Patient Activation, Depressive Symptoms, and Self-Rated Health: Care Management Intervention Effects among High-Need, Medically Complex Adults
Abstract
:1. Introduction
2. Methods
2.1. Design
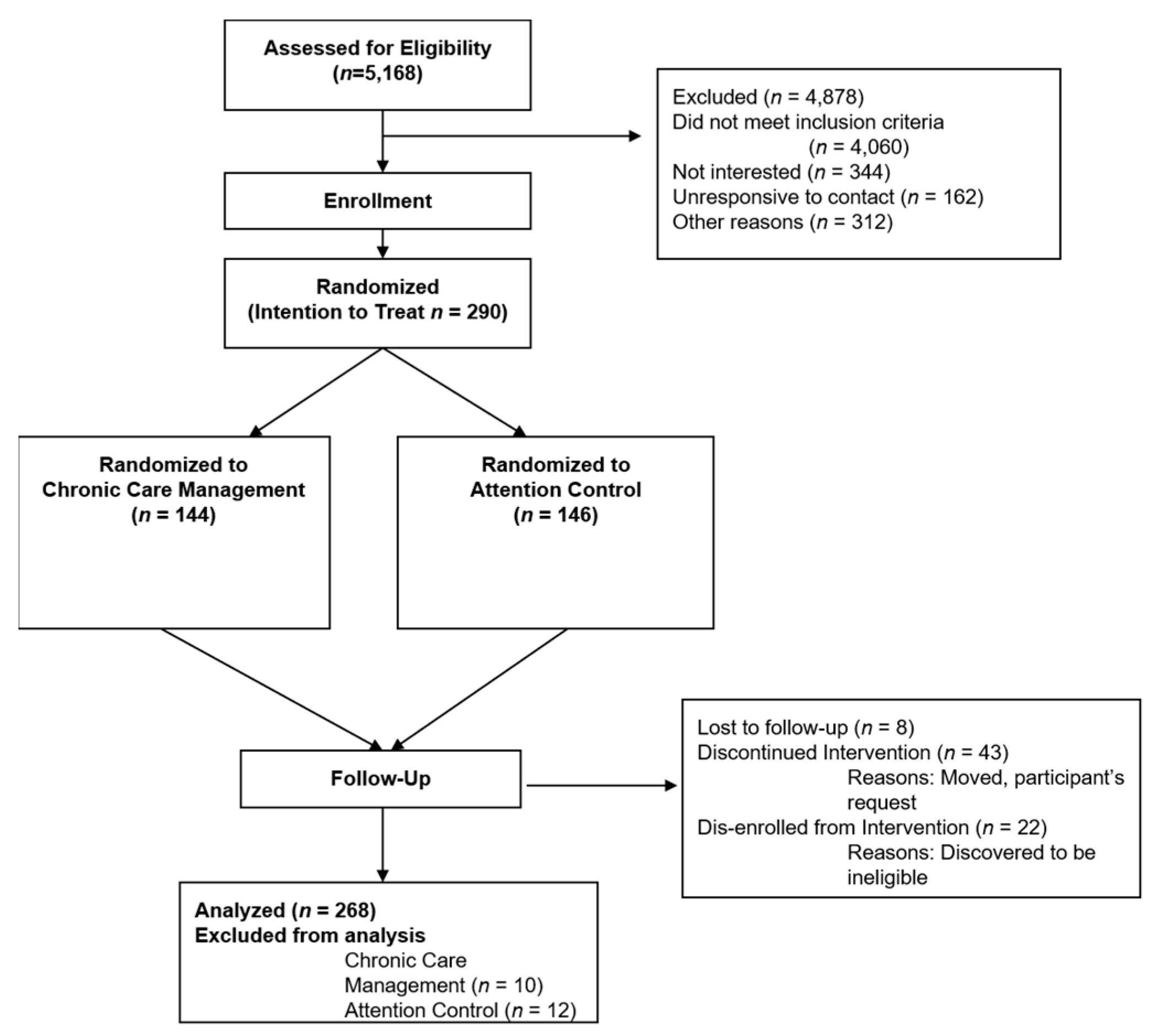
2.2. Recruitment and Randomization
2.3. Data Collection
2.4. Intervention and Protocols
2.4.1. Treatment Group Procedures
2.4.2. Attention Control Group Procedures
2.4.3. Intervention Fidelity
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations and Strengths
4.2. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blumenthal, D.; Chernof, B.; Fulmer, T.; Lumpkin, J.; Selberg, J. Caring for high-need, high-cost patients—An urgent priority. N. Engl. J. Med. 2016, 375, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Siegel, A.; Ferris, T.G. Caring for high-need, high-cost patients: What makes for a successful care management program? Commonw. Fund 2014, 19, 1–19. [Google Scholar]
- Anderson, D.R.; Olayiwola, J.N. Community health centers and the patient-centered medical home: Challenges and opportunities to reduce health care disparities in America. J. Health Care Poor Underserved 2012, 23, 949–957. [Google Scholar] [CrossRef]
- Community Health Centers: Recent Growth and the Role of the ACA. Available online: https://www.kff.org/report-section/community-health-centers-recent-growth-and-the-role-of-the-aca-issue-brief/ (accessed on 28 April 2021).
- Davy, C.; Bleasel, J.; Liu, H.; Tchan, M.; Ponniah, S.; Brown, A. Effectiveness of chronic care models: Opportunities for improving healthcare practice and health outcomes: A systematic review. BMC Health Serv. Res. 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timbie, J.W.; Setodji, C.M.; Kress, A.; Lavelle, T.A.; Friedberg, M.W.; Mendel, P.J.; Chen, E.K.; Weidmer, B.A.; Buttorff, C.; Malsberger, R.; et al. Implementation of medical homes in Federally Qualified Health Centers. N. Engl. J. Med. 2017, 377, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Shippee, N.D.; Finch, M.; Wholey, D.R. Assessing medical home mechanisms: Certification, asthma education, and outcomes. Am. J. Manag. Care 2018, 24, e79–e85. [Google Scholar]
- Nutting, P.A.; Crabtree, B.F.; Miller, W.L.; Stange, K.C.; Stewart, E.; Jaén, C. Transforming physician practices to patient-centered medical homes: Lessons from the National Demonstration Project. Health Aff. 2011, 30, 439–445. [Google Scholar] [CrossRef]
- Jortberg, B.T.; Fernald, D.H.; Hessler, D.M.; Dickinson, L.M.; Wearner, R.; Connelly, L.; Holtrop, J.S.; Fisher, L.; Dickinson, W.P. Practice characteristics associated with better implementation of self-management support. J. Am. Board Fam. Med. 2019, 32, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Dineen-Griffin, S.; Garcia-Cardenas, V.; Williams, K.; Benrimoj, S.I. Helping patients hlp themselves: A systematic review of self-management support strategies in primary health care practice. PLoS ONE 2019, 14, e0220116. [Google Scholar] [CrossRef] [Green Version]
- Dineen-Griffin, S.; Garcia-Cardenas, V.; Williams, K.; Benrimoj, S.I. Implementation of self management support for long term conditions in routine primary care settings: Cluster randomized controlled trial. BMJ 2013, 346, f2882. [Google Scholar] [CrossRef] [Green Version]
- Elissen, A.; Nolte, E.; Knai, C.; Brunn, M.; Chevreul, K.; Conklin, A.; Durand-Zaleski, I.; Erler, A.; Flamm, M.; Frølich, A.; et al. Is Europe putting theory into practice? A qualitative study of the level of self-management support in chronic care management approaches. BMC Health Serv. Res. 2013, 13, 1–19. Available online: http://www.biomedcentral.com/1472-6963/13/117 (accessed on 13 May 2021). [CrossRef] [Green Version]
- Hibbard, J.H.; Mahoney, E.R.; Stockard, J.; Tusler, M. Development and testing of a short form of the patient activation measure. Health Serv. Res. 2005, 40, 1918–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, V.M.; Davies, M.J.; Etherton-Beer, C.; McGough, S.; Schofield, D.; Jensen, J.F.; Watson, N. Increasing patient activation through diabetes self-management education: Outcomes of DESMOND in regional Western Australia. Patient Educ. Couns. 2020, 103, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, J.H.; Greene, J.; Sacks, R.; Overton, V.; Parrotta, C.D. Adding a measure of patient self-management capability to risk assessment can improve prediction of high costs. Health Aff. 2015, 35, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Greene, J.; Hibbard, J.H.; Sacks, R.; Overton, V.; Parrotta, C.D. When patient activation levels change, health outcomes and costs change, too. Health Aff. 2015, 34, 431–437. [Google Scholar] [CrossRef]
- Hibbard, J.H.; Greene, J.; Shi, Y.; Mittler, J.; Scanlon, D. Taking the long view: How well do patient activation scores predict outcomes four years later? Med. Care Res. Rev. 2015, 72, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-X.; Feng, L.-N.; Li, S.-X. The correlation between socioeconomic status and health self-management in the elderly. Int. J. Nurs. Sci. 2014, 1, 410–415. Available online: http://www.elsevier.com/ournals/inernational-journal-of-nurisng-sciences/2352-0132 (accessed on 13 May 2021). [CrossRef] [Green Version]
- Adjei Boakye, E.; Varble, A.; Rojek, R.; Peavler, O.; Trainer, A.K.; Osazuwa-Peters, N.; Hinyard, L. Sociodemographic factors associated with engagemen in diabetes self-management education among people with diabetes in the United States. Public Health Rep. 2018, 133, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardman, R.; Begg, S.; Spelten, E. What impact do chronic disease self-management support interventions have on health inequity gaps related to socioectonomic status: A systematic review. BMC Health Serv. Res. 2020, 20, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, J.; Goehring, C.; Mancuso, D. Care coordination program for Washington state Medicaid enrollees reduced inpatient hospital costs. Health Aff. 2015, 34, 653–661. [Google Scholar] [CrossRef]
- Gleason, K.T.; Tanner, E.K.; Boyd, C.M.; Saczynski, J.S.; Szanton, S.L. Factors associated with patient activation in an older adult population with functional difficulties. Patient Educ. Couns. 2016, 99, 1421–1426. [Google Scholar] [CrossRef] [Green Version]
- Hibbard, J.H.; Mahoney, E.R.; Stock, R.; Tusler, M. Do increases in patient activation result in improved self-management behaviors? Health Serv. Res. 2007, 42, 1443–1463. [Google Scholar] [CrossRef] [PubMed]
- Lorig, K.; Ritter, P.L.; Laurent, D.D.; Plant, K.; Green, M.; Jernigan, V.B.; Case, S. Online diabetes self-management program: A randomized study. Diabetes Care 2010, 33, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Deen, D.; Lu, W.H.; Rothstein, D.; Santana, L.; Gold, M.R. Asking questions: The effect of a brief intervention in community health centers on patient activation. Patient Educ. Couns. 2011, 84, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.J.; Lachin, J.M. Properties of the Urn Randomization in clinical trials. Control. Clin. Trials 1988, 9, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Hibbard, J.H.; Stockard, J.; Mahoney, E.R.; Tusler, M. Development of the Patient Activation measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv. Res. 2004, 39, 1005–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosen, D.M.; Schmittdiel, J.; Hibbard, J.; Sobel, D.; Remmers, C.; Bellows, J. Is patient activation associated with outcomes for adults with chronic conditions? J. Ambul. Care Manag. 2007, 30, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Remmers, C.; Hibbard, J.; Mosen, D.M.; Wagenfield, M.; Hoye, R.E.; Jones, C. Is patient activation associated with future health outcomes and healthcare utilization among patients with diabetes? J. Ambul. Care Manag. 2009, 32, 320–327. [Google Scholar] [CrossRef]
- Patient Activation Measure (PAM). Available online: https://www.insigniahealth.com/products/pam-survey (accessed on 28 April 2021).
- DeSalvo, K.B.; Bloser, N.; Reynolds, K.; He, J.; Muntner, P. Mortality prediction with a single general self-rated health question. A meta-analysis. J. Gen. Intern. Med. 2006, 21, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallihan, D.B.; Stump, T.E.; Callahan, C.M. Accuracy of self-reported health services use and patterns of care among urban older adults. Med. Care 1999, 37, 662–670. [Google Scholar] [CrossRef]
- Miller, T.R.; Wolinksy, F.D. Self-rated health trajectories and mortality among older adults. J. Gerontol. 2007, 62, S22–S27. [Google Scholar] [CrossRef] [Green Version]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Löwe, B.; Unützer, J.; Callahan, C.M.; Perkins, A.J.; Kroenke, K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med. Care. 2004, 42, 1194–1201. [Google Scholar] [PubMed]
- Bass, P.F.; Wilson, J.F.; Griffith, C.H. A shortened instrument for literary screening. J. Gen. Intern. Med. 2003, 18, 1036–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleich, S.N.; Sherrod, C.; Chiang, A.; Boyd, C.; Wolff, J.; DuGoff, E.; Salzberg, C.; Anderson, K.; Leff, B.; Anderson, G. Systematic review of programs treating high-need and high-cost people with multiple chronic diseases or disabilities in the United States, 2008–2014. Prev. Chronic Dis. 2015, 12, E197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, J.W. Missing data analysis: Making it work in the real world. Annu. Rev. Psychol. 2009, 60, 549–576. [Google Scholar] [CrossRef] [Green Version]
- Shafer, J.L.; Graham, J.W. Missing data: Our view of the state of the art. Psychol. Methods 2002, 7, 147–177. [Google Scholar] [CrossRef]
- Harvey, L.; Fowles, J.B.; Xi, M.; Terry, P. When activation changes, what else changes? The relationship between change in patient activation measure (PAM) and employees’ health status and health behaviors. Patient Educ. Couns. 2012, 88, 338–343. [Google Scholar] [CrossRef]
- Druss, B.G.; Ji, X.; Glick, G.; von Esenwein, S.A. Randomized trial of an electronic personal health record for patients with serious mental illnesses. Am. J. Psychiatry 2014, 171, 360–368. [Google Scholar] [CrossRef]
- Eikelenboom, N.; van Lieshout, J.; Jacobs, A.; Verhulst, F.; Lacroix, J.; van Halteren, A.; Klomp, M.; Smeele, I.; Wensing, M. Effectiveness of personalized support for self-management in primary care: A cluster randomized controlled trial. Br. J. Gen. Pract. 2016, 66, e354–e361. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, R.W.; Dickerson, F.; Lucksted, A.; Brown, C.H.; Weber, E.; Tenhula, W.N.; Kreyenbuhl, J.; Dixon, L.B. Living well: An intervention to improve self-management of medical illness for individuals with serious mental illness. Psychiatr. Serv. 2013, 64, 51–57. [Google Scholar] [CrossRef]
- Hochhalter, A.K.; Song, J.; Rush, J.; Sklar, L.; Stevens, A. Making the most of your healthcare intervention for older adults with multiple chronic illnesses. Patient Educ. Couns. 2010, 81, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hudon, C.; Chouinard, M.C.; Dubois, M.F.; Roberge, P.; Loignon, C.; Tchouaket, É.; Lambert, M.; Hudon, É.; Diadiou, F.; Bouliane, D. Case management in primary care for frequent users of health care services: A mixed methods study. Ann. Fam. Med. 2018, 16, 232–239. [Google Scholar] [CrossRef]
- Kangovi, S.; Mitra, N.; Grande, D.; Huo, H.; Smith, R.A.; Long, J.A. Community health worker support for disadvantaged patients with multiple chronic diseases: A randomized clinical trial. Am. J. Public Health 2017, 107, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Kansagara, D.; McCiain, M.; Englander, H.; Peters, D.; Morris, C.D. Patient activation measure and care transitions among socioeconomically vulnerable adults. J. Gen. Intern. Med. 2014, 29, S165. [Google Scholar]
- Napoles, T.M.; Burke, N.J.; Shim, J.K.; Davis, E.; Moskowitz, D.; Yen, I.H. Assessing patient activation among high-need, high-cost patients in urban safety net care settings. J. Urban Health 2017, 94, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Titova, E.; Salvesen, Ø.; Bentsen, S.B.; Sunde, S.; Steinshamn, S.; Henriksen, A.H. Does an integrated care intervention for CPOD patients have long-term effects on quality of life and patient activation? A prospective, open, controlled single-center intervention. PLoS ONE 2017, 12, e0167887. [Google Scholar] [CrossRef]
- Coventry, P.A.; Fisher, L.; Kenning, C.; Bee, P.; Bower, P. Capacity, responsibility, and motivation: A critical qualitative evaluation of patient and practitioner views about barriers to self-management in people with multimorbidity. BMC Health Serv. Res. 2014, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- John, J.; Tannous, K.; Jones, A. Outcomes of a 12-month patient-centered medical home model in improving patient activation and self-management behaviors among primary care patients presenting with chronic diseases in Sydney, Australia: A before-and-after-study. BMC Fam. Pract. 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Bell, J.; Turbow, S.; George, M.; Ali, M.K. Factors associated with high-utilization in a safety net setting. Health Serv. Res. 2017, 17, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bos-Touwen, I.; Schuurmans, M.; Monninkhof, E.M.; Korpershoek, Y.; Spruit-Bentvelzen, L.; Ertugrul-van der Graaf, I.; de Wit, N.; Trappenburg, J. Patient and disease characteristics associated with activation for self-management in patients with diabetes, chronic obstructive pulmonary disease, chronic heart failure, and chronic renal disease: A cross-sectional survey study. PLoS ONE 2015, 10, e0126400. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Hulen, E.; Edwards, S.; Mitchell, M.; Nicolaidis, C.; Saha, S. “It’s like riding out the chaos”: Caring for social complex patients in an ambulatory intensive care unit (A-ICU). Ann. Fam. Med. 2019, 17, 495–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, L.T.; Kaushal, R.; Garrison, K.; Carrillo, V.; Grinspan, Z.; Theis, R.; Shenkman, E.; Abramson, E. Drivers of preventable high health care utilization: A qualitative study of patients, physician, and health system leader perspectives. J. Health Serv. Res. Policy 2020, 25, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Rijken, M.; Heijmans, M.; Jansen, D.; Rademakers, J. Developments in patient activation of people with chronic illness and the impact of changes in self-reported health: Results of a nationwide longitudinal study in The Netherlands. Patient Educ. Couns. 2014, 97, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.R.; Crigler, J.; Ramirez, C.; Sisco, D.; Early, G.L. Working with socially and medically complex patients: When care transitions are circular, overlapping, and continual rather than linear and finite. J. Healthc. Qual. 2015, 37, 245–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanhope, V.; Henwood, B.F. Activating people to address their healthcare needs: Learning from people with lived experience of chronic illnesses. Community Ment. Health J. 2013, 50, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Virapongse, A.; Misky, G.J. Self-identified social determinants of health during transitions of care in the medically underserved: A narrative review. J. Gen. Intern. Med. 2018, 33, 1959–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guthrie, E.A.; Dickens, C.; Blakemore, A.; Watson, J.; Chew-Graham, C.; Lovell, K.; Afzal, C.; Kapur, N.; Tomenson, B. Depression predicts future emergency hospital admissions in primary care patients with chronic physical illness. J. Psychosom. Res. 2016, 82, 54–61. [Google Scholar] [CrossRef]
- Sacks, R.M.; Greene, J.; Hibbard, J.H.; Overton, V. How well do patient activation scores predict depression outcomes one year later? J. Affect. Disord. 2014, 169, 1–6. [Google Scholar] [CrossRef]
- McCusker, J.; Lambert, S.D.; Cole, M.G.; Ciampi, A.; Strumpf, E.; Freeman, E.E.; Belzile, E. Activation and self-efficacy in a randomized trial of a depression self-care intervention. Health Educ. Behav. 2016, 43, 716–725. [Google Scholar] [CrossRef]
- Goodwin, A.; Henschen, B.L.; O’Dwyer, L.C.; Nichols, N.; O’Leary, K.J. Interventions for frequently hospitalized patients and their effect on outcomes: A systematic review. J. Hosp. Med. 2018, 13, 853–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, A.G.; van der Pols, J.C.; Dobson, A.J. Regression to the mean: What it is and how to deal with it. Int. J. Epidemiol. 2005, 34, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Wright, P.J.; Pinto, B.M.; Corbett, C.F. Balancing internal and external validity using Precis-2 and Re-Aim: Case exemplars. West. J. Nurs. Res. 2021, 43, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality. Management of high-need, high-cost patients: A “Best Fit” framework synthesis, realist review, and systematic review. Draft Comp. Eff. Rev. 2021. Currently unavailable because the period for public review has expired; Final report forthcoming. [Google Scholar]
- Baker, J.M.; Grant, R.W.; Gopalan, A. A systematic review of care management interventions targeting multimorbidity and high care utilization. BMC Health Serv. Res. 2018, 18, 1–9. [Google Scholar] [CrossRef] [Green Version]
| Chronic Care Management Intervention (CCMI) | Attention Control Intervention (ACI) | ||
|---|---|---|---|
| Baseline characteristics | (n = 144) | (n = 146) | |
| Age, M (SD) | 55.35 (6.94) | 55.24 (7.21) | |
| Sex | |||
| Male (%) | 53 (36.8%) | 52 (35.6%) | |
| Female (%) | 91 (63.2%) | 94 (64.4%) | |
| Not Latino | 142 (98.6%) | 143 (97.9%) | |
| Race | |||
| Caucasian (%) | 124 (86.1%) | 122 (83.6%) | |
| Black (%) | 7 (4.9%) | 10 (6.8%) | |
| American Indian (%) | 3 (2.1%) | ||
| Asian (%) | 1 (.7%) | ||
| Other (%) | 12 (8.3%) | 11 (7.5%) | |
| Marital Status | |||
| Single | 23 (17.3%) | 27 (20.5%) | |
| Committed/Married | 46 (34.6%) | 39 (29.5%) | |
| Divorced/Separated | 55 (41.4%) | 53 (40.2%) | |
| Single after death of spouse | 9 (6.8%) | 13 (9.8%) | |
| Insurance | |||
| Medicare | 32 (22.5%) | 25 (17.4%) | |
| Medicaid | 93 (65.5%) | 84 (58.3%) | |
| Dual Medicare/Medicaid | 10 (7.0%) | 21 (14.6%) | |
| Other public | 1 (0.7%) | 1 (0.7%) | |
| Commercial/HMO | 5 (3.5%) | 8 (5.6%) | |
| Self-pay | 1 (0.7%) | 5 (3.5%) | |
| Education | |||
| Some high school | 15 (11.5%) | 16 (12.2%) | |
| High school | 34 (26.2%) | 44 (33.6%) | |
| Some college/technical | 55 (42.3%) | 43 (32.8%) | |
| 2-year college graduate | 13 (10.0%) | 16 (12.2%) | |
| 4-year college graduate | 10 (7.7%) | 10 (7.6%) | |
| Some post bac or greater | 3 (2.3%) | 2 (1.6%) | |
| Health literacy M (SD) | 7.0 (2.1) | 7.1 (1.9) | |
| Patient Activation Measure Score M (SD) | 55.9 (13.5) | 56.6 (11.6) | |
| Health Status Rating: n (%) at risk | 138 (95.8%) | 139 (95.2%) | |
| Patient Health Questionnaire (PHQ-9) | 11.5 (6.5) | 9.31 (5.1) | |
| Measure | Group | Baseline | 3 Months | 6 Months | 9 Months | 12 Months |
|---|---|---|---|---|---|---|
| PAM-13 | CCMI | 55.9 (13.5) | 58.2 (14.7) | 60.6 (14.5) | 58.4 (13.0) | 61.7 (14.6) |
| ACTI | 56.6 (11.6) | 59.0 (12.6) | 58.4 (11.7) | 59.2 (12.3) | 60.5 (12.7) | |
| Self-Rated Health | CCMI | 1.9 (0.8) | 2.3 (0.9) | 2.2 (0.9) | 2.2 (0.8) | 2.4 (0.9) |
| ACTI | 2.0 (0.9) | 2.2 (0.8) | 2.2 (0.9) | 2.3 (0.8) | 2.2 (0.9) | |
| Depressive Symptoms | CCMI | 11.5 (6.5) | 8.4 (5.5) | |||
| ACTI | 9.3 (5.0) | 8.0 (5.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbett, C.F.; Daratha, K.B.; McPherson, S.; Smith, C.L.; Wiser, M.S.; Vogrig, B.K.; Murphy, S.M.; Cantu, R.; Dyck, D.G. Patient Activation, Depressive Symptoms, and Self-Rated Health: Care Management Intervention Effects among High-Need, Medically Complex Adults. Int. J. Environ. Res. Public Health 2021, 18, 5690. https://doi.org/10.3390/ijerph18115690
Corbett CF, Daratha KB, McPherson S, Smith CL, Wiser MS, Vogrig BK, Murphy SM, Cantu R, Dyck DG. Patient Activation, Depressive Symptoms, and Self-Rated Health: Care Management Intervention Effects among High-Need, Medically Complex Adults. International Journal of Environmental Research and Public Health. 2021; 18(11):5690. https://doi.org/10.3390/ijerph18115690
Chicago/Turabian StyleCorbett, Cynthia F., Kenn B. Daratha, Sterling McPherson, Crystal L. Smith, Michael S. Wiser, Brenda K. Vogrig, Sean M. Murphy, Roy Cantu, and Dennis G. Dyck. 2021. "Patient Activation, Depressive Symptoms, and Self-Rated Health: Care Management Intervention Effects among High-Need, Medically Complex Adults" International Journal of Environmental Research and Public Health 18, no. 11: 5690. https://doi.org/10.3390/ijerph18115690






