2014, 9, e88418. [Google Scholar] [CrossRef]
- Assari, S. Socioeconomic status and self-rated oral health; diminished return among hispanic whites. Dent. J. 2018, 6, 11. [Google Scholar] [CrossRef]
- Park, J.B.; Han, K.; Park, Y.G.; Ko, Y. Association between socioeconomic status and oral health behaviors: The 2008-2010 Korea national health and nutrition examination survey. Exp. Ther. Med. 2016, 12, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, S.S.; Sharma, G.; Oberoi, A. A cross-sectional survey to assess the effect of socioeconomic status on the oral hygiene habits. J. Indian Soc. Periodontol. 2016, 20, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Song, J.; Han, J.; Chen, Z.; Yin, X.; Zhu, J.; Song, J. The relationship between tooth loss and mortality from all causes, cardiovascular diseases, and coronary heart disease in the general population: Systematic review and dose-response meta-analysis of prospective cohort studies. Biosci. Rep. 2019, 39, BSR20181773. [Google Scholar] [CrossRef] [PubMed]
- Koka, S.; Gupta, A. Association between missing tooth count and mortality: A systematic review. J. Prosthodont. Res. 2018, 62, 134–151. [Google Scholar] [CrossRef] [PubMed]
- Mauramo, M.; Grolimund, P.; Egli, A.; Passweg, J.; Halter, J.; Waltimo, T. Dissociations of oral foci of infections with infectious complications and survival after haematopoietic stem cell transplantation. PLoS ONE 2019, 14, e0225099. [Google Scholar] [CrossRef] [PubMed]

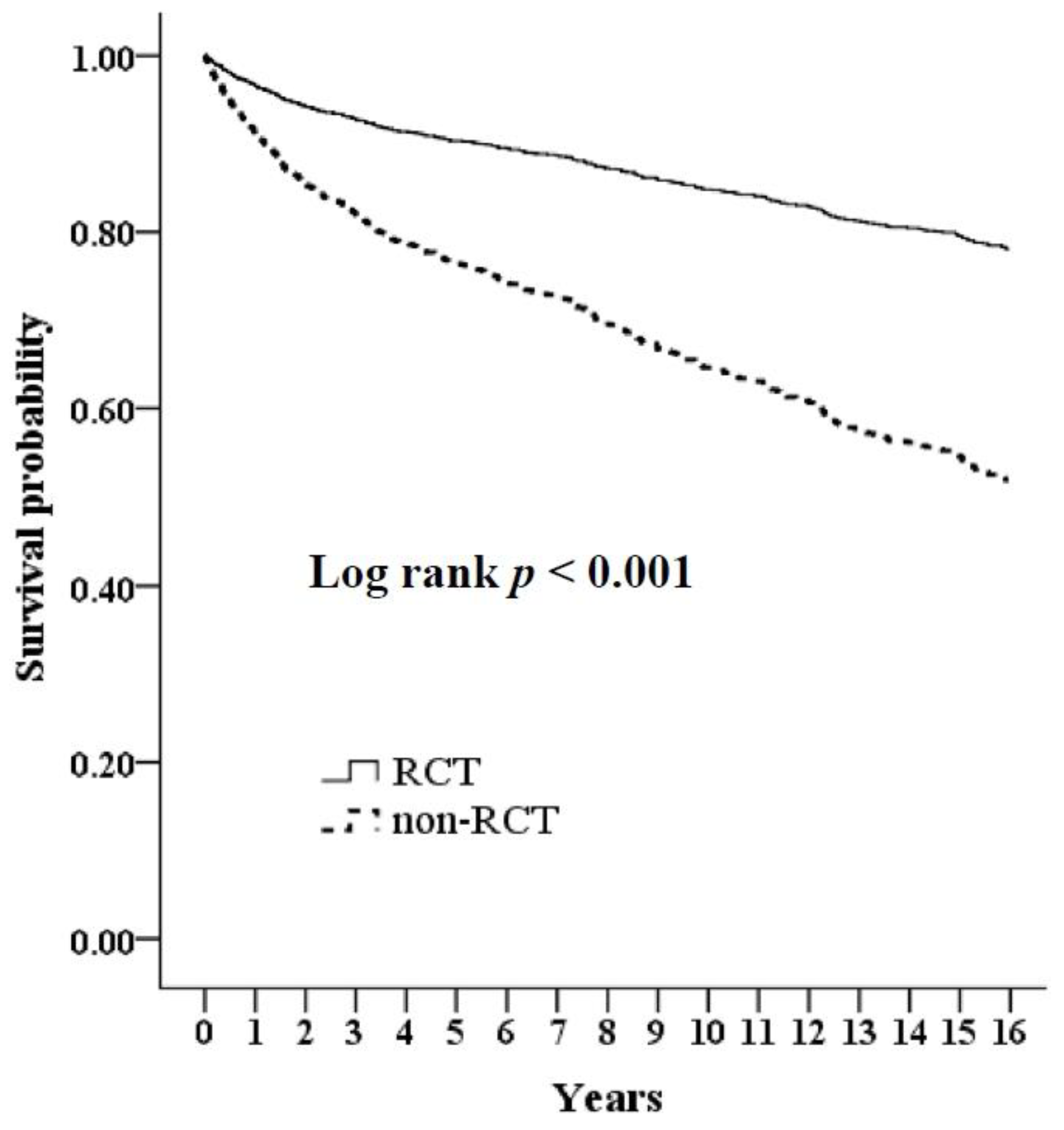
| Variables | Non-RCT | RCT | p | ||
|---|---|---|---|---|---|
| (n = 9821) | (n = 2633) | ||||
| n | % | n | % | ||
| Age (mean, sd) a | 57.02 ± 14.75 | 54.93 ± 13.84 | <0.001 * | ||
| Sex | <0.001 * | ||||
| Female | 5009 | 51.00 | 1493 | 56.70 | |
| Male | 4812 | 49.00 | 1140 | 43.30 | |
| Monthly income | 0.001 * | ||||
| No income | 1942 | 19.77 | 473 | 17.96 | |
| NT$1–15,840 | 1528 | 15.56 | 352 | 13.37 | |
| NT$15,841–25,000 | 4917 | 50.07 | 1375 | 52.22 | |
| ≥NT$25,001 | 1434 | 14.60 | 433 | 16.45 | |
| Dialysis type | 0.022 * | ||||
| HD | 8920 | 90.83 | 2352 | 89.33 | |
| PD | 901 | 9.17 | 281 | 10.67 | |
| Comorbidity | |||||
| Diabetes mellitus | 3225 | 32.84 | 584 | 22.18 | <0.001 * |
| Hypertension | 5026 | 51.18 | 1052 | 39.95 | <0.001 * |
| Hyperlipidemia | 1543 | 15.71 | 297 | 11.28 | <0.001 * |
| Gout | 1121 | 11.41 | 281 | 10.67 | 0.285 |
| Congestive heart failure | 559 | 5.69 | 125 | 4.75 | <0.001 * |
| Coronary artery disease | 1198 | 12.20 | 234 | 8.89 | <0.001 * |
| Cerebrovascular accident | 956 | 9.73 | 197 | 7.48 | <0.001 * |
| Peripheral arterial disease | 101 | 1.03 | 20 | 0.76 | 0.263 |
| Chronic lung disease | 974 | 9.92 | 235 | 8.93 | 0.127 |
| Chronic liver disease | 782 | 7.96 | 192 | 7.29 | 0.255 |
| Malignancy | 342 | 3.48 | 139 | 5.28 | <0.001 * |
| Retinopathy | 1640 | 16.70 | 308 | 11.70 | <0.001 * |
| Death | 3430 | 34.93 | 600 | 22.79 | 0.001 * |
| Follow-up years (means, sd) a | 9.89 ± 2.93 | 9.71 ± 2.89 | 0.001 * | ||
| Variables | Death | Non-Death | p | ||
|---|---|---|---|---|---|
| (n = 4030) | (n = 8424) | ||||
| n | % | n | % | ||
| Age (means, sd) a | 65.06 ± 12.19 | 52.52 ± 13.88 | <0.001 * | ||
| Sex | <0.001 * | ||||
| Female | 1990 | 49.38 | 4512 | 53.56 | |
| Male | 2,040 | 50.62 | 3912 | 46.44 | |
| Monthly income | <0.001 * | ||||
| No income | 944 | 23.42 | 1471 | 17.46 | |
| NT$1–15,840 | 613 | 15.21 | 1267 | 15.04 | |
| NT$15,841–25,000 | 2025 | 50.25 | 4267 | 50.65 | |
| ≥NT$25,001 | 448 | 11.12 | 1419 | 16.84 | |
| Dialysis type | <0.001 * | ||||
| HD | 3703 | 91.89 | 7569 | 89.85 | |
| PD | 327 | 8.11 | 855 | 10.15 | |
| Comorbidity | |||||
| Diabetes mellitus | 2066 | 51.27 | 1743 | 20.69 | <0.001 * |
| Hypertension | 2569 | 63.75 | 3509 | 41.65 | <0.001 * |
| Hyperlipidemia | 877 | 21.76 | 963 | 11.43 | <0.001 * |
| Gout | 601 | 14.91 | 801 | 9.51 | <0.001 * |
| Congestive heart failure | 432 | 10.72 | 252 | 2.99 | <0.001 * |
| Coronary artery disease | 871 | 21.61 | 561 | 6.66 | <0.001 * |
| Cerebrovascular accident | 709 | 17.59 | 444 | 5.27 | <0.001* |
| Peripheral arterial disease | 44 | 1.09 | 77 | 0.91 | 0.379 |
| Chronic lung disease | 692 | 17.17 | 517 | 6.14 | <0.001 * |
| Chronic liver disease | 478 | 11.86 | 496 | 5.89 | <0.001 * |
| Malignancy | 245 | 6.08 | 236 | 2.80 | <0.001 * |
| Retinopathy | 1009 | 25.04 | 939 | 11.15 | <0.001 * |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.09 | 1.06–1.10 | <0.001 * | 1.07 | 1.05–1.09 | <0.001 * |
| Men (Women §) | 1.15 | 0.97–1.34 | 0.082 | 1.11 | 0.91–1.29 | 0.273 |
| Monthly income (No income §) | ||||||
| NT$1–15,840 | 0.80 | 0.61–1.04 | 0.062 | 0.99 | 0.86–1.31 | 0.704 |
| NT$15,841–25,000 | 0.79 | 0.62–0.93 | 0.009 * | 0.84 | 0.78–1.24 | 0.677 |
| ≥NT$25,001 | 0.56 | 0.40–0.71 | <0.001 * | 0.79 | 0.60–1.03 | 0.125 |
| HD (PD §) | 1.23 | 0.90–1.63 | 0.172 | 0.74 | 0.58–0.97 | 0.048 * |
| Diabetes mellitus (yes vs. no) | 3.10 | 2.69–3.63 | <0.001 * | 1.92 | 1.53–2.35 | <0.001 * |
| Hypertension (yes vs. no) | 2.24 | 1.83–2.59 | <0.001 * | 1.12 | 0.89–1.49 | 0.569 |
| Hyperlipidemia (yes vs. no) | 1.93 | 1.58–2.35 | <0.001 * | 1.06 | 0.72–1.33 | 0.611 |
| Gout (yes vs. no) | 1.56 | 1.29–1.96 | <0.001 * | 0.94 | 0.60–1.35 | 0.377 |
| Congestive heart failure (yes vs. no) | 2.91 | 2.21–3.72 | <0.001 * | 1.30 | 0.99–1.66 | 0.051 |
| Coronary artery disease (yes vs. no) | 3.01 | 2.53–3.68 | <0.001 * | 1.59 | 1.28–1.92 | <0.001 * |
| Cerebrovascular accident (yes vs. no) | 2.97 | 2.31–3.51 | <0.001 * | 1.42 | 1.19–1.79 | <0.001 * |
| Peripheral arterial disease (yes vs. no) | 1.43 | 0.65–2.99 | 0.358 | 0.90 | 0.40–1.99 | 0.835 |
| Chronic lung disease (yes vs. no) | 2.64 | 2.19–3.29 | <0.001 * | 1.42 | 1.14–1.75 | 0.001 * |
| Chronic liver disease (yes vs. no) | 1.95 | 1.57–2.53 | <0.001 * | 1.59 | 1.29–2.03 | <0.001 * |
| Malignancy (yes vs. no) | 1.83 | 1.35–2.49 | <0.001 * | 1.48 | 1.18–2.18 | 0.004 * |
| Retinopathy (yes vs. no) | 2.21 | 1.82–2.61 | <0.001 * | 1.20 | 0.92–1.45 | 0.077 |
| RCT (vs. non-RCT §) | 0.59 | 0.44–0.79 | <0.001 * | 0.69 | 0.51–0.90 | 0.001 * |
| All-Cause Death | Non-RCT | RCT | 95% CI | p |
|---|---|---|---|---|
| Crude HR | 1 | 0.59 | 0.44–0.79 | <0.001 * |
| Adjusted HR a | 1 | 0.65 | 0.48–0.83 | <0.001 * |
| Adjusted HR b | 1 | 0.69 | 0.51–0.90 | 0.001 * |
| Variables | Non-RCT | RCT | p | ||
|---|---|---|---|---|---|
| (n = 3430) | (n = 600) | ||||
| n | % | n | % | ||
| Causes of death | |||||
| Coronary artery disease | 599 | 17.46 | 83 | 13.83 | 0.410 |
| Infectious disease | 1562 | 45.54 | 209 | 34.83 | 0.003 * |
| Cerebrovascular disease | 387 | 11.28 | 97 | 16.17 | 0.189 |
| Malignancy | 331 | 9.65 | 72 | 12.00 | 0.548 |
| Other | 551 | 16.06 | 139 | 23.17 | 0.049 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-C.; Chang, Y.-C.; Huang, R.-Y.; Chan, J.-S.; Chung, C.-H.; Chien, W.-C.; Kao, Y.-H.; Hsiao, P.-J. Investigation of the Impact of Endodontic Therapy on Survival among Dialysis Patients in Taiwan: A Nationwide Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 326. https://doi.org/10.3390/ijerph18010326
Chiu C-C, Chang Y-C, Huang R-Y, Chan J-S, Chung C-H, Chien W-C, Kao Y-H, Hsiao P-J. Investigation of the Impact of Endodontic Therapy on Survival among Dialysis Patients in Taiwan: A Nationwide Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(1):326. https://doi.org/10.3390/ijerph18010326
Chicago/Turabian StyleChiu, Chih-Chien, Ya-Chieh Chang, Ren-Yeong Huang, Jenq-Shyong Chan, Chi-Hsiang Chung, Wu-Chien Chien, Yung-Hsi Kao, and Po-Jen Hsiao. 2021. "Investigation of the Impact of Endodontic Therapy on Survival among Dialysis Patients in Taiwan: A Nationwide Population-Based Cohort Study" International Journal of Environmental Research and Public Health 18, no. 1: 326. https://doi.org/10.3390/ijerph18010326
APA StyleChiu, C.-C., Chang, Y.-C., Huang, R.-Y., Chan, J.-S., Chung, C.-H., Chien, W.-C., Kao, Y.-H., & Hsiao, P.-J. (2021). Investigation of the Impact of Endodontic Therapy on Survival among Dialysis Patients in Taiwan: A Nationwide Population-Based Cohort Study. International Journal of Environmental Research and Public Health, 18(1), 326. https://doi.org/10.3390/ijerph18010326







