Prevalence of Oral and Maxillofacial Disorders in Patients with Systemic Scleroderma—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Screening and Selection Process
2.3. Data Analysis
2.4. Assessment of Risk of Bias
3. Results
3.1. Results of Individual Studies
3.2. Additional Analysis—Quantitative Synthesis of Studies
4. Discussion
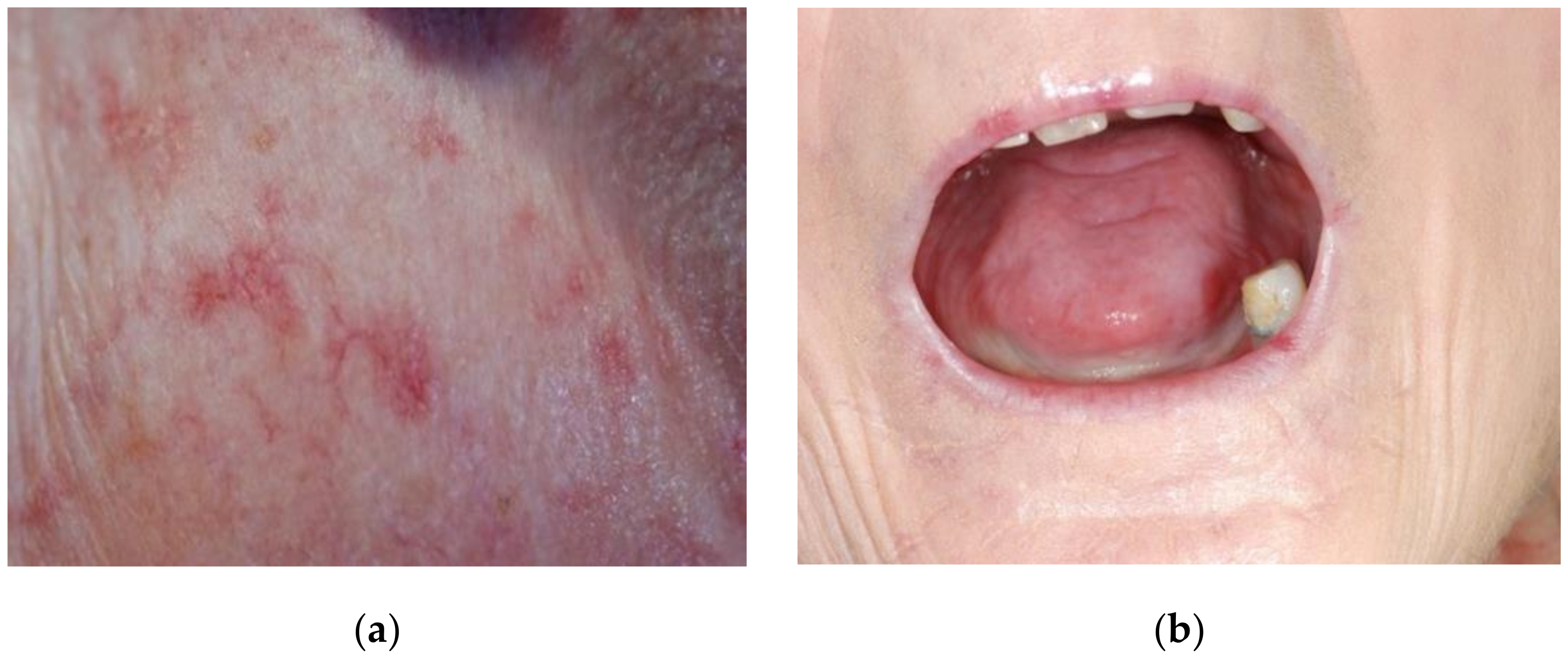
4.1. Systemic Sclerosis and Lips
4.2. Systemic Sclerosis and Salivary Glands
4.3. Systemic Sclerosis and Oral Mucosa
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Primers 2015, 1, 1–21. [Google Scholar] [CrossRef]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Rath, A. Orphanet Report Series: Prevalence and Incidence of Rare Diseases: Bibliographic Data. 2020. Available online: https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_decreasing_prevalence_or_cases.pdf (accessed on 1 January 2020).
- Berezné, A.; Valeyre, D.; Ranque, B.; Guillevin, L.; Mouthon, L. Interstitial Lung Disease Associated with Systemic Sclerosis: What Is the Evidence for Efficacy of Cyclophosphamide? Ann. N. Y. Acad. Sci. 2007, 1110, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Peoples, C.; Medsger, T.A.; Lucas, M.; Rosario, B.L.; Feghali-Bostwick, C.A.; Medsger, J.T.A. Gender differences in systemic sclerosis: Relationship to clinical features, serologic status and outcomes. J. Scleroderma Relat. Disord. 2016, 1, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Barnes, J.; Mayes, M.D. Epidemiology of systemic sclerosis: Incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr. Opin. Rheumatol. 2012, 24, 165–170. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Fransen, J.; Avouac, J.; Becker, M.; Kulak, A.; Allanore, Y.; Distler, O.; Clements, P.J.; Cutolo, M.; Czirjak, L.; et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1327–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, E.C.; Medsger, T.A. Criteria for the classification of early systemic sclerosis. J. Rheumatol. 2001, 28, 1573–1576. [Google Scholar]
- Leroy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; A Medsger, T.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [PubMed]
- Steen, V.D. Autoantibodies in Systemic Sclerosis. Semin. Arthritis Rheum. 2005, 35, 35–42. [Google Scholar] [CrossRef]
- Steen, V.D.; Medsger, T.A., Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000, 43, 2437–2444. [Google Scholar] [CrossRef]
- Rannou, F.; Poiraudeau, S.; Berezné, A.; Baubet, T.; Le-Guern, V.; Cabane, J.; Guillevin, L.; Revel, M.; Fermanian, J.; Mouthon, L. Assessing disability and quality of life in systemic sclerosis: Construct validities of the Cochin Hand Function Scale, Health Assessment Questionnaire (HAQ), Systemic Sclerosis HAQ, and Medical Outcomes Study 36-Item Short Form Health Survey. Arthritis Rheum. 2007, 57, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hunzelmann, N.; Krieg, T. Scleroderma: From pathophysiology to novel therapeutic approaches. Exp. Derm. 2010, 19, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Scardina, G.A.; Messina, P. Systemic sclerosis: Description and diagnostic role of the oral phenomena. Gen. Derm. 2004, 52, 42–47. [Google Scholar]
- Hunzelmann, N.; Genth, E.; Krieg, T.; Lehmacher, W.; Melchers, I.; Meurer, M.; Moinzadeh, P.; Muller-Ladner, U.; Pfeiffer, C.; Riemekasten, G.; et al. The registry of the German Network for Systemic Scleroderma: Frequency of disease subsets and patterns of organ involvement. Rheumatology 2008, 47, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scardina, G.A.; Pizzigatti, M.E.; Messina, P. Periodontal microcirculatory abnormalities in patients with systemic sclero-sis. J. Periodontol. 2005, 76, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Gomes da Silva, G.S.; de Melo, M.L.M.; Leão, J.C.; Carvalho, A.T.; Porter, S.; Duarte, A.L.B.P.; Dantas, A.T.; Gueiros, L.A. Oral features of systemic sclerosis: A case-control study. Oral Dis. 2019, 25, 1995–2002. [Google Scholar] [CrossRef]
- Mouthon, L.; Rannou, F.; Berezne, A.; Pagnoux, C.; Arene, J.; Fois, E.; Cabane, J.; Guillevin, L.; Revel, M.; Fermanian, J.; et al. Development and validation of a scale for mouth handicap in systemic sclerosis: The Mouth Handicap in Systemic Sclerosis scale. Ann. Rheum. Dis. 2007, 66, 1651–1655. [Google Scholar] [CrossRef] [Green Version]
- Albilia, J.B.; Lam, D.K.; Blanas, N.; Clokie, C.M.L.; Sándor, G.K.B. Small mouths. Big problems? A review of scleroderma and its oral health implications. J. Can. Dent. Assoc. 2007, 73, 831–836. [Google Scholar]
- Derk, C.T.; Rasheed, M.; Spiegel, J.R.; Jimenez, S.A. Increased incidence of carcinoma of the tongue in patients with systemic sclerosis. J. Rheumatol. 2005, 32, 637–641. [Google Scholar] [PubMed]
- Derk, C.T.; Rasheed, M.; Artlett, C.M.; A Jimenez, S. A cohort study of cancer incidence in systemic sclerosis. J. Rheumatol. 2006, 33, 1113–1116. [Google Scholar]
- Kuo, C.-F.; Luo, S.-F.; Yu, K.-H.; Chou, I.-J.; Tseng, W.-Y.; Chang, H.-C.; Fang, Y.-F.; Chiou, M.-J.; See, L.-C. Cancer risk among patients with systemic sclerosis: A nationwide population study in Taiwan. Scand. J. Rheumatol. 2011, 41, 44–49. [Google Scholar] [CrossRef]
- German Federal Ministry of Health. Available online: http://www.bmg.bund.de/themen/praevention/gesundheitsgefahren/seltene-erkrankungen.html (accessed on 30 September 2020).
- Naylor, W.; Douglass, C.W.; Mix, E. The nonsurgical treatment of microstomia in scleroderma: A pilot study. Oral Surg. Oral Med. Oral Pathol. 1984, 57, 508–511. [Google Scholar] [CrossRef]
- Rannou, F.; Boutron, I.; Mouthon, L.; Sanchez, K.; Tiffreau, V.; Hachulla, E.; Thoumie, P.; Cabane, J.; Chatelus, E.; Sibilia, J.; et al. Personalized Physical Therapy Versus Usual Care for Patients With Systemic Sclerosis: A Randomized Controlled Trial. Arthritis Rheum. 2016, 69, 1050–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osial, J.R.; Whiteside, T.L.; Buckingham, R.B.; Singh, G.; Barnes, E.L.; Pierce, J.M.; Rodnan, G.P. Clinical and serologic study of Sjögren’s syndrome in patients with progressive systemic sclerosis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1983, 26, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Segovia, D.; Ibánez, G.; Hernández-Ortíz, J.; Velázquez-Forero, F.; González-Jiménez, Y. Sjögren’s syndrome in progressive systemic sclerosis (scleroderma). Am. J. Med. 1974, 57, 78–85. [Google Scholar] [CrossRef]
- Clegg, D.; Reading, J.C.; Mayes, M.D.; Seibold, J.R.; Harris, C.; Wigley, F.M.; Ward, J.R.; Pisko, E.J.; Weisman, M.H.; Lee, P. Comparison of aminobenzoate potassium and placebo in the treatment of scleroderma. J. Rheumatol. 1994, 21, 105–110. [Google Scholar]
- Pizzo, G.; Scardina, G.A.; Messina, P. Effects of a nonsurgical exercise program on the decreased mouth opening in patients with systemic scleroderma. Clin. Oral Investig. 2003, 7, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Sordet, C.; Depinay, C.; Ardizonne, M.; Vacher-Lavenu, M.C.; Sibilia, J.; Kahan, A.; Allanore, Y. Systemic sclerosis-associated Sjögren’s syndrome and relationship to the limited cutaneous subtype: Results of a prospective study of sicca syndrome in 133 consecutive patients. Arthritis Rheum. 2006, 54, 2243–2249. [Google Scholar] [CrossRef]
- Sumanth, M.K.; Sharma, V.K.; Khaitan, B.K.; Kapoor, A.; Tejasvi, T. Evaluation of oral methotrexate in the treatment of systemic sclerosis. Int. J. Derm. 2007, 46, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.; Conte, C.; Brewer, C.; Good, C.C.; Perella, D.; Rossie, K.M.; Steen, V. Oral hygiene in scleroderma: The effectiveness of a multi-disciplinary intervention program. Disabil. Rehabil. 2009, 32, 379–384. [Google Scholar] [CrossRef]
- Yuen, H.K.; Weng, Y.; Bandyopadhyay, D.; Reed, S.G.; Leite, R.S.; Silver, R.M. Effect of a multi-faceted intervention on gingival health among adults with systemic sclerosis. Clin. Exp. Rheumatol. 2011, 29, S26–S32. [Google Scholar] [PubMed]
- Del Papa, N.; Caviggioli, F.; Sambataro, D.; Zaccara, E.; Vinci, V.; Di Luca, G.; Parafioriti, A.; Armiraglio, E.; Maglione, W.; Polosa, R.; et al. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell Transpl. 2015, 24, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.L.; Brewer, I.; Leonardi, R.; Roberts, N.; Bagnato, G. Pain threshold and temporomandibular function in systemic sclerosis: Comparison with psoriatic arthritis. Clin. Rheumatol. 2018, 37, 1861–1867. [Google Scholar] [CrossRef]
- Tarpley, T.M.; Anderson, L.G., Jr.; White, C.L. Minor salivary gland involvement in Sjogren’s syndrome. Oral Surg. Oral Med. Oral Pathol. 1974, 37, 64–74. [Google Scholar] [CrossRef]
- Vital, S. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helkimo, M.I.; Bailey, J.O., Jr.; Ash, M.M., Jr. Correlations of electromyographic silent period duration and the Helkimo dysfunction index. Acta Odontol. Scand. 1979, 37, 51–56. [Google Scholar] [CrossRef]
- Knippschild, S.; Baulig, C.; Krummenauer, F. Heterogeneity in meta-analyses—Do not compare apples and oranges. Z Zahnärztl Impl. 2015, 31, 224–229. [Google Scholar]
- Blettner, M.; Sauerbrei, W.; Schlehofer, B.; Scheuchenpflug, T.; Friedenreich, C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int. J. Epidemiol. 1999, 28, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Devgire, V.; Hughes, M. Raynaud’s phenomenon. Br. J. Hosp. Med. 2019, 80, 658–664. [Google Scholar] [CrossRef]
- Jung, S.; Martin, T.; Schmittbuhl, M.; Huck, O. The spectrum of orofacial manifestations in systemic sclerosis: A challenging management. Oral Dis. 2016, 23, 424–439. [Google Scholar] [CrossRef]
- Baron, M.; Hudson, M.; Tatibouet, S.; Steele, R.; Lo, E.; Gravel, S.; Gyger, G.; Sayegh, T.E.; Pope, J.; Fontaine, A.; et al. The Canadian systemic sclerosis oral health study: Orofacial manifestations and oral health-related quality of life in systemic sclerosis compared with the general population. Rheumatology 2014, 53, 1386–1394. [Google Scholar] [CrossRef] [Green Version]
- Alantar, A.; Cabane, J.; Hachulla, E.; Princ, G.; Ginisty, D.; Hassin, M.; Sorel, M.; Maman, L.; Pilat, A.; Mouthon, L. Recommendations for the care of oral involvement in patients with systemic sclerosis. Arthritis Rheum. 2011, 63, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Veale, B.J.; Jablonski, R.Y.; Frech, T.M.; Pauling, J.D. Orofacial manifestations of systemic sclerosis. Br. Dent. J. 2016, 221, 305–310. [Google Scholar] [CrossRef]
- Paquette, D.L.; Falanga, V. Cutaneous concerns of scleroderma patients. J. Derm. 2003, 30, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Crincoli, V.; Fatone, L.; Fanelli, M.; Rotolo, R.P.; Chialà, A.; Favia, G.; Lapadula, G. Orofacial Manifestations and Temporomandibular Disorders of Systemic Scleroderma: An Observational Study. Int. J. Mol. Sci. 2016, 17, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couderc, M.; Tournadre, A.; Mathieu, S.; Pereira, B.; Soubrier, M.; Dubost, J.J. Do the salivary glands of patients with systemic sclerosis show ultrasonographic modifications suggestive of Sjögren’s syndrome? Ann. Rheum. Dis. 2020, 79, e137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobak, S.; Oksel, F.; Aksu, K.; Kabasakal, Y. The frequency of sicca symptoms and S jögren’s syndrome in patients with systemic sclerosis. Int. J. Rheum. Dis. 2013, 16, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Airò, P.; Dieude, P.; Caramaschi, P.; Tiev, K.; Diot, E.; Sibilia, J.; Cappelli, S.; Granel, B.; Vacca, A.; et al. Associated Autoimmune Diseases in Systemic Sclerosis Define a Subset of Patients with Milder Disease: Results from 2 Large Cohorts of European Caucasian Patients. J. Rheumatol. 2010, 37, 608–614. [Google Scholar] [CrossRef]
- Bassel, M.; Hudson, M.; Taillefer, S.S.; Schieir, O.; Baron, M.; Thombs, B.D. Frequency and impact of symptoms experienced by patients with systemic sclerosis: Results from a Canadian National Survey. Rheumatology 2010, 50, 762–767. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, S.; Goldblatt, F.; Dugar, M.; Gordon, T.P.; Roberts-Thomson, P.J. Prevalence of sicca symptoms in a South Australian cohort with systemic sclerosis. Intern. Med. J. 2008, 38, 897–903. [Google Scholar] [CrossRef]
- Baron, M.; Hudson, M.; Tatibouet, S.; Steele, R.; Lo, E.; Gravel, S.; Gyger, G.; El Sayegh, T.; Pope, J.; Fontaine, A.; et al. Relationship Between Disease Characteristics and Orofacial Manifestations in Systemic Sclerosis: Canadian Systemic Sclerosis Oral Health Study III. Arthritis Rheum. 2015, 67, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janin, A.; Gosselin, B.; Gosset, D.; Hatron, P.Y.; Sauvezie, B. Histological criteria of Sjögren’s syndrome in scleroderma. Clin. Exp. Rheumatol. 1989, 7, 167–169. [Google Scholar]
- Porter, S.R.; Leao, J.C. Review article: Oral ulcers and its relevance to systemic disorders. Aliment. Pharm. 2005, 21, 295–306. [Google Scholar] [CrossRef]
- Derk, C.T.; Conway, R.T.; A Jimenez, S. Primary B-cell lymphoma of the tongue in a patient with systemic sclerosis. Oral Oncol. 2004, 40, 103–106. [Google Scholar] [CrossRef]
- Acartürk, T.O.; Maldonado, A.A.; Ereso, A. Intraoral reconstruction with “thinned” peroneal artery perforator flaps: An alternative to classic donor areas in comorbid patients. Microsurgery 2014, 35, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Jackowski, J.; Peters, H.; Magnin, P.; Dirschka, T. Development of a Check-List for orofacial defects associated with scleroderma. J. Dent. Res. 2003, 82, C-458–C-587. [Google Scholar]





| Inclusion Criteria |
|---|
|
| Exclusion Criteria |
|
| Search algorithm | (“systemic sclerosis” OR “scleroderma”) AND (“oral” OR “mouth” OR “gingiva” OR “intraoral” OR “perioral” OR “tongue” OR “alveolar” OR “periodontium” OR “periodontal” OR “jaw” OR “jaws” OR “mandible” OR “maxilla” OR “gnathic” OR “facial” OR “craniofacial” OR “maxillofacial” OR “temporomandibular joint” OR “palate” OR “palatal” OR “palatine” OR “palatum” OR “palatinal” OR “palatopharyngeal” OR “zygomatic” OR “uvula” OR “salivary” OR “parotid” OR “sublingual” OR “Sjogren’s syndrome” OR “Sjogren syndrome” OR “oral hygiene”) AND (“treatment” OR “therapy”) |
| Main Group | Subgroups | Main Group | Subgroups |
|---|---|---|---|
| Oral Tissue | Oral mucosa | Tongue | Mobility |
| Palatinal rugae | Plasticity | ||
| Palatopharyngeal arch | Swallowing disorders | ||
| Hard palate | Consistence | ||
| Soft palate | Colour | ||
| Uvula | Atrophy | ||
| Base of the mouth | Raynaud-phenomenon | ||
| Gingiva | Fibrosis | ||
| Intraoral telangiectasias | Lingual frenulum/scleroglosson | ||
| Xerostomia | |||
| Capillary system | |||
| Atrophy | |||
| Lips | Microstomia | Periodontal status | Periodontal ligament |
| Microcheilia | Periodontitis | ||
| Colour | |||
| Consistence | |||
| Ulcerations | |||
| Mouth angle | |||
| Perioral wrinkles | |||
| Capillary system | |||
| Labial frenulum | |||
| Bone | Alveolar bone | Other | Muscles |
| Jaw bone | Oral cancer | ||
| Ascending ramus | Teeth | ||
| Jaw angle | Trigeminal nerve | ||
| Zygomatic arch | Salivary glands | ||
| TMJ and coronoid process | |||
| Dysgnathic alterations |
| Author and Year | Cases | Age | Sex | Disease Duration (Years) | Methods | Results |
|---|---|---|---|---|---|---|
| Osial et al., 1983 [26] | 58 | 51 ± 2 | F: 86% M: 14% | 6.1 ± 1.0 | Clinical examination of the patients and histopathological assessment of the labial small salivary glands to investigate Sjögren’s syndrome | Enlargement of the labial salivary glands (2), fibrosis of the salivary glands (19), Sjögren’s syndrome (17), enlargement of the parotid salivary gland |
| Naylor et al., 1984 [24] | 9 | NA | NA | NA | Non-surgical improvement of the mouth opening by forming two groups and comparing two exercises by applying a double-blind procedure | Mean mouth opening improvement in control group: 3.0 mm; mean mouth opening improvement in test group: 5.6 mm |
| Drosos et al., 1988 [27] | 44 | 49.2 ± 13.3 | F: 95% M: 5% | 8.0 ± 7.4 | Evaluation of simultaneous existence of Sjögren’s syndrome via lip biopsy, dry keratoconjunctivitis, and/or xerostomia | Labial salivary gland score: 2 + (10), 1 + (3); mild to moderate fibrosis (17); normal tissue (14); Sjögren’s syndrome (9); enlargement of the parotid gland (20); |
| Clegg et al., 1994 [28] | 146 | 49 ± 13 | F: 83% M: 17% | 104 months | Comparison between potassium aminobenzoate and a placebo in the treatment of the dermal manifestations | No significant improvement as to mouth opening, skin thickness, and lip mobility through medication |
| Pizzo et al., 2003 [29] | 10 | 56.8 ± 11.19 | F: 100% | NA | Investigation of the effect of an 18-month, non-surgical, domestic-exercise-based intervention on mouth opening | All subjects showed improved mouth opening |
| Avouac et al., 2006 [30] | 133 | 55 ± 13 | F: 86% M: 14% | 6.5 ± 6 | Subjective mouth dryness questionnaire, Schirmer I test for measuring the salivary flow rate; labial biopsy after positive questionnaire or Schirmer test | Subjective sicca symptomatology (85); positive Schirmer I test (61); biopsy (91) |
| Sumanth et al., 2007 [31] | 33 | 31 ± 9 | F: 88% M: 12% | 5.6 ± 4.5 | Effects of methotrexate administration on oral mouth health | Statistically significant improvement of mouth opening (33.92 mm ± 1.70 mm, p = 0.024) (25); no statistical differences in histopathological assessment |
| Poole et al., 2010 [32] | 17 | 53.9 | F: 89% M: 11% | 10.75 | Effect of oral hygiene measures and domestic exercises on oral mouth health | Significant reduction of oral inflammation parameters (BOP) after 6 study months |
| Yuen et al., 2011 [33] | 48 | 50.7 ± 13.0 | F: 79% M: 21% | 7.6 ± 6.1 | Effect of an oral hygiene regime on gum health | Significant improvement of gingival index (GI) |
| Del Papa et al., 2015 [34] | 20 | 35 ± 15 | F: 100% | 11 ± 10 | Effect of autologous fat transplantation to treat perioral fibrosis | Significant improvement of mouth opening and function |
| Rannou et al., 2017 [25] | 218 | 52.7 ± 14.8 (Group 1) 53.1 ± 14.4 (Group 2) | F: 86 % M: 14% (Group 1) F: 80% M: 20% (Group 2) | 6.5 ± 6.5 (Group 1) 6.7 ± 8.6 (Group 2) | Group 1: Customized physiotherapy in addition to regular therapy; Group 2: No customized physiotherapy in addition to regular therapy | No significant improvement through additional measures after 12 months |
| Lo Giudice et al., 2018 [35] | 30 | 60 | F: 100% | 10 | To investigate whether a lower pain threshold is associated with increased temporomandibular dysfunction in systemic sclerosis (SSc) compared with psoriasis arthritis (PsA) and healthy controls | The temporomandibular apparatus is functionally impaired in comparison with control group |
| Group | N Studies | N Included Patients | Pooled Prevalence Estimate | N Added Studies | Adjusted Pooled Prevalence Estimate |
|---|---|---|---|---|---|
| Lip | 6 | 340 | 63.1% [95% CI: 48.9–75.3] | 2 | 57.7% [95% CI: 40.8–72.09] |
| Oral mucosa | 3 | 235 | 35.5% [95% CI: 15.7–62.0] | 0 | 35.5% [95% CI: 15.7–62.0] |
| Other | 4 | 283 | 25.4% [95% CI: 14.2–41.3] | 0 | 25.4% [95% CI: 14.2–41.3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benz, K.; Baulig, C.; Knippschild, S.; Strietzel, F.P.; Hunzelmann, N.; Jackowski, J. Prevalence of Oral and Maxillofacial Disorders in Patients with Systemic Scleroderma—A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 5238. https://doi.org/10.3390/ijerph18105238
Benz K, Baulig C, Knippschild S, Strietzel FP, Hunzelmann N, Jackowski J. Prevalence of Oral and Maxillofacial Disorders in Patients with Systemic Scleroderma—A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(10):5238. https://doi.org/10.3390/ijerph18105238
Chicago/Turabian StyleBenz, Korbinian, Christine Baulig, Stephanie Knippschild, Frank Peter Strietzel, Nicolas Hunzelmann, and Jochen Jackowski. 2021. "Prevalence of Oral and Maxillofacial Disorders in Patients with Systemic Scleroderma—A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 10: 5238. https://doi.org/10.3390/ijerph18105238
APA StyleBenz, K., Baulig, C., Knippschild, S., Strietzel, F. P., Hunzelmann, N., & Jackowski, J. (2021). Prevalence of Oral and Maxillofacial Disorders in Patients with Systemic Scleroderma—A Systematic Review. International Journal of Environmental Research and Public Health, 18(10), 5238. https://doi.org/10.3390/ijerph18105238






