Short-Time Recurrences of Plasmodium vivax Malaria as a Public Health Proxy for Chloroquine-Resistance Surveillance: A Spatio-Temporal Study in the Brazilian Amazon
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Study Design and Data Collection
2.3. Data Processing and Recurrence Identification
2.4. Variables of Interest
2.5. Statistical Analysis
2.6. Software
2.7. Ethical Considerations
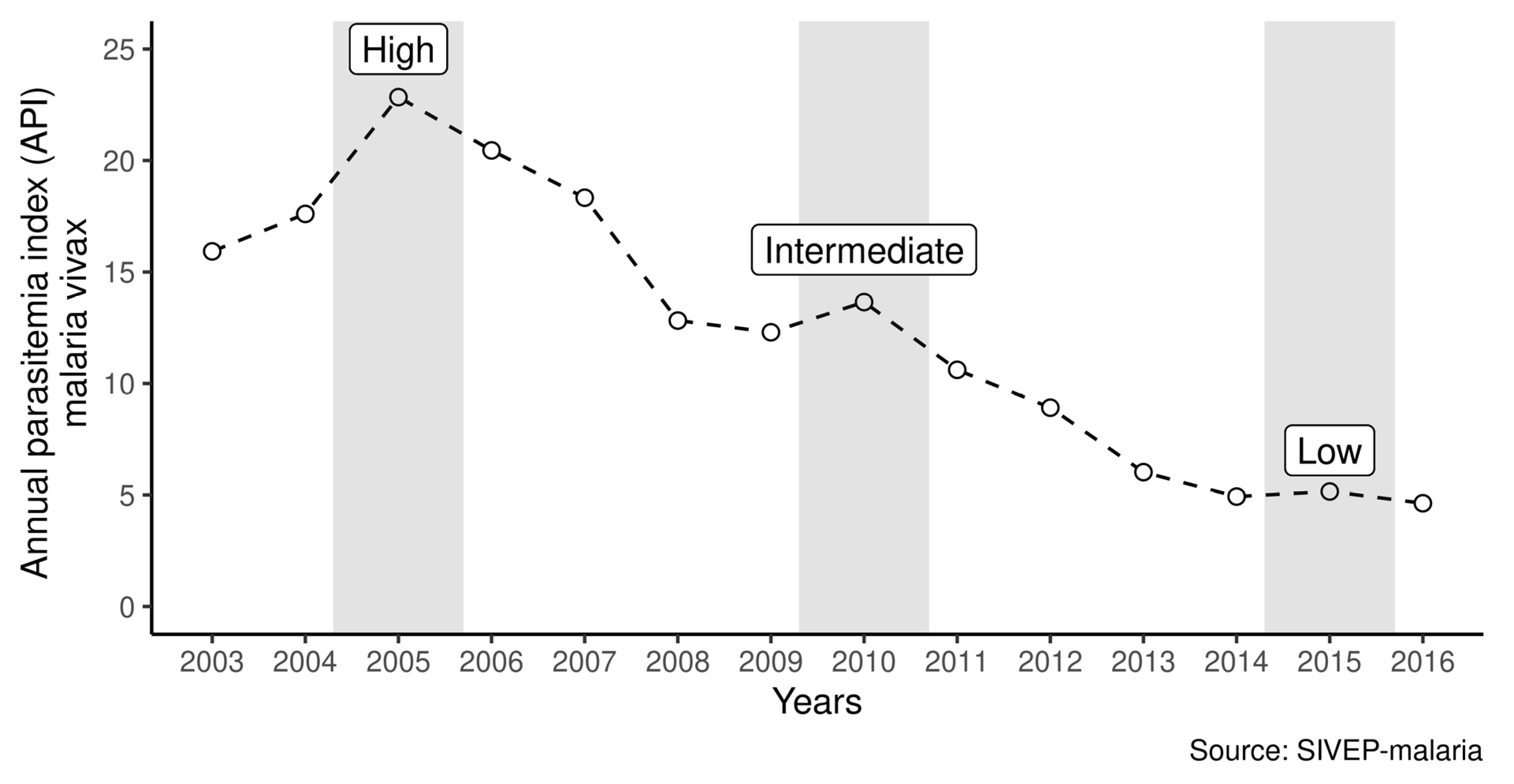
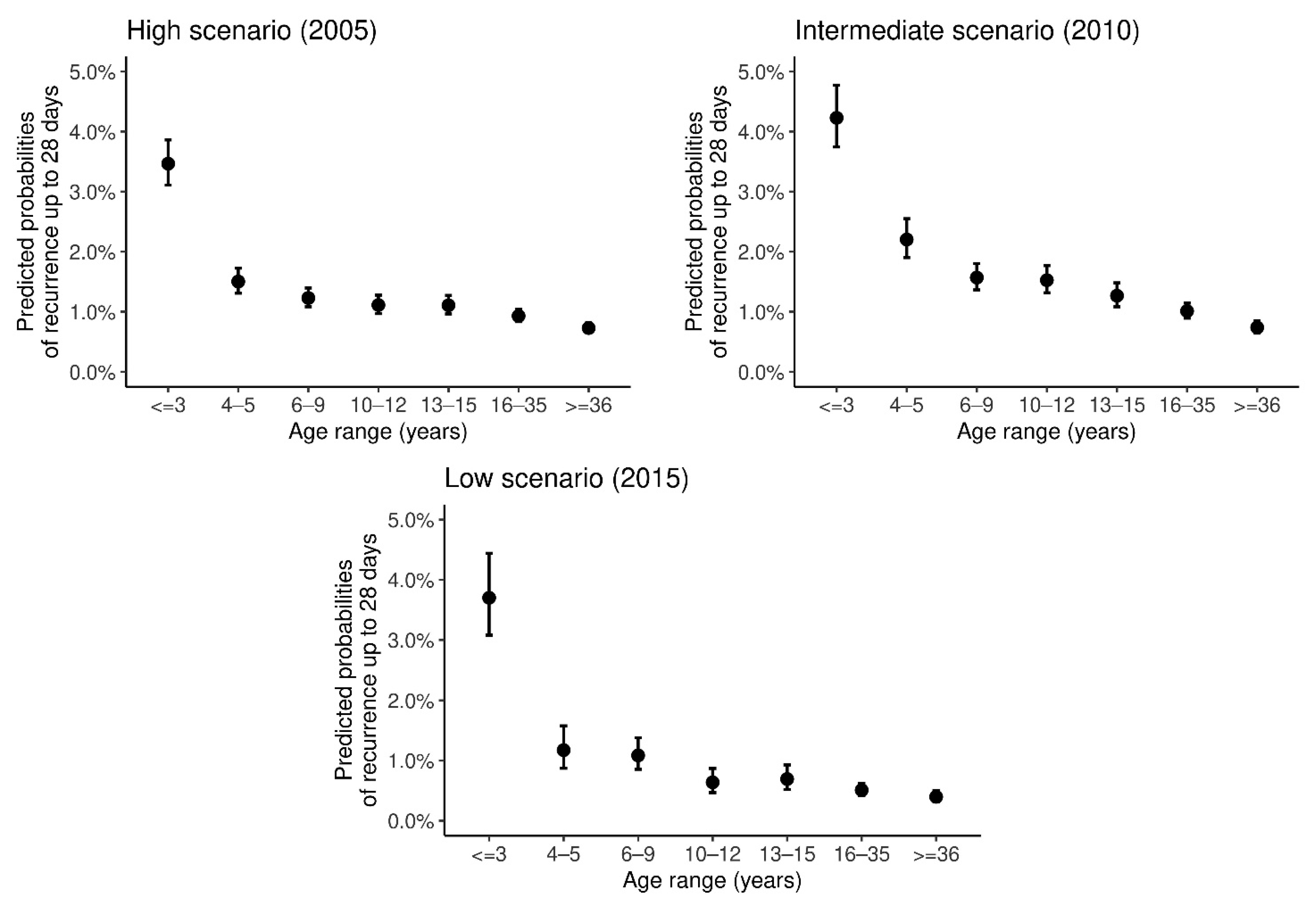
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soto, J.; Toledo, J.; Gutierrez, P.; Luzz, M.; Llinas, N.; Cedeño, N.; Dunne, M.; Berman, J. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am. J. Trop. Med. Hyg. 2001, 65, 90–93. [Google Scholar] [CrossRef] [Green Version]
- Ruebush, T.K.; Zegarra, J.; Cairo, J.; Andersen, E.M.; Green, M.; Pillai, D.R.; Marquiño, W.; Huilca, M.; Arévalo, E.; Garcia, C.; et al. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am. J. Trop. Med. Hyg. 2003, 69, 548–552. [Google Scholar] [CrossRef]
- Musset, L.; Heugas, C.; Naldjinan, R.; Blanchet, D.; Houze, P.; Abboud, P.; Volney, B.; Walter, G.; Lazrek, Y.; Epelboin, L.; et al. Emergence of P. vivax resistance to chloroquine in French Guiana. Antimicrob. Agents Chemother. 2019, 63, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratcliff, A.; Siswantoro, H.; Kenangalem, E.; Wuwung, M.; Brockman, A.; Edstein, M.D.; Laihad, F.; Ebsworth, E.P.; Anstey, N.M.; Tjitra, E.; et al. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine–pyrimethamine in southern Papua, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Commons, R.J.; Simpson, J.A.; Thriemer, K.; Humphreys, G.S.; Abreha, T.; Alemu, S.G.; Añez, A.; Anstey, N.M.; Awab, G.R.; Baird, J.K.; et al. The effect of chloroquine dose and primaquine on Plasmodium vivax recurrence: A WorldWide Antimalarial Resistance Network systematic review and individual patient pooled meta-analysis. Lancet Infect. Dis. 2018, 18, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Ladeia-Andrade, S.; Menezes, M.J.; de Sousa, T.N.; Silvino, A.C.R.; de Carvalho, J.F.; Salla, L.C.; Nery, O.A.; De Melo, G.N.P.; Corder, R.M.; Rodrigues, P.T.; et al. Monitoring the Efficacy of Chloroquine-Primaquine Therapy for Uncomplicated Plasmodium vivax Malaria in the Main Transmission Hot Spot of Brazil. Antimicrob. Agents Chemother. 2019, 63, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, A.M.; Mesones-Lapouble, O.; Marchesini, P.; Sampaio, V.D.S.; Brasil, P.; Tauil, P.L.; Fontes, C.J.; Costa, F.T.M.; Daniel-Ribeiro, C.T.; Lacerda, M.V.G.; et al. Plasmodium vivax landscape in Brazil: Scenario and challenges. Am. J. Trop. Med. Hyg. 2016, 95, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canelas, T.; Ribeiro, H.; Castillo-Salgado, C. Analyzing the Local Epidemiological Profile of Malaria Transmission in the Brazilian Amazon between 2010 and 2015. PLoS Curr. 2018, 10. [Google Scholar] [CrossRef]
- Daher, A.; Silva, J.C.A.L.; Stevens, A.; Marchesini, P.; Fontes, C.J.; Ter Kuile, F.O.; Lalloo, D.G. Evaluation of Plasmodium vivax malaria recurrence in Brazil. Malar. J. 2019, 18, 18. [Google Scholar] [CrossRef]
- Pongtavornpinyo, W.; Yeung, S.; Hastings, I.M.; Dondorp, A.M.; Day, N.P.; White, N.J. Spread of anti-malarial drug resistance: Mathematical model with implications for ACT drug policies. Malar. J. 2008, 7, 229. [Google Scholar] [CrossRef] [Green Version]
- Simões, L.R.; Alves, E.R., Jr.; Ribatski-Silva, D.; Gomes, L.T.; Nery, A.F.; Fontes, C.J.F. Factors associated with recurrent Plasmodium vivax malaria in Porto Velho, Rondônia State, Brazil, 2009. Cad. Saude Publica 2014, 30, 1403–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guidelines for the Treatment of Malaria, 3rd ed.; WHO: Geneva, Switzerland, 2015; Volume 317, p. 290. [Google Scholar]
- De Santana Filho, F.S.; de Arcanjo, A.R.L.; Chehuan, Y.M.; Costa, M.R.; Martinez-Espinosa, F.E.; Vieira, J.L.; das Barbosa, M.G.V.; Alecrim, W.D.; das Alecrim, M.G.C. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg. Infect. Dis. 2007, 13, 1125–1126. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.M.; Costa, M.R.F.; Santana Filho, F.S.; Vieira, J.L.F.; Nascimento, M.T.S.; Brasil, L.W.; Nogueira, F.; Silveira, H.; Reyes-Lecca, R.C.; Monteiro, W.M.; et al. Plasmodium vivax Chloroquine Resistance and Anemia in the Western Brazilian Amazon. Antimicrob. Agents Chemother. 2014, 58, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.R.; Almeida, A.C.G.; da Silva, G.A.V.; Ramasawmy, R.; Lopes, S.C.P.; Siqueira, A.M.; Costa, G.L.; Sousa, T.N.; Vieira, J.L.F.; Lacerda, M.V.G.; et al. Chloroquine resistance is associated to multi-copy pvcrt-o gene in Plasmodium vivax malaria in the Brazilian Amazon. Malar. J. 2018, 17, 267. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, A.M.; Alencar, A.C.; Melo, G.C.; Magalhaes, B.L.; Machado, K.; Alencar Filho, A.C.; Kuehn, A.; Marques, M.M.; Manso, M.C.; Felger, I.; et al. Fixed-Dose Artesunate-Amodiaquine Combination vs Chloroquine for Treatment of Uncomplicated Blood Stage P. vivax Infection in the Brazilian Amazon: An Open-Label Randomized, Controlled Trial. Clin. Infect. Dis. 2017, 64, 166–174. [Google Scholar] [CrossRef]
- Popovici, J.; Pierce-Friedrich, L.; Kim, S.; Bin, S.; Run, V.; Lek, D.; Hee, K.H.D.; Lee Soon-U, L.; Cannon, M.V.; Serre, D.; et al. Recrudescence, Reinfection, or Relapse? A More Rigorous Framework to Assess Chloroquine Efficacy for Plasmodium vivax Malaria. J. Infect. Dis. 2019, 219, 315–322. [Google Scholar] [CrossRef]
- Taylor, A.R.; Watson, J.A.; Chu, C.S.; Puaprasert, K.; Duanguppama, J.; Day, N.P.; Nosten, F.; Neafsey, D.E.; Buckee, C.O.; Imwong, M.; et al. Resolving the cause of recurrent Plasmodium vivax malaria probabilistically. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moreira Braz, R.; Luiz Tauil, P.; Carolina Faria e Silva Santelli, A.; Jesus Fernandes Fontes, C. Avaliação da completude e da oportunidade das notificações de malária na Amazônia Brasileira, 2003–2012. Epidemiol. Serviços Saúde 2016, 25, 10–11. [Google Scholar] [CrossRef]
- Allévius, B.; Höhle, M. An expectation-based space-time scan statistic for ZIP-distributed data. arXiv 2017, arXiv:1712.09188. [Google Scholar]
- Baird, J.K.; Leksana, B.; Masbar, S.; Fryauff, D.J.; Sutanihardja, M.A.; Wignall, F.S.; Hoffman, S.L. Diagnosis of resistance to chloroquine by Plasmodium vivax: Timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 1997, 56, 621–626. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO|Methods for Surveillance of Antimalarial Drug Efficacy; WHO: Geneva, Switzerland, 2009; Volume 90, pp. 11–26. [Google Scholar]
- White, N.J. Drug Resistance in Malaria; Oxford Academic: Oxford, UK, 1998; Volume 54. [Google Scholar]
- White, N.J. The Treatment of Malaria. N. Engl. J. Med. 1996, 335, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Tjitra, E.; Anstey, N.M.; Sugiarto, P.; Warikar, N.; Kenangalem, E.; Karyana, M.; Lampah, D.A.; Price, R.N. Multidrug-Resistant Plasmodium vivax Associated with Severe and Fatal Malaria: A Prospective Study in Papua, Indonesia. PLoS Med. 2008, 5, e128. [Google Scholar] [CrossRef] [PubMed]
- Lana, R.; Nekkab, N.; Siqueira, A.M.; Peterka, C.; Marchesini, P.; Lacerda, M.; Mueller, I.; White, M.; Villela, D. The top 1%: Quantifying the unequal distribution of malaria in Brazil. Malar. J. 2021, 20, 1–11. [Google Scholar] [CrossRef]
- Abdulla, S.; Sagara, I. Dispersible Formulation of artemether/lumefantrine: Specifically Developed for Infants and Young Children. Malar. J. 2009, 8, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Källander, K.; Nsungwa-Sabiiti, J.; Peterson, S. Symptom overlap for malaria and pneumonia—policy implications for home management strategies. Acta Trop. 2004, 90, 211–214. [Google Scholar] [CrossRef]
- White, N.J. Antimalarial drug resistance. J. Clin. Investig. 2004, 113, 1084. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar. J. 2011, 10, 297. [Google Scholar] [CrossRef] [Green Version]
- Bartoloni, A.; Zammarchi, L. Clinical Aspects of Uncomplicated and Severe Malaria. Mediterr. J. Hematol. Infect. Dis. 2012, 4, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balieiro, A.A.S.; Siqueira, A.M.; Melo, G.C.; Monteiro, W.M.; Sampaio, V.S.; Mueller, I.; Lacerda, M.V.G.; Villela, D.A.M. Short-Time Recurrences of Plasmodium vivax Malaria as a Public Health Proxy for Chloroquine-Resistance Surveillance: A Spatio-Temporal Study in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2021, 18, 5061. https://doi.org/10.3390/ijerph18105061
Balieiro AAS, Siqueira AM, Melo GC, Monteiro WM, Sampaio VS, Mueller I, Lacerda MVG, Villela DAM. Short-Time Recurrences of Plasmodium vivax Malaria as a Public Health Proxy for Chloroquine-Resistance Surveillance: A Spatio-Temporal Study in the Brazilian Amazon. International Journal of Environmental Research and Public Health. 2021; 18(10):5061. https://doi.org/10.3390/ijerph18105061
Chicago/Turabian StyleBalieiro, Antonio A. S., Andre M. Siqueira, Gisely C. Melo, Wuelton M. Monteiro, Vanderson S. Sampaio, Ivo Mueller, Marcus V. G. Lacerda, and Daniel A. M. Villela. 2021. "Short-Time Recurrences of Plasmodium vivax Malaria as a Public Health Proxy for Chloroquine-Resistance Surveillance: A Spatio-Temporal Study in the Brazilian Amazon" International Journal of Environmental Research and Public Health 18, no. 10: 5061. https://doi.org/10.3390/ijerph18105061
APA StyleBalieiro, A. A. S., Siqueira, A. M., Melo, G. C., Monteiro, W. M., Sampaio, V. S., Mueller, I., Lacerda, M. V. G., & Villela, D. A. M. (2021). Short-Time Recurrences of Plasmodium vivax Malaria as a Public Health Proxy for Chloroquine-Resistance Surveillance: A Spatio-Temporal Study in the Brazilian Amazon. International Journal of Environmental Research and Public Health, 18(10), 5061. https://doi.org/10.3390/ijerph18105061






