Prenatal HIV Test Uptake and Its Associated Factors for Prevention of Mother to Child Transmission of HIV in East Africa
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design and Data Sources
2.2. Outcome Variables
2.3. Explanatory Variables
2.4. Statistical Analyses
3. Results
3.1. Characteristics of the Study Participants in East Africa
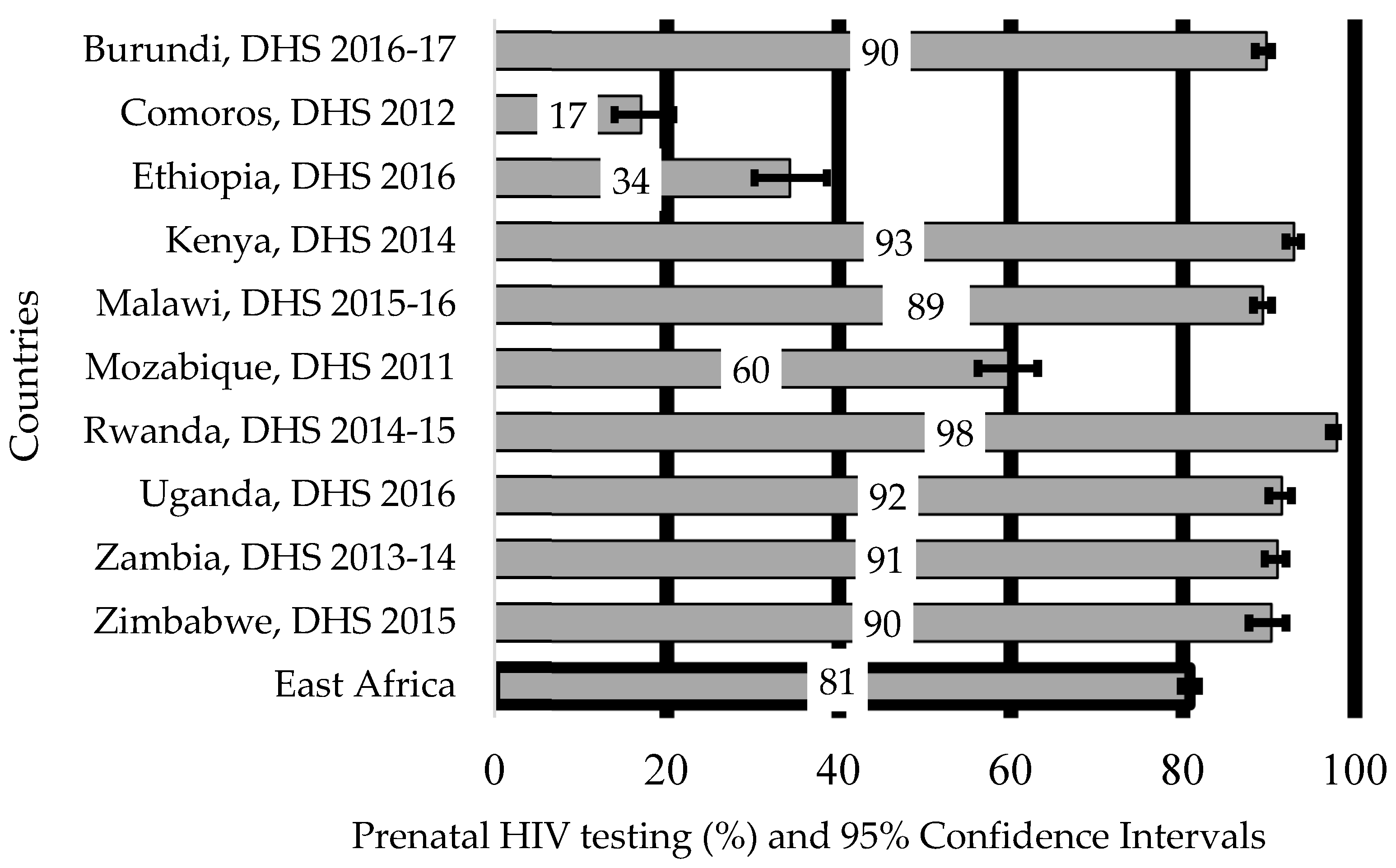
3.2. The Proportion of Women Who Used HIV Test Services for PMTCT of HIV in East African Countries by Each Study Factor
3.3. Factors Associated with Prenatal HIV Testing for PMTCT of HIV in East African Countries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNICEF. Prevention of Mother to Child Transmission (PMTCT). 2017. Available online: https://www.unicef.org/supply/index_42855.html (accessed on 20 January 2020).
- World Health Organization. Monitoring The Building Blocks of Health Systems: A Handbook of Indicators and Their Measurement Strategies. Available online: https://www.who.int/workforcealliance/knowledge/toolkit/26.pdf?ua=1 (accessed on 20 January 2020).
- Organization WH. Prevention of Mother-to-Child Transmission (PMTCT). 2018. Available online: https://www.who.int/gho/hiv/epidemic_response/PMTCT_text/en/ (accessed on 20 January 2020).
- Mutabazi, J.C.; Zarowsky, C.; Trottier, H. The Impact of Programs for Prevention of Mother-to-Child Transmission of HIV on Health Care Services and Systems in Sub-Saharan Africa-A Review. Public Health Rev. 2017, 38, 1–27. [Google Scholar] [CrossRef]
- Tudor Car, L.; van Velthoven, M.H.M.M.T.; Brusamento, S.; Elmoniry, H.; Car, J.; Majeed, A.; Tugwell, P.; Welch, V.; Marusic, A.; Atun, R. Integrating Prevention of Mother-to-Child HIV Transmission Programs to Improve Uptake: A Systematic Review. PLoS ONE 2012, 7, e35268. [Google Scholar]
- World Health Organization. PMTCT Strategic Vision 2010–2015 Geneva: WHO. 2010. Available online: https://www.who.int/hiv/pub/mtct/strategic_vision.pdf?ua=1 (accessed on 20 January 2020).
- Center for Disease Control and Prevention. Prevent Mother to Child Transmission CDC. 2020. Available online: https://www.cdc.gov/hiv/basics/hiv-prevention/mother-to-child.html (accessed on 2 May 2021).
- D’Ippolito, M.; Read, J.S.; Korelitz, J.; Joao, E.C.; Mussi-Pinhata, M.; Rocha, N.; NISDI Perinatal Study Group. Missed Opportunities for Prevention of Mother-to-Child Transmission of Human Immunodeficiency Virus Type 1 in Latin America and the Caribbean: The NISDI Perinatal Study. Pediatric Infect. Dis. J. 2007, 26, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Joint United Nations Programme on HIV/AIDS. Unaids Data. Unaids. 2019. Available online: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf (accessed on 10 May 2020).
- Joint United Nations Programme on HIV/AIDS. Ending AIDS: Progress Towards the 90–90–90 Targets: Unaids. 2017. Available online: https://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf (accessed on 10 May 2020).
- Joint United Nations Programme on HIV/AIDS. UNAIDS FACT SHEET–GLOBAL HIV STATISTICS WORLD AIDS DAY 2019 Geneva: UNAIDS. 2019. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 21 July 2020).
- Edmonds, A.; Yotebieng, M.; Lusiama, J.; Matumona, Y.; Kitetele, F.; Napravnik, S.; Cole, S.R.; van Rie, A.; Behets, F. The Effect of Highly Active Antiretroviral Therapy on the Survival of HIV-Infected Children in a Resource-Deprived Setting: A Cohort Study. PLoS Med. 2011, 8, e1001044. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antiretrovirals for Reducing the Risk of Mother-to-Child Transmission of HIV Infection Geneva: WHO. 2008. Available online: https://extranet.who.int/rhl/topics/hiv-aids/hiv-prevention/antiretrovirals-reducing-risk-mother-child-transmission-hiv-infection (accessed on 12 August 2020).
- Pellowski, J.; Wedderburn, C.; Stadler, J.A.M.; Barnett, W.; Stein, D.; Myer, L.; Zar, H.J. Implementation of Prevention of Mother-to-Child Transmission (PMTCT) in South Africa: Outcomes from a Population-based Birth Cohort Study in Paarl, Western Cape. BMJ Open 2019, 9, e033259. [Google Scholar] [CrossRef]
- Ghoma Linguissi, L.S.; Sagna, T.; Soubeiga, S.T.; Gwom, L.C.; Nkenfou, C.N.; Obiri-Yeboah, D.; Ouattara, A.K.; Pietra, V.; Simpore, J. Prevention of Mother-to-Child Transmission (PMTCT) of HIV: A Review of the Achievements and Challenges in Burkina-Faso. HIV AIDS 2019, 11, 165–177. [Google Scholar] [CrossRef]
- Sinunu, M.A.; Schouten, E.J.; Wadonda-Kabondo, N.; Kajawo, E.; Eliya, M.; Moyo, K.; Chimbwandira, F.; Strunin, L.; Kellerman, S.E. Evaluating the Impact of Prevention of Mother-to-Child Transmission of HIV in Malawi through Immunization Clinic-based Surveillance. PLoS ONE 2014, 9, e100741. [Google Scholar] [CrossRef]
- The United Nations Children’s Fund. Children, HIV and AIDS Regional Snapshot: Sub-Saharan Africa: UNICEF. 2019. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/Children%2C%20HIV%20and%20AIDS%20Regional%20snapshot%20-%20Sub-Saharan%20Africa%20%28December%202019%29.pdf (accessed on 19 September 2020).
- UNAIDS. Seizing the Moment: Tackling Entrenched Inequalities to End Epidemics. 2020. Available online: https://www.thejakartapost.com/academia/2020/07/08/seizing-the-moment-tackling-entrenched-inequalities-to-end-epidemics.html (accessed on 23 February 2021).
- World Health Organization. End HIV/AIDS by 2030 HIV/AIDS: Framework for Action in the WHO African Region, 2016–2020 Geneva: WHO. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/259638/EndAIDS-eng.pdf?sequence=1 (accessed on 26 May 2020).
- Kenyan Ministry of Health. Free Maternal Care and Removal of User Fees at Primary-Level Facilities in Kenya: Monitoring the Implementation and Impact: Baseline Report. 2014. Available online: https://www.healthpolicyproject.com/pubs/400_KenyaUserFeesBaselineReportFINAL.pdf (accessed on 17 April 2020).
- Ministry of Health-Ethiopia. Essential Health Services Package of Ethiopia. 2019. Available online: https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/essential_health_services_package_of_ethiopia_2019.pdf (accessed on 17 April 2020).
- National Consolidated Guidelines for Comprehensive HIV Prevention, Care and Treatment. Continuum of HIV Services Refers to a Comprehensive Package of HIV Prevention, Diagnostic, Treatment, Care and Support Services Provided for People at Risk of HIV Infection or Living with HIV and Their Families. 2018. Available online: https://www.afro.who.int/sites/default/files/2019-04/National%20Comprehensive%20HIV%20Care%20%20Guideline%202018.pdf (accessed on 17 April 2020).
- Zegeye, E.; Mbonigaba, J.; Dimbuene, Z. Factors Associated with the Utilization of Antenatal Care and Prevention of Mother-to-Child HIV Transmission Services in Ethiopia: Applying a Count Regression Model. BMC Womens Health 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Mariama Mustapha, V.M.; Sabrina, B.-K.; Joseph, R.; Nicolette, N.-B. Utilization of “Prevention of Mother-to-Child Transmission” of HIV Services by Adolescent and Young Mothers in Mulago Hospital, Uganda. BMC Infect. Dis. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Gebresillassie, B.M.; Emiru, Y.K.; Erku, D.A.; Mersha, A.G.; Mekuria, A.B.; Ayele, A.A.; Tegegn, H.G. Utilization of Provider-Initiated HIV Testing and Counseling as an Intervention for PMTCT Services Among Pregnant Women Attending Antenatal Clinic in a Teaching Hospital in Ethiopia. Front. Public Health 2019, 7, 205. [Google Scholar] [CrossRef]
- Kinuthia, J.; Kiarie, J.N.; Farquhar, C.; Richardson, B.A.; Nduati, R.; Mbori-Ngacha, D.; John-Stewart, G. Uptake of Prevention of Mother to Child Transmission Interventions in Kenya: Health Systems are more Influential than Stigm. J. Int. AIDS Soc. 2011, 14, 1–9. [Google Scholar] [CrossRef][Green Version]
- Alemu, Y.M.; Ambaw, F.; Wilder-Smith, A. Utilization of HIV Testing Services among Pregnant Mothers in Low Income Primary Care Settings in Northern Ethiopia: A Cross Sectional Study. BMC Pregnancy Childbirth 2017, 17, 199. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Zhang, Y.; Li, X.M.; Menon, J.A. Facilitators and Barriers for HIV-testing in Zambia: A Systematic Review of Multi-level Factors. PLoS ONE 2018, 13, e0192327. [Google Scholar] [CrossRef]
- Kohler, P.K.; Ondenge, K.; Mills, L.A.; Okanda, J.; Kinuthia, J.; Olilo, G.; Odhiambo, F.; Laserson, K.F.; Zierler, B.; Voss, J.; et al. Shame, Guilt, and Stress: Community Perceptions of Barriers to Engaging in Prevention of Mother to Child Transmission (PMTCT) Programs in Western Kenya. AIDS Patient Care STDS 2014, 28, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Dwyer-Lindgren, L.; Cork, M.A.; Sligar, A.; Steuben, K.M.; Wilson, K.F.; Provost, N.R.; Mayala, B.K.; VanderHeide, J.D.; Collison, M.L.; Hall, J.B.; et al. Mapping HIV Prevalence in Sub-Saharan Africa between 2000 and 2017. Nature 2019, 570, 189–193. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs Statistical Division. Standard Country or Area Codes for Statistical Use. 2020. Available online: https://unstats.un.org/unsd/methodology/m49/ (accessed on 6 December 2020).
- United Nation. World Population Review. 2020. Available online: https://worldpopulationreview.com/country-rankings/east-african-countries (accessed on 6 December 2020).
- Joint United Nations Programme on HIV/AIDS. Fast-Track—Ending the AIDS Epidemic by 2030. 2014. Available online: https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf (accessed on 7 August 2020).
- USAID. The DHS Program. Demographic and Health Survey Maryland: ICF International. 2020. Available online: https://dhsprogram.com/data/available-datasets.cfm (accessed on 7 December 2020).
- The DHS program, Demographic Health Surveys. Available online: https://www.dhsprogram.com/What-We-Do/Survey-Types/DHS.cfm (accessed on 29 January 2020).
- Demographic and Health Survey 2016, Central Statistical Agency (CSA) [Ethiopia] and ICF. Addis Ababa, Ethiopia, and Rockville, Maryland, USA. 2016. Available online: https://dhsprogram.com/pubs/pdf/FR328/FR328.pdf (accessed on 21 January 2020).
- Demographic and Health Survey 2016: Uganda Bureau of Statistics Kampala, Uganda and The DHS Program ICF Rockville, Maryland, USA. 2016. Available online: https://dhsprogram.com/pubs/pdf/FR333/FR333.pdf (accessed on 21 January 2020).
- Veloso, V.G.; Bastos, F.I.; Portela, M.C.; Grinsztejn, B.; Joao, E.C.; Pilotto, J.H.D.S.; Araújo, A.B.B.; Santos, B.R.; Fonseca, R.C.D.; Kreitchmann, R.; et al. HIV Rapid Testing as a Key Strategy for Prevention of Mother-to-Child Transmission in Brazil. Rev. Saude Publica 2010, 44, 803–811. [Google Scholar] [CrossRef][Green Version]
- European Centre for Disease Prevention and Control. Antenatal Screening Approaches Effective in Preventing Motherchild-Transmission of HIV, Hepatitis B, Syphilis and Rubella in Vulnerable Populations. 2017. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/antenatal-screening-approaches-to-prevent-MTCT-of-HIV-HBV-syphilis-rubella-lit-review-2017.pdf (accessed on 21 January 2020).
- Andersen, R.M. Revisiting the Behavioral Model and Access to Medical Care: Does it Matter? J. Health Soc. Behav. 1995, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saad-Haddad, G.; DeJong, J.; Terreri, N.; Restrepo-Mendez, M.C.; Perin, J.; Vaz, L.; Newby, H.; Amouzou, A.; Barros, A.J.D.; Bryce, J. Patterns and Determinants of Antenatal Care Utilization: Analysis of National Survey Data in Seven Countdown Countries. J. Glob. Health 2016, 6, 010404. [Google Scholar] [CrossRef]
- Tolera, H.; Gebre-Egziabher, T.; Kloos, H. Using Andersen’s Behavioral Model of Health Care Utilization in a Decentralized Program to Examine the Use of Antenatal Care in Rural Western Ethiopia. PLoS ONE 2020, 15, e0228282. [Google Scholar] [CrossRef]
- Mbalinda, S.N.; Kaye, D.K.; Nyashanu, M.; Kiwanuka, N. Using Andersen’s Behavioral Model of Health Care Utilization to Assess Contraceptive Use among Sexually Active Perinatally HIV-Infected Adolescents in Uganda. Int. J. Reprod. Med. 2020, 2020, 8016483. [Google Scholar] [CrossRef]
- Mekonnen, T.; Dune, T.; Perz, J.; Ogbo, F.A. Trends and Determinants of Antenatal Care Service Use in Ethiopia between 2000 and 2016. Int. J. Environ. Res. Public Health 2019, 16, 748. [Google Scholar] [CrossRef]
- Ejigu, Y.; Tadesse, B. HIV Testing during Pregnancy for Prevention of Mother-to-Child Transmission of HIV in Ethiopia. PLoS ONE 2018, 13, e0201886. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, M.; Cawthorne, A.; Nattabi, B.; Ayella, E.O.; Ogwang, M.; Declich, S. Investigating Factors Associated with Uptake of HIV Voluntary Counselling and Testing among Pregnant Women Living in North Uganda. AIDS Care 2007, 19, 733–739. [Google Scholar] [CrossRef] [PubMed]
- DHS Comparative Reports. The DHS Wealth Index. Available online: https://dhsprogram.com/pubs/pdf/CR6/CR6.pdf (accessed on 11 January 2021).
- Ezeh, O.K.; Ogbo, F.A.; Stevens, G.J.; Tannous, W.K.; Uchechukwu, O.L.; Ghimire, P.R.; Agho, K.E.; Maternal, Global and Child Health Research Collaboration (GloMACH). Factors Associated with the Early Initiation of Breastfeeding in Economic Community of West African States (ECOWAS). Nutrients 2019, 11, 2765. [Google Scholar] [CrossRef] [PubMed]
- Agho, K.E.; Ezeh, O.K.; Ghimire, P.R.; Uchechukwu, O.L.; Stevens, G.J.; Tannous, W.K.; Fleming, C.; Ogbo, F.A. Maternal, Global Exclusive Breastfeeding Rates and Associated Factors in 13 “Economic Community of West African States” (ECOWAS) Countries. Nutrients 2019, 11, 3007. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection Recommendations for a Public Health Approach-Second Edition: WHO. 2016. Available online: https://www.who.int/hiv/pub/arv/arv-2016/en/ (accessed on 21 January 2021).
- Health Kmo. Kenya Aids Response Progress Report 2016. 2016. Available online: http://nacc.or.ke/wp-content/uploads/2016/11/Kenya-AIDS-Progress-Report_web.pdf (accessed on 20 February 2021).
- Larsson, E.C.; Thorson, A.E.; Pariyo, G.; Waiswa, P.; Kadobera, D.; Marrone, G.; Ekström, A.M. Missed Opportunities: Barriers to HIV Testing during Pregnancy from a Population based Cohort Study in Rural Uganda. PLoS ONE 2012, 7, e37590. [Google Scholar]
- United Nations Programme on HIV/AIDS. Policy Statement: UNAIDS/WHO Policy Statement on HIV Testing: Unaids. 2004. Available online: http://data.unaids.org/una-docs/hivtestingpolicy_en.pdf (accessed on 21 February 2021).
- Africa OSIFE. HIV/AIDS, Human Rights, and Legal Services in Uganda: A Country Assessment. 2008. Available online: https://www.refworld.org/pdfid/4cdcead32.pdf (accessed on 22 February 2021).
- Kasenga, F.; Byass, P.; Emmelin, M.; Hurtig, A.K. The Implications of Policy Changes on the Uptake of a PMTCT Programme in Rural Malawi: First Three Years of Experience. Glob. Health Action 2009, 2, 1883. [Google Scholar] [CrossRef]
- Tudor, C.L.; Brusamento, S.; Elmoniry, H.; van Velthoven, M.H.; Pape, U.J.; Welch, V.; Tugwell, P.; Majeed, A.; Rudan, I.; Car, J.; et al. The Uptake of Integrated Perinatal Prevention of Mother-to-Child HIV Transmission Programs in Low-and Middle-income Countries: A Systematic Review. PLoS ONE 2013, 8, e56550. [Google Scholar] [CrossRef] [PubMed]
- Tlhajoane, M.; Masoka, T.; Mpandaguta, E.; Rhead, R.; Church, K.; Wringe, A.; Kadzura, N.; Arinaminpathy, N.; Nyamukapa, C.; Schur, N.; et al. A Longitudinal Review of National HIV Policy and Progress Made in Health Facility Implementation in Eastern Zimbabwe. Health Res. Policy Syst. 2018, 16, 92. [Google Scholar] [CrossRef]
- Druce, N.; Nolan, A. Seizing the Big Missed Opportunity: Linking HIV and Maternity Care Services in Sub-Saharan Africa. Reprod. Health Matters 2007, 15, 190–201. [Google Scholar] [CrossRef]
- Creek, T.; Ntumy, R.; Mazhani, L.; Moore, J.; Smith, M.; Han, G.; Shaffer, N.; Kilmarx, P.H. Factors Associated with Low Early Uptake of a National Program to Prevent Mother to Child Transmission of HIV (PMTCT): Results of a Survey of Mothers and Providers, Botswana, 2003. AIDS Behav. 2009, 13, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.K.L.; Asaolu, I.O.; Center, K.E.; Gibson, S.J.; Wightman, P.; Ezeanolue, E.E.; Ehiri, J.E. Antenatal Care and Uptake of HIV Testing among Pregnant Women in Sub-Saharan Africa: A Cross-sectional Study. J. Int. Aids Soc. 2016, 19, 20605. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, O. Access to Health Care in Developing Countries: Breaking Down Demand Side Barriers. Cad. Saude Publica 2007, 23, 2820–2834. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Workforce: WHO. 2015. Available online: https://www.who.int/healthsystems/topics/workforce/en/ (accessed on 14 March 2021).
- Sahn, D.E.; Stifel, D.C. Urban-rural Inequality in Living Standards in Africa. J. Afr. Econ. 2003, 12, 564–597. [Google Scholar] [CrossRef]
- Kyei, N.N.A.; Campbell, O.M.R.; Gabrysch, S. The Influence of Distance and Level of Service Provision on Antenatal Care Use in Rural Zambia. PLoS ONE 2012, 7, e46475. [Google Scholar] [CrossRef] [PubMed]
- Assefa, Y.; Gilks, C.F.; Dean, J.; Tekle, B.; Lera, M.; Balcha, T.T.; Getaneh, Y.; Van Damme, W.; Hill, P.S. Towards Achieving the Fast-track Targets and Ending the Epidemic of HIV/AIDS in Ethiopia: Successes and Challenges. Int. J. Infect. Dis. 2019, 78, 57–64. [Google Scholar] [CrossRef]
- Kuwawenaruwa, A.; Borghi, J.; Remme, M.; Mtei, G. An Assessment of Equity in the Distribution of Non-financial Health Care Inputs Across Public Primary Health Care Facilities in Tanzania. Int. J. Equity Health 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Wellings, K.; Jones, K.G.; Mercer, C.H.; Tanton, C.; Clifton, S.; Datta, J.; Copas, A.J.; Erens, B.; Gibson, L.J.; Macdowall, W.; et al. The Prevalence of Unplanned Pregnancy and Associated Factors in Britain: Findings from the Third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Lancet 2013, 382, 1807–1816. [Google Scholar] [CrossRef]
- Wasswa, R.; Kabagenyi, A.; Atuhaire, L. Determinants of Unintended Pregnancies among Currently Married Women in Uganda. J. Health Popul. Nutr. 2020, 39, 15. [Google Scholar] [CrossRef]
- Kawakatsu, Y.; Sugishita, T.; Oruenjo, K.; Wakhule, S.; Kibosia, K.; Were, E.; Honda, S. Determinants of Health Facility Utilization for Childbirth in Rural Western Kenya: Cross-sectional Study. BMC Pregnancy Childbirth 2014, 14, 265. [Google Scholar] [CrossRef]
- Peltzer, K.; Mlambo, G.; Phaweni, K. Factors Determining Prenatal HIV Testing for Prevention of Mother to Child Transmission of HIV in Mpumalanga, South Africa. AIDS Behav. 2010, 14, 1115–1123. [Google Scholar] [CrossRef]
- Kominami, M.; Kawata, K.; Ali, M.; Meena, H.; Ushijima, H. Factors Determining Prenatal HIV Testing for Prevention of Mother to Child Transmission in Dar Es Salaam, Tanzania. Pediatr. Int. 2007, 49, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Skinner, D.; Mfecane, S.; Gumede, T.; Henda, N.; Davids, A. Barriers to Accessing PMTCT Services in a Rural Area of South Africa. Afr. J. AIDS Res. 2005, 4, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, B.; Nel, R.; Lategan-Potgieter, R. Implementation of the Prevention of Mother-to-Child Transmission (PMTCT) Program in the Northern Cape, South Africa. Curr. Hiv. Res. 2017, 15, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Bateganya, M.H.; Abdulwadud, O.A.; Kiene, S.M. Home-based HIV Voluntary Counseling and Testing in Developing Countries. Cochrane Database Syst. Rev. 2007, 4, CD006493. [Google Scholar]
- Jurgensen, M.; Sandoy, I.F.; Michelo, C.; Fylkesnes, K.; Mwangala, S.; Blystad, A. The Seven Cs of the High Acceptability of Home-based VCT: Results from a Mixed Methods Approach in Zambia. Soc. Sci. Med. 2013, 97, 210–219. [Google Scholar] [CrossRef]
- Demographic and Health Survey. Protecting the Privacy of DHS Survey Respondents. Available online: https://www.dhsprogram.com/What-We-Do/Protecting-the-Privacy-of-DHS-Survey-Respondents.cfm (accessed on 14 March 2021).

| Burundi | Comoros | Ethiopia | Kenya | Malawi | Mozambique | Rwanda | Uganda | Zambia | Zimbabwe | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes # n (%) | Yes # n (%) | Yes # n (%) | Yes # n (%) | Yes # n (%) | Yes # n (%) | Yes # n (%) | Yes # n (%) | Yes # n (%) | Yes # n (%) | Yes # n (%) | |
| Community level factors | |||||||||||
| Residency | |||||||||||
| Urban | 471 (8.7) | 96 (7.4) | 410 (9.5) | 2530 (34.4) | 848 (12.6) | 1050 (21.4) | 554 (17.1) | 1214 (20.6) | 1656 (32.6) | 653 (26.6) | 16,238 (20.3) |
| Rural | 4384 (81.0) | 125 (9.6) | 1069 (24.8) | 4306 (58.5) | 5131 (76.7) | 1884 (38.3) | 2612 (80.7) | 4185 (70.9) | 2962 (58.4) | 1563 (63.7) | 38,289 (60.5) |
| Predisposing factors | |||||||||||
| Maternal age | |||||||||||
| 15–24 | 1255 (23.2) | 68 (5.3) | 453 (10.5) | 2688 (36.5) | 2717 (40.6) | 1223 (24.9) | 852 (26.3) | 2290 (38.8) | 1816 (35.8) | 882 (36.0) | 14,244 (30.6) |
| 25–34 | 2481 (45.9) | 111 (8.5) | 766 (17.8) | 3186 (43.3) | 2361 (35.3) | 1250 (25.5) | 1634 (50.5) | 2306 (39.1) | 1998 (39.4) | 1029 (41.9) | 17,120 (36.7) |
| 35–49 | 1117 (20.6) | 42 (3.2) | 260 (6.0) | 963 (13.1) | 897 (13.4) | 455 (9.3) | 680 (21.0) | 799 (13.6) | 804 (15.8) | 305 (12.4) | 6321 (13.5) |
| Maternal education | |||||||||||
| No education | 2096 (38.7) | 64 (4.9) | 612 (14.2) | 615 (8.4) | 673 (10.0) | 817 (16.6) | 417 (12.9) | 499 (8.5) | 424 (8.4) | 28 (1.1) | 6244 (13.4) |
| Primary | 2155 (39.8) | 46 (3.5) | 571 (13.3) | 3775 (51.3) | 3983 (59.5) | 1552 (31.6) | 2276 (70.3) | 3200 (54.2) | 2464 (48.6) | 654 (26.7) | 20,676 (44.3) |
| Secondary and higher | 603 (11.2) | 111 (8.6) | 296 (6.8) | 2445(33.2) | 1323 (19.8) | 565 (11.5) | 474 (14.6) | 1699 (28.8) | 1725 (34.0) | 1534 (62.5) | 10,777 (23.1) |
| Maternal occupation | |||||||||||
| Not working | 386 (7.1) | 123 (9.8) | 776 (18.0) | 1146 (32.4) | 1846 (27.6) | 1728 (53.0) | 254 (7.8) | 1001 (17.0) | 2084 (41.6) | 1216 (49.7) | 10,560 (25.7) |
| Professional work | 457 (8.5) | 40 (3.2) | 321 (7.4) | 547 (15.5) | 515 (7.7) | 413 (12.6) | 417 (12.9) | 1161 (19.7) | 971 (19.4) | 687 (28.1) | 5528 (13.5) |
| Nonprofessional work | 4012 (74.1) | 40 (3.2) | 382 (8.9) | 1584 (44.9) | 3619 (54.0) | 76 (2.3) | 2494 (77.1) | 3234 (54.8) | 1503 (30.0) | 306 (12.5) | 17,249 (42.0) |
| Partner education | |||||||||||
| No education | 1589 (32.3) | 39 (3.1) | 461 (11.3) | 220 (6.3) | 522 (9.3) | 662 (14.3) | 449 (15.5) | 399 (8.0) | 360 (8.0) | 20 (1.0) | 4723 (12.4) |
| Primary | 2270 (46.2) | 43 (3.5) | 545 (13.4) | 1500 (47.1) | 2735 (48.9) | 1359 (29.2) | 2007 (69.5) | 2342 (47.1) | 1605 (35.5) | 418 (20.0) | 14,825 (38.9) |
| Secondary and higher | 559 (11.4) | 117 (9.4) | 393 (9.6) | 1237 (38.9) | 1803 (32.2) | 697 (15.0) | 372 (12.9) | 1804 (36.3) | 2141 (47.3) | 1449 (69.3) | 10,574 (27.7) |
| History of Sexual violence | |||||||||||
| No | 2513 (68.0) | 168 (16.3) | 549 (29.4) | 1151 (83.2) | 1372 (73.5) | 1437 (53.2) | 654 (89.8) | 2267 (72.0) | 2927 (76.4) | 1522 (80.6) | 14,560 (65.7) |
| Yes | 799 (21.6) | 1 (0.1) | 62 (3.3) | 129 (9.3) | 298 (16.0) | 119 (4.4) | 58 (7.9) | 629 (19.9) | 538 (14.1) | 175 (9.3) | 2808 (12.7) |
| Read newspapers or magazines | |||||||||||
| No | 4643 (85.8) | 154 (11.9) | 1266 (29.4) | 4753 (64.6) | 4970 (74.2) | 2503 (50.9) | 2479 (76.7) | 4321 (73.2) | 3333 (65.7) | 1413 (57.6) | 29,834 (63.9) |
| Yes | 212 (3.9) | 67 (5.2) | 213 (4.9) | 2081 (28.3) | 1010 (15.1) | 431 (8.8) | 682 (21.1) | 1079 (18.3) | 1285 (25.3) | 803 (32.7) | 7862 (16.9) |
| Listened to the radio | |||||||||||
| No | 2664 (49.2) | 92 (7.1) | 888 (20.6) | 1317 (17.9) | 3127 (46.7) | 977 (19.9) | 576 (17.8) | 1429 (24.2) | 1932 (38.1) | 980 (39.9) | 13,982 (29.9) |
| Yes | 2191 (40.5) | 129 (9.9) | 591 (13.7) | 5516 (75.0) | 2852 (42.6) | 1957 (39.8) | 2590 (80.0) | 3971 (67.3) | 2686 (52.9) | 1235 (50.4) | 23,719 (50.9) |
| Watched television | |||||||||||
| No | 4488 (82.3) | 51 (3.9) | 986 (22.9) | 3813 (51.9) | 5028 (75.1) | 1883 (38.3) | 1977 (61.2) | 3866 (65.5) | 2973 (58.6) | 1352 (55.1) | 26,417 (56.6) |
| Yes | 366 (6.8) | 170 (13.1) | 493 (11.4) | 3018 (41.1) | 951 (14.2) | 1051 (21.4) | 1184 (36.7) | 1533 (26.0) | 1645 (32.4) | 864 (35.2) | 11,276 (24.2) |
| Enabling factors | |||||||||||
| Household wealth index | |||||||||||
| Poor | 2090 (38.6) | 53 (4.1) | 388 (9.0) | 2888 (29.3) | 2826 (42.2) | 961 (19.6) | 1421 (43.9) | 2278 (38.6) | 2095 (41.3) | 964 (39.3) | 15,965 (34.2) |
| Middle | 1938 (35.8) | 111 (8.6) | 612 (14.2) | 2507 (34.1) | 2176 (32.5) | 1314 (26.7) | 1176 (36.3) | 1989 (33.7) | 1805 (35.6) | 912 (37.2) | 14540 (31.2) |
| Rich | 826 (15.3) | 57 (4.3) | 479 (11.1) | 1441 (19.6) | 977 (14.6) | 660 (13.4) | 569 (17.6) | 1132 (19.2) | 717 (14.1) | 340 (13.8) | 7198 (15.4) |
| Household decision making | |||||||||||
| Not Involved | 2155 (44.0) | 143 (12.3) | 412 (10.1) | 1744 (59.8) | 3156 (57.6) | 1390 (33.7) | 1110 (42.5) | 3055 (62.2) | 2054 (50.4) | 678 (32.4) | 15,899 (43.7) |
| Involved | 2250 (46.0) | 54 (4.6) | 986 (24.2) | 970 (33.2) | 1810 (33.0) | 1002 (24.3) | 1453 (55.6) | 1437 (29.2) | 1660 (40.8) | 1205 (57.6) | 12,827 (35.3) |
| Health facility distance | |||||||||||
| Challenging | 1590 (29.4) | 98 (7.5) | 635 (14.7) | 841 (23.8) | 3379 (50.5) | 1499 (30.5) | 722 (22.3) | 2188 (37.1) | 1983 (39.1) | 847 (34.5) | 13,781 (32.2) |
| Not challenging | 3265 (60.3) | 123 (9.5) | 844 (16.6) | 2448 (69.1) | 2601 (38.9) | 1435 (29.2) | 2444 (75.5) | 3212 (54.4) | 2634 (51.9) | 1368 (55.8) | 20,375 (47.6) |
| Aware MTCT during pregnancy | |||||||||||
| No | 657 (12.5) | 78 (6.3) | 343 (8.8) | 3173 (22.5) | 1153 (17.6) | 479 (10.0) | 992 (30.7) | 1558 (26.5) | 1666 (32.9) | 174 (7.2) | 10,273 (22.5) |
| Yes | 4198 (79.9) | 143 (11.5) | 1136 (29.2) | 3660 (60.1) | 4826 (73.8) | 2455 (50.0) | 2172 (67.2) | 3841 (65.2) | 2950 (58.4) | 2042 (84.1) | 27,423 (60.1) |
| Aware MTCT during birth | |||||||||||
| No | 246 (4.7) | 100 (8.1) | 286 (7.4) | 1428 (19.5) | 898 (13.7) | 463 (9.6) | 100 (3.1) | 340 (5.8) | 456 (9.0) | 208 (8.6) | 4524 (9.9) |
| Yes | 4609 (87.7) | 121 (9.7) | 1193 (30.6) | 5408 (73.8) | 5081 (77.7) | 2471 (51.5) | 3066 (94.8) | 5060 (85.9) | 4161 (82.3) | 2008 (82.7) | 33,177 (72.7) |
| Aware MTCT during breastfeeding | |||||||||||
| No | 436 (8.3) | 90 (7.3) | 226 (5.8) | 691 (9.4) | 536 (8.2) | 335 (7.0) | 162 (5.0) | 513 (8.7) | 343 (6.8) | 306 (12.6) | 3639 (8.0) |
| Yes | 4419 (84.1) | 131 (10.5) | 1253 (32.2) | 6145 (83.8) | 5444 (83.3) | 2599 (54.0) | 3004 (92.9) | 4886 (82.9) | 4273 (84.6) | 1909 (78.7) | 34,062 (74.6) |
| Need Factors | |||||||||||
| A desire for the pregnancy | |||||||||||
| Wanted pregnancy | 4387 (81.1) | 197 (15.2) | 1407 (32.6) | 2946 (83.2) | 5326 (79.6) | 2828 (57.6) | 2752 (85.1) | 4916 (83.3) | 4332 (85.4) | 2041 (83.2) | 31,132 (72.7) |
| Unwanted pregnancy | 468 (8.6) | 24 (1.9) | 71 (1.7) | 345 (9.7) | 654 (9.8) | 106 (2.2) | 413 (12.8) | 484 (8.2) | 283 (5.6) | 175 (7.1) | 3022 (7.1) |
| Characteristic | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AOR | 95%CI | p Value | AOR | 95%CI | p Value | AOR | 95%CI | p Value | AOR | 95%CI | p Value | |||||
| Community level factors | ||||||||||||||||
| Countries | ||||||||||||||||
| Burundi | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||
| Comoros | 0.01 | 0.01 | 0.02 | <0.001 | 0.007 | 0.005 | 0.01 | <0.001 | 0.007 | 0.005 | 0.01 | <0.001 | 0.007 | 0.005 | 0.01 | <0.001 |
| Ethiopia | 0.05 | 0.04 | 0.06 | <0.001 | 0.05 | 0.03 | 0.06 | <0.001 | 0.04 | 0.03 | 0.05 | <0.001 | 0.04 | 0.03 | 0.05 | <0.001 |
| Kenya | 1.19 | 0.99 | 1.42 | 0.058 | 0.75 | 0.55 | 1.01 | <0.061 | 0.71 | 0.50 | 1.00 | 0.055 | 0.73 | 0.51 | 1.03 | 0.078 |
| Malawi | 0.92 | 0.78 | 1.09 | 0.382 | 0.71 | 0.54 | 0.93 | 0.014 | 0.72 | 0.53 | 0.99 | 0.043 | 0.73 | 0.53 | 1.03 | 0.06 |
| Mozambique | 0.13 | 0.11 | 0.16 | <0.001 | 0.17 | 0.13 | 0.23 | <0.001 | 0.15 | 0.11 | 0.20 | <0.001 | 0.15 | 0.11 | 0.20 | <0.001 |
| Rwanda | 4.91 | 3.62 | 6.66 | <0.001 | 3.18 | 1.79 | 5.64 | <0.001 | 2.28 | 1.28 | 4.05 | 0.005 | 2.30 | 1.29 | 4.08 | 0.004 |
| Uganda | 1.11 | 0.90 | 1.36 | 0.296 | 0.74 | 0.57 | 0.96 | 0.023 | 0.61 | 0.45 | 0.81 | 0.001 | 0.61 | 0.46 | 0.82 | 0.001 |
| Zambia | 0.93 | 0.75 | 1.15 | 0.514 | 0.59 | 0.45 | 0.77 | <0.001 | 0.54 | 0.40 | 0.73 | <0.001 | 0.54 | 0.39 | 0.73 | 0.001 |
| Zimbabwe | 0.90 | 0.67 | 1.21 | 0.505 | 0.39 | 0.26 | 0.57 | <0.001 | 0.42 | 0.28 | 0.64 | <0.001 | 0.42 | 0.28 | 0.64 | 0.001 |
| Residence | ||||||||||||||||
| Urban | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||
| Rural | 0.28 | 0.24 | 0.33 | <0.001 | 0.53 | 0.42 | 0.66 | <0.001 | 0.66 | 0.51 | 0.85 | 0.002 | 0.66 | 0.51 | 0.85 | 0.002 |
| Predisposing factors | ||||||||||||||||
| Maternalage | ||||||||||||||||
| 15–24 | 1.00 | 1.00 | 1.00 | |||||||||||||
| 25–34 | 1.02 | 0.90 | 1.15 | 0.752 | 0.92 | 0.80 | 1.05 | 0.245 | 0.93 | 0. 81 | 1.07 | 0.324 | ||||
| 35–49 | 1.03 | 0.88 | 1.22 | 0.650 | 0.89 | 0.74 | 1.06 | 0.223 | 0.94 | 0.78 | 1.13 | 0.523 | ||||
| Maternaleducation | ||||||||||||||||
| No education | 1.00 | 1.00 | 1.00 | |||||||||||||
| Primary | 1.42 | 1.23 | 1.63 | <0.001 | 1.29 | 1.10 | 1.50 | 0.001 | 1.29 | 1.10 | 1.50 | <0.001 | ||||
| Secondary and higher | 2.50 | 1.99 | 3.13 | <0.001 | 1.97 | 1.54 | 2.52 | <0.001 | 1.96 | 1.53 | 2.51 | <0.001 | ||||
| Maternaloccupation | ||||||||||||||||
| Not working | 1.00 | 1.00 | 1.00 | |||||||||||||
| Professional work | 1.05 | 0.87 | 1.27 | 0.569 | 0.90 | 0.74 | 1.10 | 0.329 | 0.90 | 0.74 | 1.09 | 0.294 | ||||
| Nonprofessional work | 1.08 | 0.91 | 1.27 | 0.347 | 1.02 | 0.85 | 1.22 | 0.762 | 1.02 | 0.85 | 1.22 | 0.785 | ||||
| Partner education | ||||||||||||||||
| No education | 1.00 | 1.00 | 1.00 | |||||||||||||
| Primary | 1.29 | 1.12 | 1.49 | <0.001 | 1.24 | 1.05 | 1.45 | 0.008 | 1.24 | 1.06 | 1.45 | 0.007 | ||||
| Secondary and higher | 1.65 | 1.36 | 2.01 | <0.001 | 1.55 | 1.25 | 1.93 | <0.001 | 1.56 | 1.26 | 1.94 | <0.001 | ||||
| History of Sexual violence | ||||||||||||||||
| No | 1.00 | 1.00 | 1.00 | |||||||||||||
| Yes | 0.92 | 0.79 | 1.07 | 0.306 | 0.92 | 0.78 | 1.09 | 0.366 | 0.93 | 0.78 | 1.10 | 0.408 | ||||
| Read newspapers or magazines | ||||||||||||||||
| No | 1.00 | 1.00 | 1.00 | |||||||||||||
| Yes | 1.29 | 1.05 | 1.60 | 0.014 | 1.31 | 1.04 | 1.65 | 0.020 | 1.31 | 1.04 | 1.65 | 0.021 | ||||
| Listened to the radio | ||||||||||||||||
| No | 1.00 | 1.00 | 1.00 | |||||||||||||
| Yes | 1.25 | 1.11 | 1.41 | <0.001 | 1.10 | 0.97 | 1.26 | 0.121 | 1.13 | 1.01 | 1.29 | 0.047 | ||||
| Watched television | ||||||||||||||||
| No | 1.00 | 1.00 | 1.00 | |||||||||||||
| Yes | 1.67 | 1.39 | 2.01 | <0.001 | 1.46 | 1.19 | 1.79 | <0.001 | 1.46 | 1.20 | 1.79 | <0.001 | ||||
| Enabling factors | ||||||||||||||||
| Household wealth index | ||||||||||||||||
| Poor | 1.00 | 1.00 | ||||||||||||||
| Middle | 1.29 | 1.11 | 1.50 | 0.001 | 1.29 | 1.11 | 1.50 | 0.001 | ||||||||
| Rich | 1.57 | 1.16 | 2.11 | 0.003 | 1.57 | 1.17 | 2.11 | 0.003 | ||||||||
| Health facility distance | ||||||||||||||||
| Challenging | 0.79 | 0.69 | 0.90 | 0.001 | 0.80 | 0.69 | 0.91 | 0.001 | ||||||||
| Not challenging | 1.00 | 1.00 | ||||||||||||||
| Household Decision making | ||||||||||||||||
| Not involved | 0.88 | 0.78 | 1.00 | 0.065 | 0.88 | 0.78 | 1.01 | 0.072 | ||||||||
| Involved | 1.00 | 1.00 | ||||||||||||||
| Aware of MTCT during pregnancy | ||||||||||||||||
| No | 1.00 | 1.00 | ||||||||||||||
| Yes | 0.87 | 0.75 | 1.01 | 0.073 | 1.19 | 1.00 | 1.41 | 0.06 | ||||||||
| Aware of MTCT duringbirth | ||||||||||||||||
| No | 1.00 | 1.00 | ||||||||||||||
| Yes | 1.74 | 1.43 | 2.11 | <0.001 | 1.73 | 1.42 | 2.10 | <0.001 | ||||||||
| Aware of MTCT during breastfeeding | ||||||||||||||||
| No | 1.00 | 1.00 | ||||||||||||||
| Yes | 1.40 | 1.16 | 1.70 | <0.001 | 1.41 | 1.16 | 1.71 | <0.001 | ||||||||
| Need factors | ||||||||||||||||
| Desire for pregnancy | ||||||||||||||||
| Wanted pregnancy | 1.00 | |||||||||||||||
| Unwanted pregnancy | 0.79 | 0.63 | 1.04 | 0.117 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astawesegn, F.H.; Stulz, V.; Agho, K.E.; Mannan, H.; Conroy, E.; Ogbo, F.A. Prenatal HIV Test Uptake and Its Associated Factors for Prevention of Mother to Child Transmission of HIV in East Africa. Int. J. Environ. Res. Public Health 2021, 18, 5289. https://doi.org/10.3390/ijerph18105289
Astawesegn FH, Stulz V, Agho KE, Mannan H, Conroy E, Ogbo FA. Prenatal HIV Test Uptake and Its Associated Factors for Prevention of Mother to Child Transmission of HIV in East Africa. International Journal of Environmental Research and Public Health. 2021; 18(10):5289. https://doi.org/10.3390/ijerph18105289
Chicago/Turabian StyleAstawesegn, Feleke Hailemichael, Virginia Stulz, Kingsley E. Agho, Haider Mannan, Elizabeth Conroy, and Felix Akpojene Ogbo. 2021. "Prenatal HIV Test Uptake and Its Associated Factors for Prevention of Mother to Child Transmission of HIV in East Africa" International Journal of Environmental Research and Public Health 18, no. 10: 5289. https://doi.org/10.3390/ijerph18105289
APA StyleAstawesegn, F. H., Stulz, V., Agho, K. E., Mannan, H., Conroy, E., & Ogbo, F. A. (2021). Prenatal HIV Test Uptake and Its Associated Factors for Prevention of Mother to Child Transmission of HIV in East Africa. International Journal of Environmental Research and Public Health, 18(10), 5289. https://doi.org/10.3390/ijerph18105289







