Monitoring of Environmental Hg Occurrence in Tunisian Coastal Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Area
2.2. Sample Collection
2.3. Hg Analysis
3. Results
4. Discussion
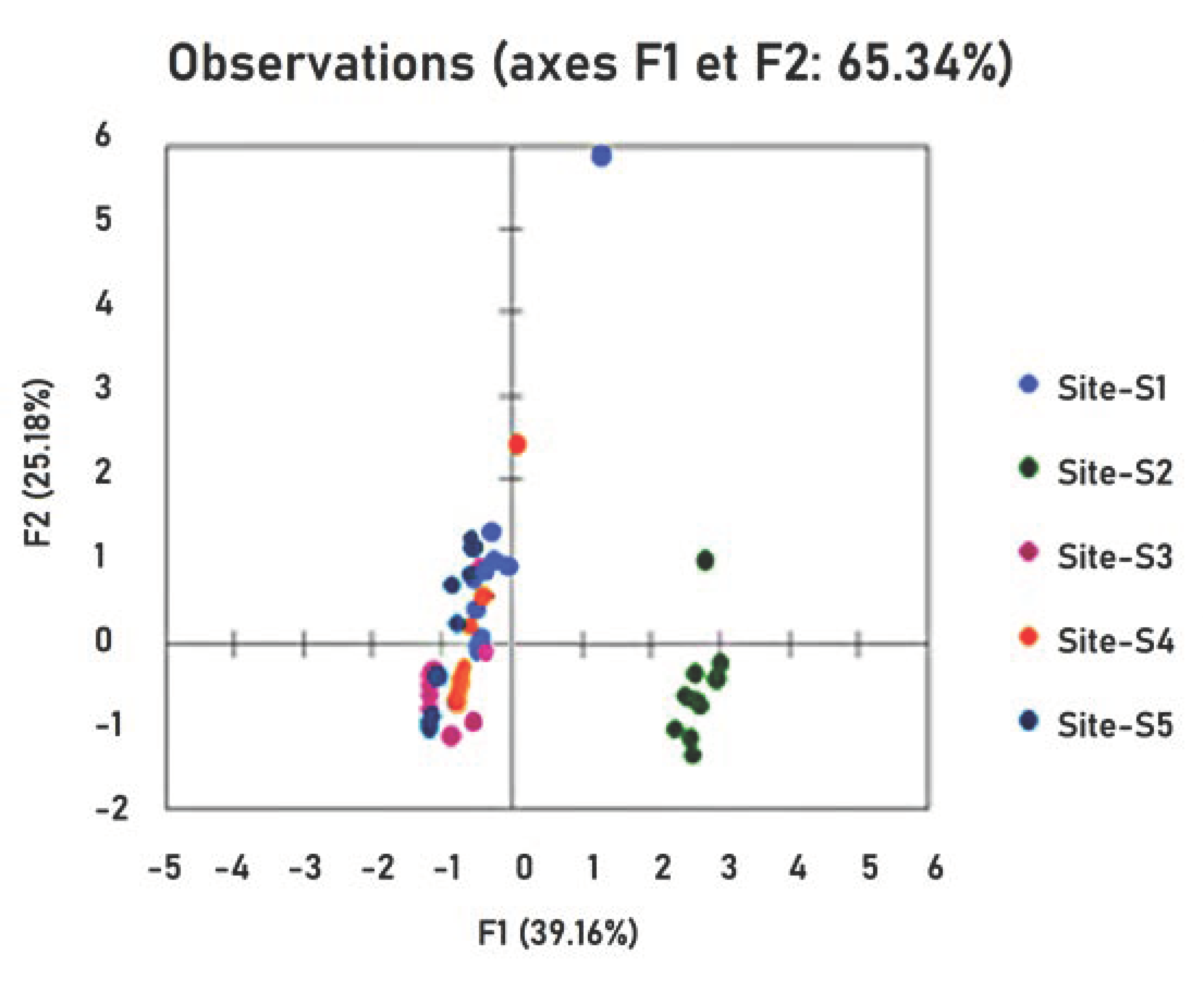
4.1. Hg Correlation between Samples and Sites
4.2. Health Risk Assessment
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alcoverro, T.; Cerbiān, E.; Ballesteros, E. The photosynthetic capacity of the seagrass Posidonia oceanica: Influence of nitrogen and light. J. Exp. Mar. Biol. Ecol. 2001, 261, 107–120. [Google Scholar] [CrossRef]
- Kim, B.-M.; Lee, B.-E.; Hong, Y.-C.; Park, H.; Ha, M.; Kim, Y.-J.; Kim, Y.; Chang, N.; Kim, B.-N.; Oh, S.-Y.; et al. Hg levels in maternal and cord blood and attained weight through the 24 months of life. Sci. Total Environ. 2011, 410–411, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef]
- Coelho, J.; Pimenta, J.; Gomes, R.; Barroso, C.; Pereira, M.; Pardal, M.; Duarte, A. Can Nassarius reticulatus be used as a bioindicator for Hg contamination? Results from a longitudinal study of the Portuguese coastline. Mar. Pollut. Bull. 2006, 52, 674–680. [Google Scholar] [CrossRef]
- Beldowska, M.; Falkowska, L. Hg in marine fish, mammals, seabirds, and human hair in the coastal zone of the southern Baltic. Water Air Soil Pollut. 2016, 227, 52. [Google Scholar] [CrossRef] [PubMed]
- Maulvault, A.L.; Custódio, A.; Anacleto, P.; Repolho, T.; Pousão, P.; Nunes, M.L.; Diniz, M.; Rosa, R.; Marques, A. Bioaccumulation and elimination of Hg in juvenile seabass (Dicentrarchus labrax) in a warmer environment. Environ. Res. 2016, 149, 77–85. [Google Scholar] [CrossRef]
- Cammilleri, G.; Vazzana, M.; Arizza, V.; Giunta, F.; Vella, A.; Dico, G.L.; Giaccone, V.; Giofrè, S.V.; Giangrosso, G.; Cicero, N.; et al. Hg in fish products: What’s the best for consumers between bluefin tuna and yellowfin tuna? Nat. Prod. Res. 2018, 32, 457–462. [Google Scholar] [CrossRef]
- Murata, Y.; Finkelstein, D.B.; Lamborg, C.H.; Finkelstein, M.E. Tuna Consumption, Hg Exposure, and Knowledge about Hg Exposure Risk from Tuna Consumption in University Students. Environ. Toxicol. Chem. 2019, 38, 1988–1994. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Hg Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef]
- Licata, P.; Trombetta, D.; Cristani, M.; Naccari, C.; Martino, D.; Caló, M.; Naccari, F. Heavy metals in liver and muscle of bluefin tunA (Thunnus thynnus) caught in the Straits of Messina (Sicily, Italy). Environ. Monit. Assess. 2005, 107, 239–248. [Google Scholar] [CrossRef]
- Parrino, V.; Costa, G.; Cannavà, C.; Fazio, E.; Bonsignore, M.; Concetta, S.; Piccione, G.; Fazio, F. Flow cytometry and micro-Raman spectroscopy: Identification of hemocyte populations in the mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) from Faro Lake and Tyrrhenian Sea (Sicily, Italy). Fish Shellfish Immunol. 2019, 87, 1–8. [Google Scholar] [CrossRef]
- Xu, X.; Yekeen, T.A.; Xiao, Q.; Wang, Y.; Lu, F.; Huo, X. Placental IGF-1 and IGFBP-3 expression correlate with umbilical cord blood PAH and PBDE levels from prenatal exposure to electronic waste. Environ. Pollut. 2013, 182, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L. The toxicology of Hg and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Magos, L.; Clarkson, T.W. Overview of the clinical toxicity of Hg. Ann. Clin. Biochem. 2006, 43, 257–268. [Google Scholar] [CrossRef]
- Guzzi, G.; La Porta, C.A. Molecular mechanisms triggered by Hg. Toxicology 2008, 244, 1–12. [Google Scholar] [CrossRef]
- Turco, V.L.; Di Bella, G.; Furci, P.; Cicero, N.; Pollicino, G.; Dugo, G. Heavy metals content by ICP-OES in Sarda sarda, Sardinella aurita and Lepidopus caudatus from the Strait of Messina (Sicily, Italy). Nat. Prod. Res. 2013, 27, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Salvo, A.; Potorti, A.G.; Cicero, N.; Bruno, M.; Turco, V.L.; Di Bella, G.; Dugo, G. Statistical characterisation of heavy metal contents in Paracentrotus lividus from Mediterranean Sea. Nat. Prod. Res. 2014, 28, 718–726. [Google Scholar] [CrossRef]
- Giangrosso, G.; Cammilleri, G.; Macaluso, A.; Vella, A.; D’Orazio, N.; Graci, S.; Dico, G.M.L.; Galvano, F.; Giangrosso, M.; Ferrantelli, V. Hair Hg Levels Detection in Fishermen from Sicily (Italy) by ICP-MS Method after Microwave-Assisted Digestion. Bioinorg. Chem. Appl. 2016, 2016, 5408014. [Google Scholar] [CrossRef]
- Conti, M.E.; Cecchetti, G. A biomonitoring study: Trace metals in algae and molluscs from Tyrrhenian coastal areas. Environ. Res. 2003, 93, 99–112. [Google Scholar] [CrossRef]
- Mergler, D.; Anderson, H.A.; Chan, L.H.M.; Mahaffey, K.R.; Murray, M.; Sakamoto, M.; Stern, A.H. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio 2007, 36, 3–11. [Google Scholar] [CrossRef]
- Parrino, V.; Kesbiç, O.S.; Acar, Ü.; Fazio, F. Hot pepper (Capsicum sp.) oil and its effects on growth performance and blood parameters in rainbow trout (Oncorhynchus mykiss). Nat. Prod. Res. 2020, 34, 3226–3230. [Google Scholar] [CrossRef]
- Mezghani-Chaari, S.; Hamza, A.; Hamza-Chaffai, A. Hg contamination in human hair and some marine species from Sfax coasts of Tunisia: Levels and risk assessment. Environ. Monit. Assess. 2011, 180, 477–487. [Google Scholar] [CrossRef]
- Ben Salem, Z.; Ayadi, H. First investigation of trace metal distribution in surface seawater and copepods of the south coast of Sfax (Tunisia). Environ. Sci. Pollut. Res. Int. 2017, 24, 19662–19670. [Google Scholar] [CrossRef] [PubMed]
- Bhagure, G.R.; Mirgane, S.R. Heavy metal concentrations in groundwaters and soils of Thane Region of Maharashtra, India. Environ. Monit. Assess. 2011, 173, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Khemis, I.B.; Aridh, N.B.; Hamza, N.; M’Hetli, M.; Sadok, S. Heavy metals and minerals contents in pikeperch (Sander lucioperca), carp (Cyprinus carpio) and flathead grey mullet (Mugil cephalus) from Sidi Salem Reservoir (Tunisia): Health risk assessment related to fish consumption. Environ. Sci. Pollut. Res. Int. 2017, 24, 19494–19507. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, S.; Amairia, S.; Chaabouni, M.; Oueslati, W.; Chine, O.; Nachi Mkaouar, A.; Cheikhsbouii, A.; Ghorbel, R.; Zrelli, M. Contamination of Fishery Products with Hg, Cadmium, and Lead in Tunisia: Level’s Estimation and Human Health Risk Assessment. Biol. Trace Elem. Res. 2021, 199, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.; Berducci, M.T.; Bianchi, J.; Giani, M.; Campanella, L. MethylHg determination in marine sediment and organisms by Direct Hg Analyser. Anal. Chim. Acta 2009, 641, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, G.; Bravo, J.C.; Fernández, C.; Tarazona, J.V. A new method for total Hg and methyl Hg analysis in muscle of seawater fish. Bull. Environ. Contam Toxicol. 2009, 83, 210–213. [Google Scholar] [CrossRef]
- Dominik, J.; Tagliapietra, D.; Bravo, A.G.; Sigovini, M.; Spangenberg, J.E.; Amouroux, D.; Zonta, R. Hg in the food chain of the Lagoon of Venice, Italy. Mar. Pollut. Bull. 2014, 88, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Govers, L.L.; Lamers, L.P.; Bouma, T.J.; Eygensteyn, J.; de Brouwer, J.H.; Hendriks, A.J.; Huijbers, C.M.; van Katwijk, M.M. Seagrasses as indicators for coastal trace metal pollution: A global meta-analysis serving as a benchmark, and a Caribbean case study. Environ. Pollut. 2014, 195, 210–217. [Google Scholar] [CrossRef]
- Papp, R.; Aleksandar, P.; Nancy, K.; Richard, T.-G. Pharmacokinetics of Cefovecin in squirrel monkey (Saimiri sciureus), rhesus macaques (Macaca mulatta), and cynomolgus macaques (Macaca fascicularis). J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 805–808. [Google Scholar] [PubMed]
- Östlund, U.; Kidd, L.; Wengström, Y.; Rowa-Dewar, N. Combining qualitative and quantitative research within mixed method research designs: A methodological review. Int. J. Nurs. Stud. 2011, 48, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Habib, Z.A. Bisphosphonates in the treatment of osteoporosis: A review of skeletal safety concerns. Expert Rev. Endocrinol. Metab. 2017, 12, 59–71. [Google Scholar] [CrossRef] [PubMed]

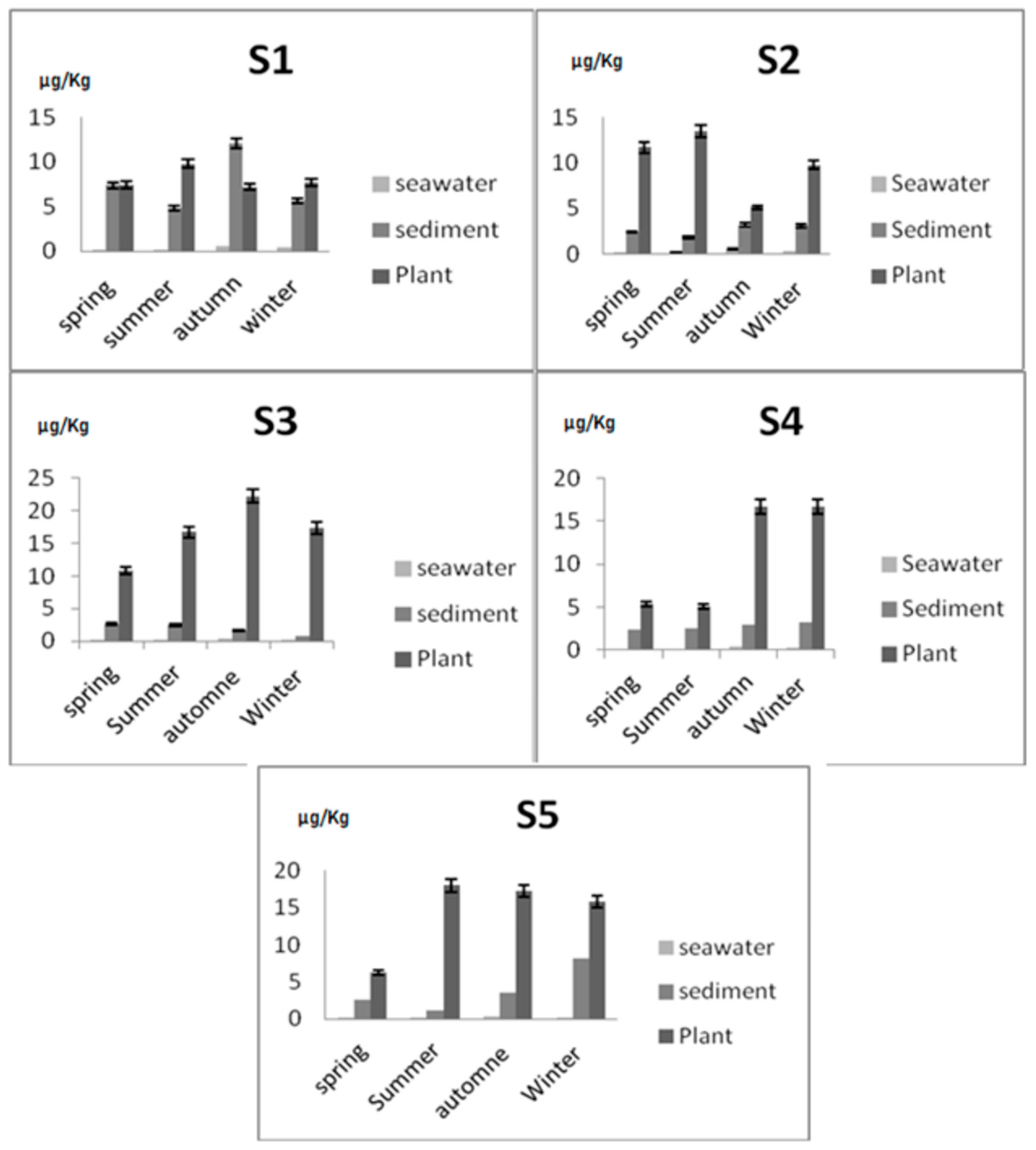

| Environmental Samples | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|
| Seawater (μg/kg) (n = 5 × 33) | 0.32 ± 0.19 | 0.25 ± 0.16 | 0.24 ± 0.15 | 0.21 ± 0.12 | 0.19 ± 0.05 |
| Sediment (μg/kg) (n = 5 × 33) | 7.48 ± 5.96 | 5.83 ± 3.57 | 1.88 ± 0.83 | 2.43 ± 1.58 | 3.85 ± 3.19 |
| Plant (μg/kg) (n = 5 × 12) | 9.27 ± 3.10 | 11.74 ± 2.85 | 16.76 ± 4.48 | 5.33 ± 0.05 | 14.34 ± 4.45 |
| Sparus aurata (μg/kg) (n = 5 × 6) %TWI | 774.78 ± 570.71 64.6% | 1853.71 ± 655.61 154.5% | 438.54 ± 208.76 36.5% | 826.16 ± 232.041 68.8% | 498 ± 470.71 41.5% |
| Sarpa salpa (μg/kg) (n = 5 × 6) %TWI | 65.11 ± 54.03 5.4% | 2518.71 ± 231.8 209.8% | 403.53 ± 431.61 33.6% | 14.14 ± 0.46 1.2% | 35.79 ± 21.69 2.9% |
| Variables | Seawater | Sediment | Plant | S. aurata | S. salpa | Site-S1 | Site-S2 | Site-S3 | Site-S4 | Site-S5 |
|---|---|---|---|---|---|---|---|---|---|---|
| seawater | 1 | 0.304 | 0.032 | 0.050 | 0.053 | 0.138 | 0.034 | −0.018 | −0.067 | −0.087 |
| sediment | 0.304 | 1 | −0.065 | 0.192 | 0.082 | 0.424 | 0.119 | −0.298 | −0.212 | −0.034 |
| plant | 0.032 | −0.065 | 1 | −0.154 | 0.181 | −0.169 | 0.063 | 0.449 *** | −0.632 | 0.289 |
| S. aurata | 0.050 | 0.192 | −0.154 | 1 | 0.898 | −0.107 | 0.954 *** | −0.428 | −0.049 | −0.370 |
| S. salpa | 0.053 | 0.082 | 0.181 | 0.898 | 1 | −0.273 | 0.971 *** | −0.109 | −0.300 | −0.290 |
| Posdidonia oceanica | Sparus aurata | Sarpa salpa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCF | BSAF | BCF | BSAF | BCF | BSAF | |||||||
| Site | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| S1 | 1.65 ± 0.48 | 0.78–2.41 | 0.19 ± 0.36 | <0.01–0.85 | 3.52 ± 0.29 | 3.02–4.09 | 2.03 ± 0.29 | 1.88–2.80 | 2.34 ± 0.55 | 1.36–3.28 | 0.87 ± 0.65 | 0.01–1.98 |
| S2 | 1.82 ± 0.45 | 0.98–2.45 | 0.45 ± 0.36 | 0.06–1.12 | 4.02 ± 0.43 | 3.26–4.58 | 2.65 ± 0.34 | 2.22–3.25 | 4.16 ± 0.44 | 3.37–4.74 | 2.78 ± 0.35 | 2.38–3.41 |
| S3 | 1.98 ± 0.40 | 1.38–2.25 | 0.92 ± 0.28 | 0.48–1.36 | 3.45 ± 0.44 | 4.58–2.68 | 2.39 ± 0.22 | 2.13–2.76 | 3.10 ± 0.56 | 2.23–4.04 | 2.05 ± 0.59 | 1.27–2.84 |
| S4 | 1.52 ± 0.36 | 0.85–2.09 | 0.44 ± 0.34 | <0.01–0.79 | 3.71 ± 0.36 | 3.04–4.28 | 2.63 ± 0.34 | 1.98–2.97 | 1.95 ± 0.36 | 1.29–2.52 | 0.8 ± 0.66 | 0.23–1.22 |
| S5 | 1.96 ± 0.46 | 1.17–2.57 | 0.66 ± 0.43 | 0.18–1.56 | 3.53 ± 0.40 | 2.87–4.06 | 2.23 ± 0.40 | 1.72–3.02 | 2.33 ± 0.45 | 1.69–3.06 | 1.02 ± 0.50 | 0.34–2.03 |
| Mean | 1.79 | 0.53 | 3.65 | 2.39 | 2.77 | 1.52 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jebara, A.; Lo Turco, V.; Faggio, C.; Licata, P.; Nava, V.; Potortì, A.G.; Crupi, R.; Mansour, H.B.; Di Bella, G. Monitoring of Environmental Hg Occurrence in Tunisian Coastal Areas. Int. J. Environ. Res. Public Health 2021, 18, 5202. https://doi.org/10.3390/ijerph18105202
Jebara A, Lo Turco V, Faggio C, Licata P, Nava V, Potortì AG, Crupi R, Mansour HB, Di Bella G. Monitoring of Environmental Hg Occurrence in Tunisian Coastal Areas. International Journal of Environmental Research and Public Health. 2021; 18(10):5202. https://doi.org/10.3390/ijerph18105202
Chicago/Turabian StyleJebara, Amel, Vincenzo Lo Turco, Caterina Faggio, Patrizia Licata, Vincenzo Nava, Angela Giorgia Potortì, Rosalia Crupi, Hedi Ben Mansour, and Giuseppa Di Bella. 2021. "Monitoring of Environmental Hg Occurrence in Tunisian Coastal Areas" International Journal of Environmental Research and Public Health 18, no. 10: 5202. https://doi.org/10.3390/ijerph18105202
APA StyleJebara, A., Lo Turco, V., Faggio, C., Licata, P., Nava, V., Potortì, A. G., Crupi, R., Mansour, H. B., & Di Bella, G. (2021). Monitoring of Environmental Hg Occurrence in Tunisian Coastal Areas. International Journal of Environmental Research and Public Health, 18(10), 5202. https://doi.org/10.3390/ijerph18105202










