Event-Based Prospective Memory Deficit in Children with ADHD: Underlying Cognitive Factors and Association with Symptoms
Abstract
:1. Introduction
1.1. Prospective Memory in ADHD
1.2. Cognitive Factors Underlying Prospective Memory Performance
1.3. The Current Study
- (a)
- Children and adolescents with ADHD would show reduced performance compared to controls in several event-based PM tasks, expressed as reduced accuracy and increased response times for both the ongoing and the PM conditions. We also predicted that their performance would be modulated by the cognitive factors manipulated in the PM tasks.
- (b)
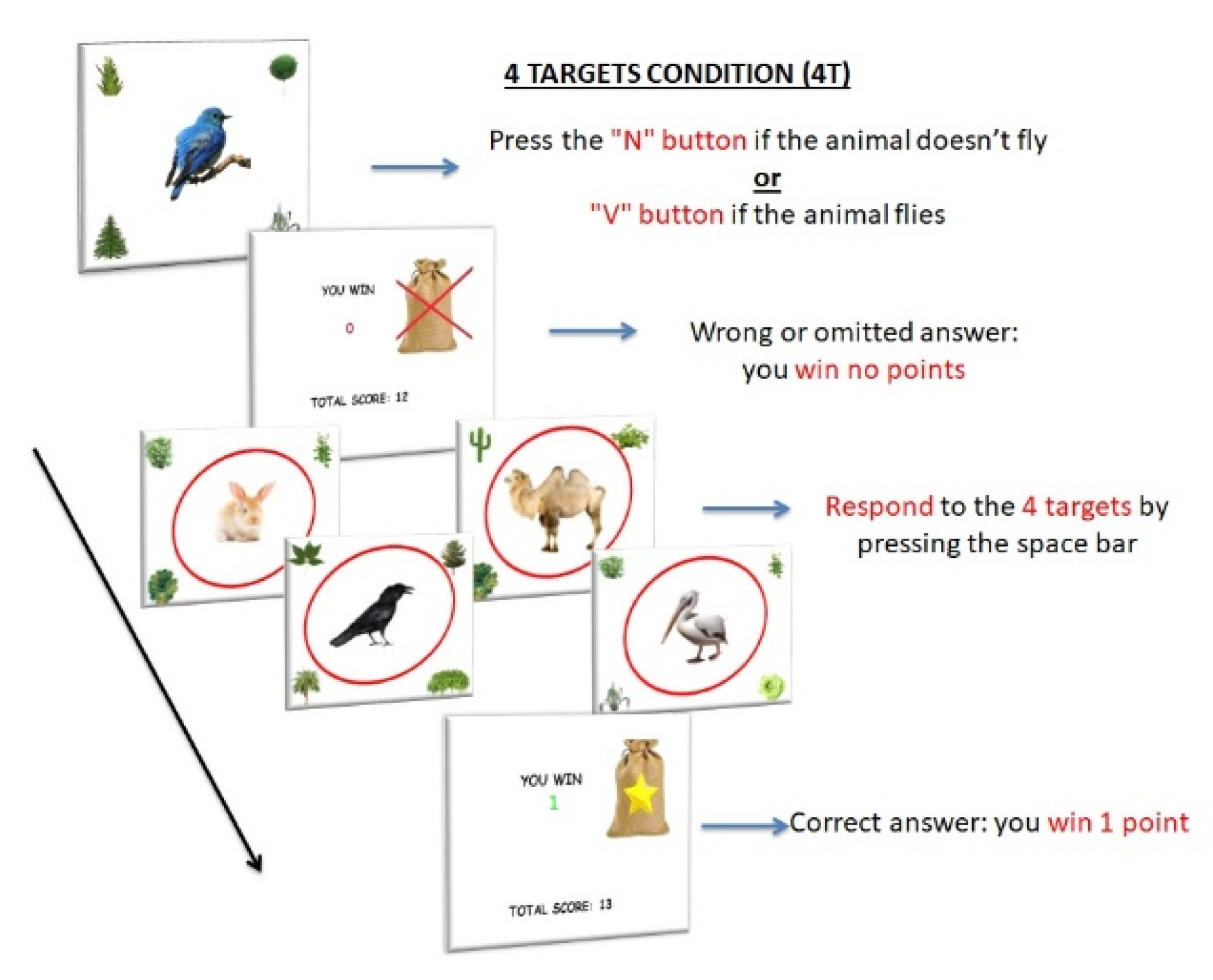
- The increase of WM load (number of PM targets) would specifically affect the performance of children with ADHD by reducing the response accuracy and increasing the response times for both the ongoing and the PM task compared to the other PM conditions.
- (c)
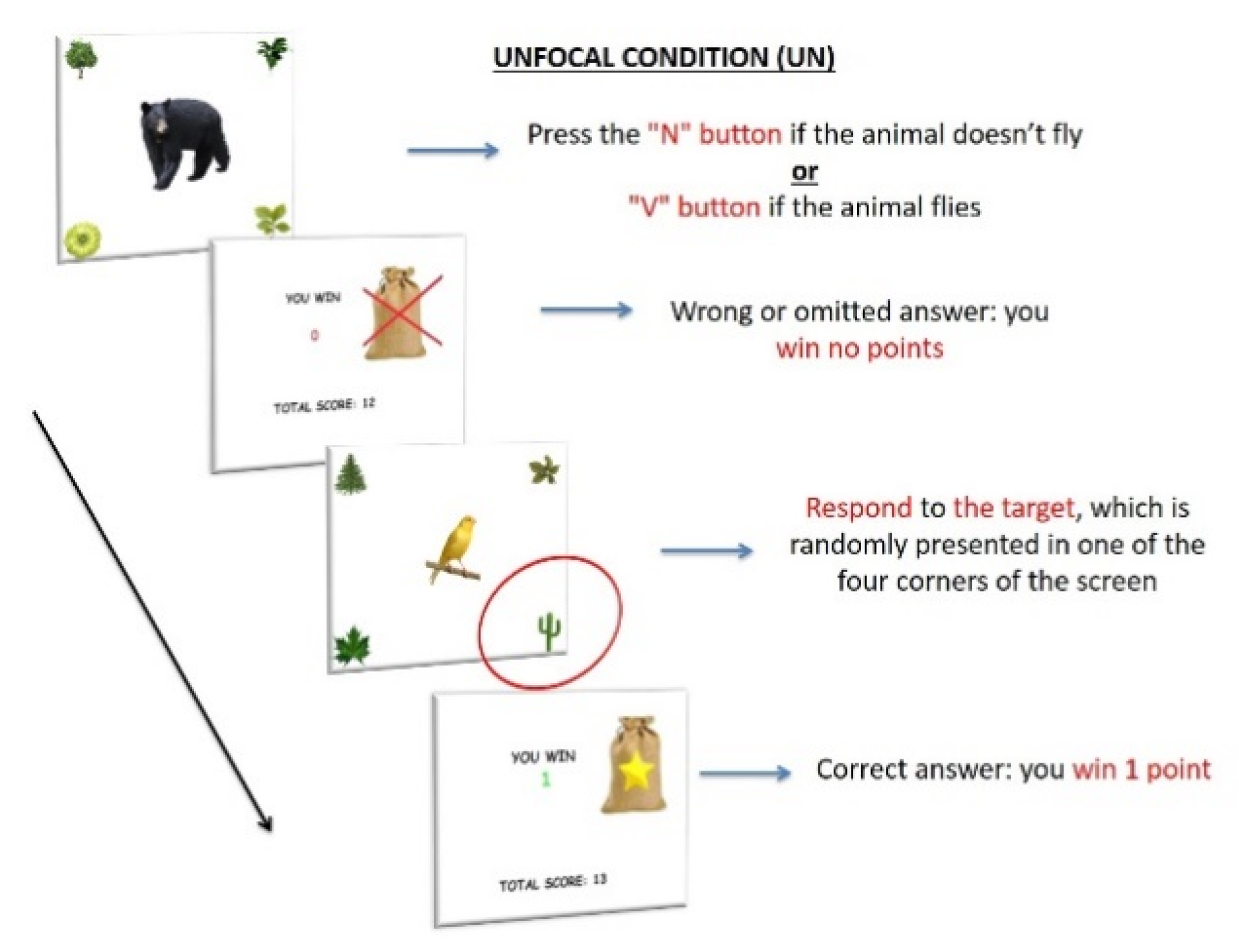
- The occurrence of the PM targets outside the central focus of attention would affect the performance of children with ADHD by reducing the response accuracy and increasing the response times for both the ongoing and the PM task compared to the other PM conditions.
- (d)
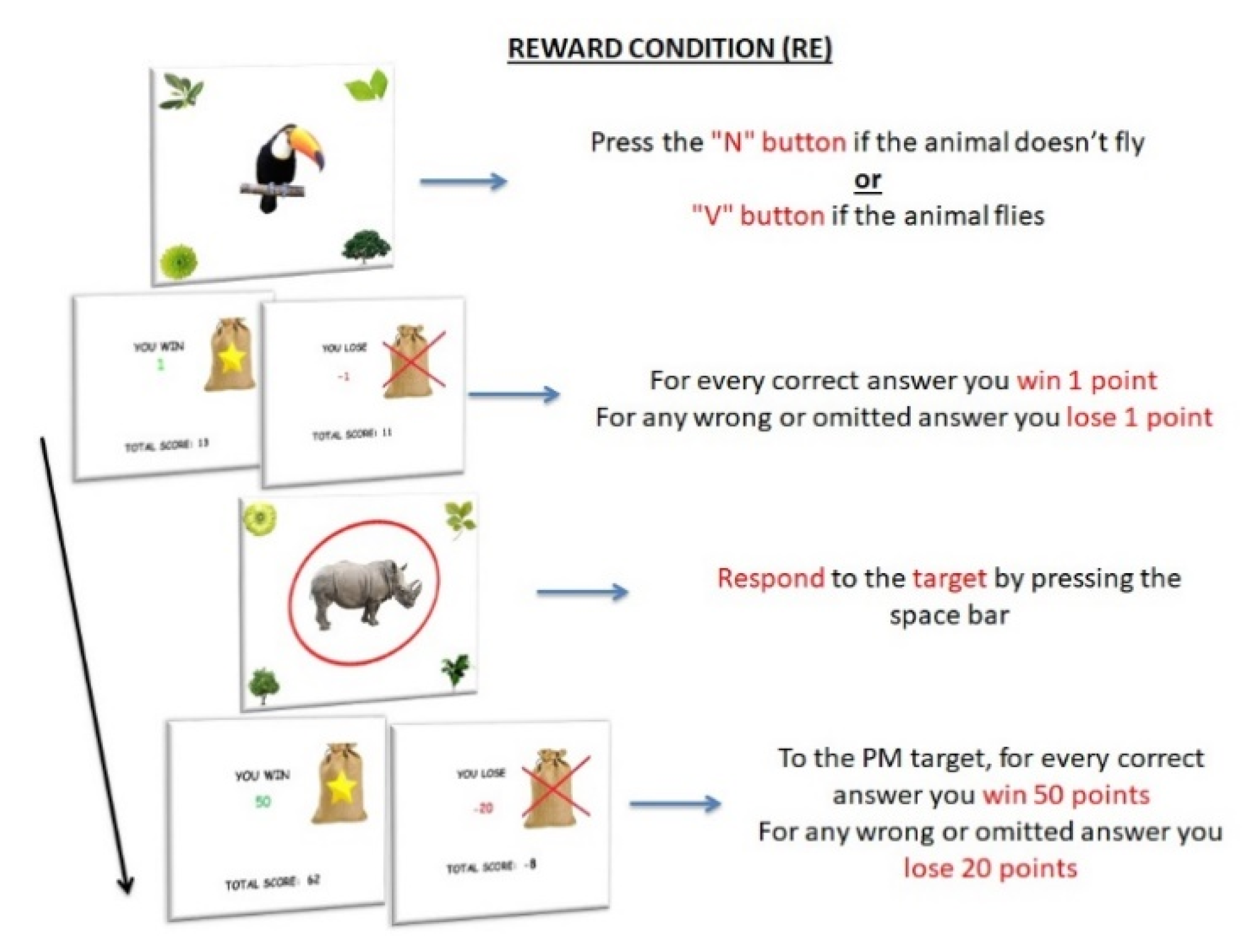
- The increase in reward amount would positively influence the PM performance of children with ADHD in comparison with the other PM conditions.
- (e)
- A negative relationship would emerge between PM performance and the ADHD symptoms.
2. Materials and Methods
2.1. Participants
2.2. Study Design and Procedure
2.2.1. Assessment
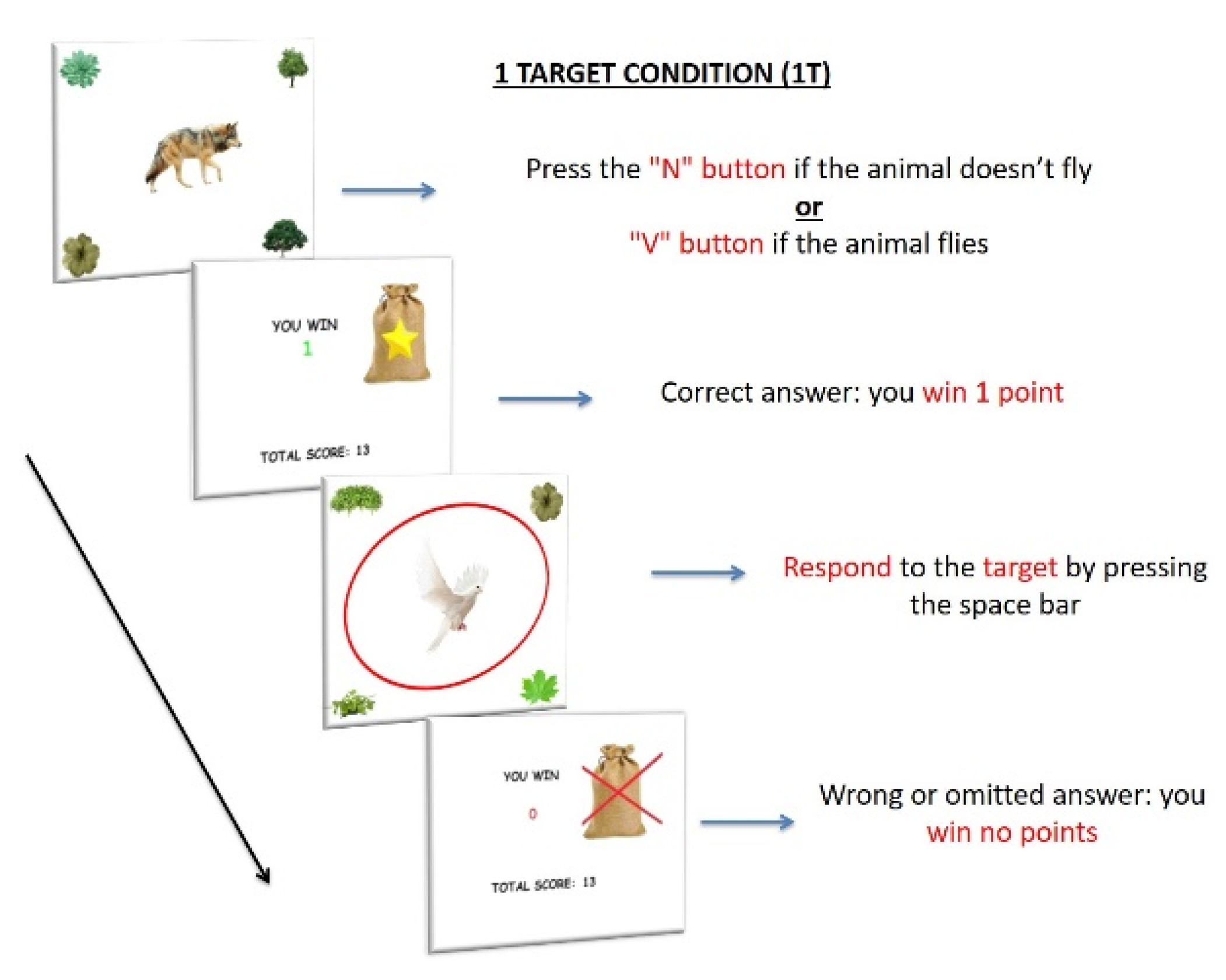
2.2.2. Experimental Paradigm
Stimuli and Procedure
Baseline Condition
PM Conditions
2.3. Statistical Analyses
3. Results
3.1. PM Procedure
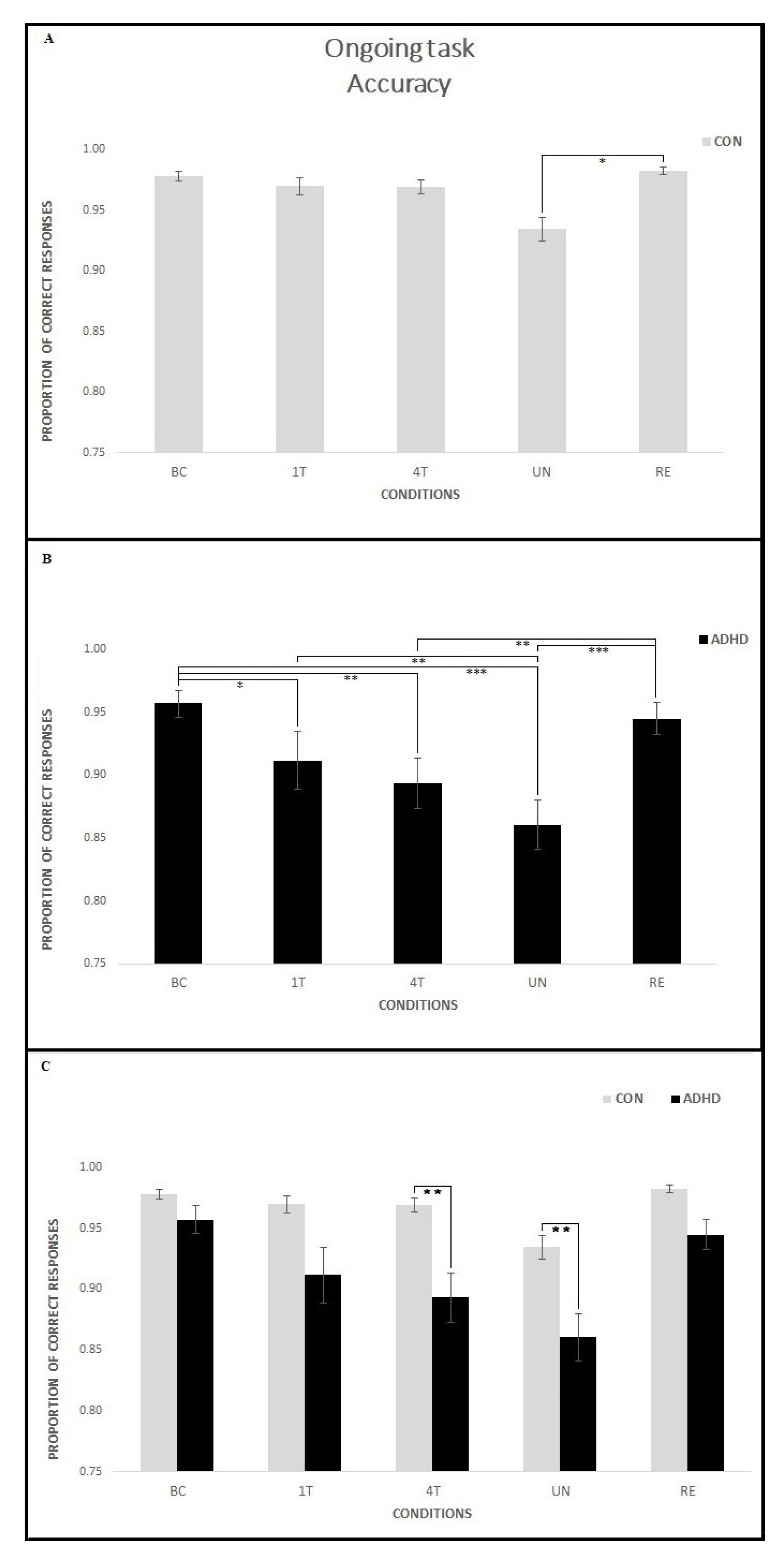
3.1.1. Ongoing Task—Accuracy
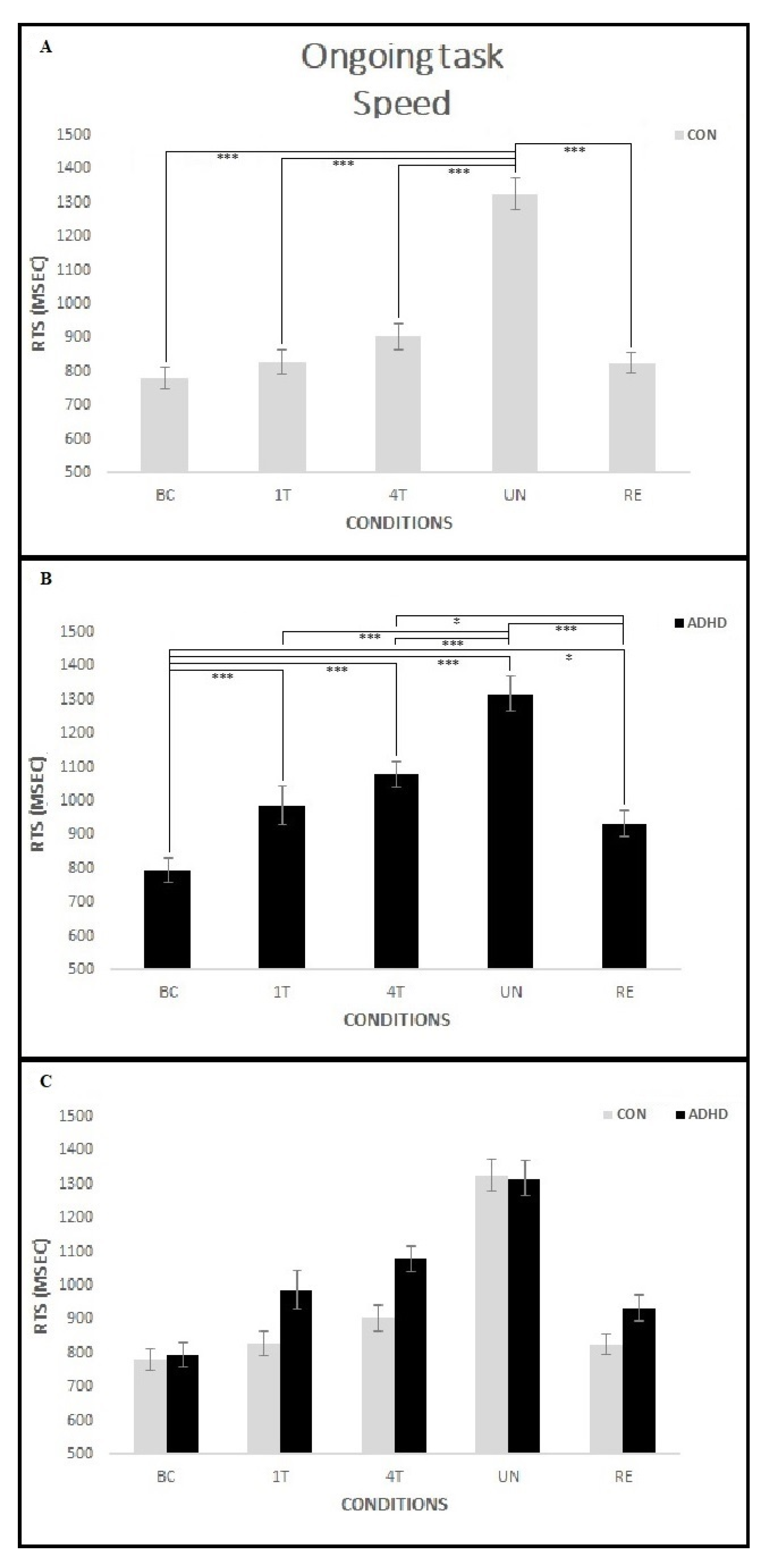
3.1.2. Ongoing Task—Speed
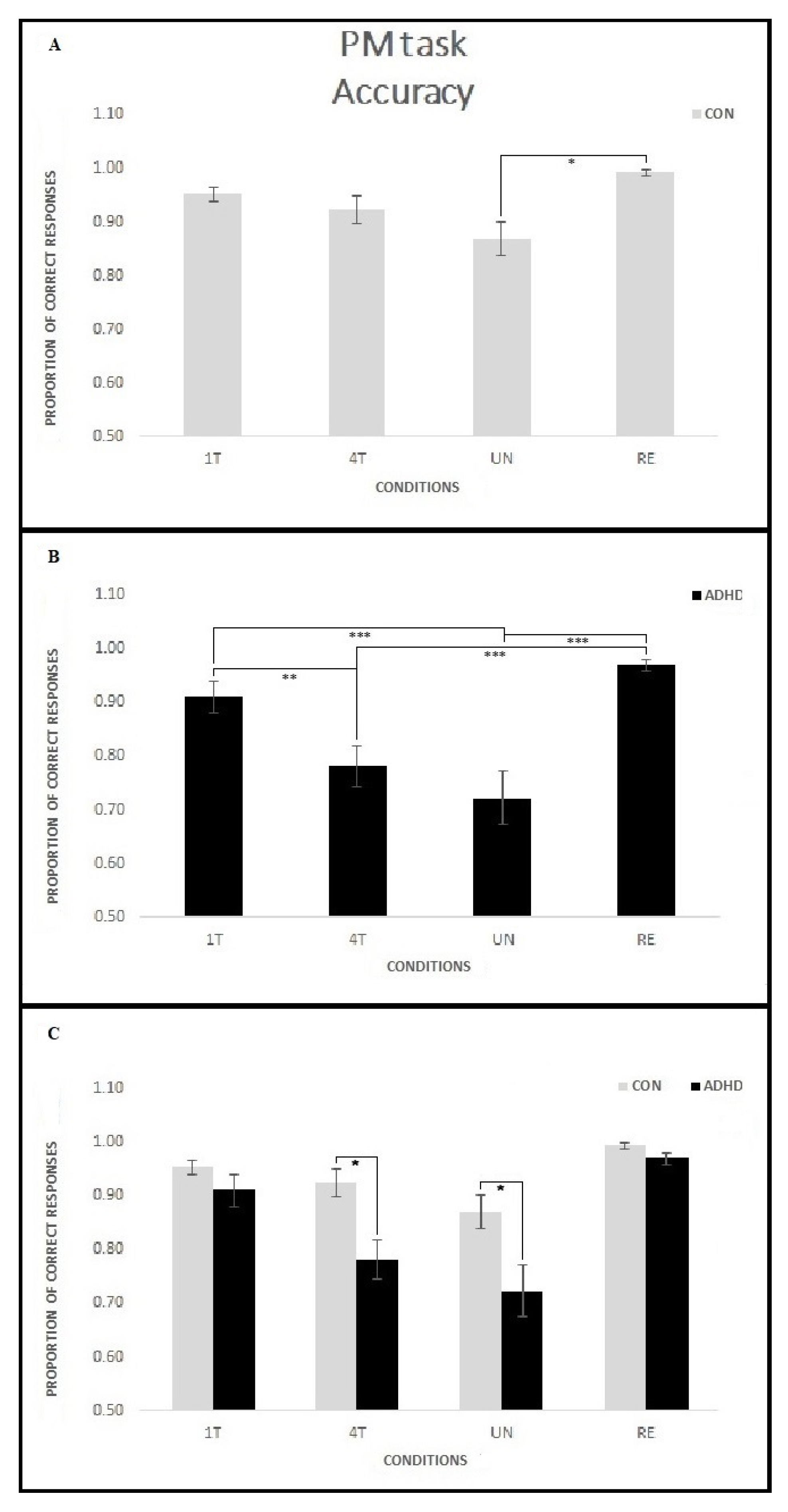
3.1.3. PM Task—Accuracy
3.1.4. PM Task—Speed
3.2. Correlation Analysis
4. Discussion
4.1. Event-Based PM Tasks Interfere with Ongoing Task Execution in Children with ADHD
4.2. Manipulation of the Reward Positively Influences Ongoing Task Performance in ADHD Children
4.3. PM Performance Is Related to ADHD Symptoms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Polanczyk, G.; Rohde, L.A. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr. Opin. Psychiatry 2007, 20, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Simon, V.; Czobor, P.; Bálint, S.; Mészáros, A.; Bitter, I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: Meta-analysis. Br. J. Psychiatry 2009, 194, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Barnett, R. Attention deficit hyperactivity disorder. Lancet 2016, 387, 737. [Google Scholar] [CrossRef] [Green Version]
- Cottini, M.; Basso, D.; Saracini, C.; Palladino, P. Performance predictions and postdictions in prospective memory of school-aged children. J. Exp. Child Psychol. 2019, 179, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Brandimonte, M.A.; Einstein, G.O.; McDaniel, M.A. Prospective Memory: Theory and Applications; Lawrence Erlbaum: Mahwah, NJ, USA, 1996. [Google Scholar]
- Einstein, G.O.; McDaniel, M.A. Retrieval processes in prospective memory: Theoretical approaches and some new findings. In Prospective Memory: Theory and Applications; Brandimonte, M.A., Einstein, G.O., McDaniel, M.A., Eds.; Lawrence Erlbaum: Mahwah, NJ, USA, 1996; pp. 115–142. [Google Scholar]
- Kidder, D.P.; Park, D.C.; Hertzog, C.; Morrell, R.W. Prospective memory and aging: The effects of working memory and prospective memory task load. Aging Neuropsychol. Cogn. 1997, 4, 93–112. [Google Scholar] [CrossRef]
- Hicks, J.L.; Marsh, R.L.; Cook, G.I. Task interference in time-based, event based, and dual intention prospective memory conditions. J. Mem. Lang. 2005, 53, 430–444. [Google Scholar] [CrossRef]
- Marsh, R.L.; Hicks, J.L.; Cook, G.I. Task interference from prospective memories covaries with contextual associations of fulfilling them. Mem. Cognit. 2006, 34, 1037–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guynn, M.J. A two-process model of strategic monitoring in event-based prospective memory: Activation/retrieval mode and checking. Int. J. Psychol. 2003, 38, 245–256. [Google Scholar] [CrossRef]
- Marsh, R.L.; Hicks, J.L.; Cook, G.I.; Hansen, J.S.; Pallos, A.L. Interference to ongoing activities covaries with the characteristics of an event-based intention. J. Exp. Psychol. Learn. Mem. Cogn. 2003, 29, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Burgess, P.W.; Gonen-Yaacovi, G.; Volle, E. Functional neuroimaging studies of prospective memory: What have we learnt so far? Neuropsychologia 2011, 49, 2246–2257. [Google Scholar] [CrossRef]
- Burgess, P.W.; Quayle, A.; Frith, C.D. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia 2001, 39, 545–555. [Google Scholar] [CrossRef]
- Hashimoto, T.; Umeda, S.; Kojima, S. Neural substrates of implicit cueing effect on prospective memory. NeuroImage 2011, 54, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Halahalli, H.N.; John, J.P.; Lukose, A.; Jain, S.; Kutty, B.M. Endogenous-cue prospective memory involving incremental updating of working memory: An fMRI study. Brain Struct. Funct. 2014. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.M.; Ruge, H.; Walser, M.; Goschke, T. The functional neuroanatomy of spontaneous retrieval and strategic monitoring of delayed intentions. Neuropsychologia 2014, 52, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Cona, G.; Scarpazza, C.; Sartori, G.; Moscovitch, M.; Bisiacchi, P.S. Neural bases of prospective memory: A meta-analysis and the “Attention to Delayed Intention” (AtoDI) model. Neurosci. Biobehav. Rev. 2015, 52, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.J. Decoding the content of delayed intentions. J. Neurosc. 2011, 31, 2888–2894. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, M.A.; Lamontagne, P.; Beck, S.M.; Scullin, M.K.; Braver, T.S. Dissociable neural routes to successful prospective memory. Psychol. Sci. 2013, 24, 1791–1800. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.; Prior, M.; Kinsella, G.J. Do executive function deficits differentiate between adolescents with ADHD and oppositional defiant/conduct disorder? A neuropsychological study using the Six Elements Test and Hayling Sentence Completion Test. J. Abnorm. Child Psychol. 2000, 28, 403–414. [Google Scholar] [CrossRef]
- Kerns, K.A.; Price, K.J. An investigation of prospective memory in children with ADHD. Child Neuropsychol. 2001, 7, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kliegel, M.; Ropeter, A.; Mackinlay, R. Complex prospective memory in children with ADHD. Child Neuropsychol. 2006, 12, 407–419. [Google Scholar] [CrossRef]
- Talbot, K.D.; Kerns, K.A. Event- and time-triggered remembering: The impact of attention deficit hyperactivity disorder on prospective memory performance in children. J. Exp. Child Psychol. 2014, 127, 126–143. [Google Scholar] [CrossRef]
- Zinke, K.; Altgassen, M.; Mackinlay, R.J.; Rizzo, P.; Drechsler, R.; Kliegel, M. Time-based prospective memory performance and time-monitoring in children with ADHD. Child Neuropsychol. 2010, 16, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Altgassen, M.; Scheres, A.; Edel, M.A. Prospective memory (partially) mediates the link between ADHD symptoms and procrastination. Atten. Defic. Hyperact. Disord. 2019, 11, 59–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuermaier, A.B.M.; Tucha, L.; Koerts, J.; Aschenbrenner, S.; Westermann, C.; Weisbrod, M.; Lange, K.W.; Tucha, O. Complex Prospective Memory in Adults with Attention Deficit Hyperactivity Disorder. PLoS ONE 2013, 8, e58338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altgassen, M.; Koch, A.; Kliegel, M. Do Inhibitory Control Demands Affect Event-Based Prospective Memory Performance in ADHD? J. Atten. Disord. 2019, 23, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Talbot, K.S.; Müller, U.; Kerns, K.A. Prospective memory in children with attention deficit hyperactivity disorder: A review. Clin. Neuropsychol. 2018, 32, 783–815. [Google Scholar] [CrossRef] [PubMed]
- Mahy, C.E.; Moses, L.J.; Kliegel, M. The development of prospective memory in children: An executive framework. Dev. Rev. 2014, 34, 305–326. [Google Scholar] [CrossRef]
- Smith, R.E. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. J. Exp. Psychol. Learn. Mem. Cogn. 2003, 29, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Voigt, B.; Mahy, C.E.; Ellis, J.; Schnitzspahn, K.; Krause, I.; Altgassen, M.; Kliegel, M. The development of time-based prospective memory in childhood: The role of working memory updating. Dev. Psychol. 2014, 50, 2393–2404. [Google Scholar] [CrossRef] [Green Version]
- Mahy, C.E.; Voigt, B.; Ballhausen, N.; Schnitzspahn, K.; Ellis, J.; Kliegel, M. The impact of cognitive control on children’s goal monitoring in time-based prospective memory task. Child Neuropsyc. 2015, 21, 823–839. [Google Scholar] [CrossRef] [Green Version]
- Einstein, G.O.; Holland, L.J.; McDaniel, M.A.; Guynn, M.J. Age-related deficits in prospective memory: The influence of task complexity. Psychol. Aging 1992, 7, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Cheie, L.; MacLeod, C.; Miclea, M.; Visu-Petra, L. When children forget to remember: Effects of reduced working memory availability on prospective memory performance. Mem. Cognit. 2017, 45, 651–663. [Google Scholar] [CrossRef]
- Fronda, G.; Monti, C.; Sozzi, M.; Corbo, M.; Balconi, M. Prospective memory and working memory in comparison. New experimental paradigms. Int. J. Neurosci. 2020, 130, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Mioni, G.; Santon, S.; Stablum, F.; Cornoldi, C. Time-based prospective memory difficulties in children with ADHD and the role of time perception and working memory. Child Neuropsychol. 2017, 23, 588–608. [Google Scholar] [CrossRef]
- Hicks, J.L.; Cook, G.I.; Marsh, R.L. Detecting event-based prospective memory cues occurring within and outside the focus of attention. Am. J. Psychol. 2005, 118, 1–11. [Google Scholar] [PubMed]
- Smith, Z.R.; Langberg, J.M. Review of the Evidence for Motivation Deficits in Youth with ADHD and Their Association with Functional Outcomes. Clin. Child Fam. Psychol. Rev. 2018, 21, 500–526. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.J.; Taylor, E.; Heptinstall, E. Hyperactivity and delay aversion--II. The effect of self versus externally imposed stimulus presentation periods on memory. J. Child Psychol. Psychiatry 1992, 33, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.J.; Sergeant, J.A.; Nigg, J.; Willcutt, E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: Nosologic and diagnostic implications. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Hauser, J.; Lange, K.M.; Makulska-Gertruda, E.; Takano, T.; Takeuchi, Y.; Tucha, L.; Tucha, O. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten. Defic. Hyperact. Disord. 2014, 6, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A. Attention-Deficit Hyperactive Disorder: A handbook for Diagnosis and Treatment, 3rd ed.; Guilford: New York, NY, USA, 2006. [Google Scholar]
- Cheung, C.H.; Rijdijk, F.; McLoughlin, G.; Faraone, S.V.; Asherson, P.; Kuntsi, J. Childhood predictors of adolescent and young adult outcome in ADHD. J. Psychiatr. Res. 2015, 62, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliegel, M.; Jäger, T. The effect of age and cue-action reminders on event-based prospective memory performance in preschoolers. Cogn. Dev. 2007, 22, 33–46. [Google Scholar] [CrossRef]
- Yang, T.X.; Chan, R.C.; Shum, D. The development of prospective memory in typically developing children. Neuropsychology 2011, 25, 342–352. [Google Scholar] [CrossRef]
- Spiess, M.A.; Meier, B.; Roebers, C. Development and longitudinal relationships between children’s executive functions, prospective memory, and metacognition. Cogn. Dev. 2016, 38, 99–113. [Google Scholar] [CrossRef]
- Kaufman, J.; Schweder, A.E. The Schedule for Affective Disorders and Schizophrenia for School-age Children: Present and Lifetime Version (K-SADS-PL). In Comprehensive Handbook of Psychological Assessment, Personality Assessment; Hersen, M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Conners, C.K. Conners’ Rating Scales—Revised: Technical Manual; MHS: New York, NY, USA, 2000. [Google Scholar]
- Raven, J. The Raven’s Progressive Matrices: Change and Stability over Culture and Time. Cogn. Psychol. 2000, 41, 1–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision; American Psychiatric Association: Washington, DC, USA, 2000; pp. 771–774. [Google Scholar]
- Kliegel, M.; Mahy, C.E.; Voigt, B.; Henry, J.D.; Rendell, P.G.; Aberle, I. The development of prospective memory in young schoolchildren: The impact of ongoing task absorption, cue salience, and cue centrality. J. Exp. Child Psychol. 2013, 116, 792–810. [Google Scholar] [CrossRef] [PubMed]
- Brandimonte, M.A.; Filippello, P.; Coluccia, E.; Altgassen, M.; Kliegel, M. To do or not to do? Prospective memory versus response inhibition in autism spectrum disorder and attention-deficit/hyperactivity disorder. Memory 2011, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Dörrenbächer, S.; Kray, J. Impairments in resource allocation and executive control in children with ADHD. Clin. Child Psychol. Psychiatry 2019, 24, 462–481. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.J.; Castellanos, F.X. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007, 31, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.X.; Margulies, D.S.; Kelly, C.; Uddin, L.Q.; Ghaffari, M.; Kirsch, A.; Shaw, D.; Shehzad, Z.; Di Martino, A.; Biswal, B.; et al. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psych. 2008, 63, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Fassbender, C.; Schweitzer, J.B. there evidence for neural compensation in attention deficit hyperactivity disorder? A review of the functional neuroimaging literature. Clin. Psychol. Rev. 2006, 26, 445–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killeen, P.R.; Russell, V.A.; Sergeant, J.A. A behavioral neuroenergetics theory of ADHD. Neurosci. Biobehav. Rev. 2013, 37, 625–657. [Google Scholar] [CrossRef]
- Dolan, R.J. Emotion, cognition, and behaviour. Science 2002, 298, 1191–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kensinger, E.A.; Corkin, S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proc. Natl. Acad. Sci. USA 2004, 101, 3310–3315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luman, M.; Oosterlaan, J.; Sergeant, J.A. The impact of reinforcement contingencies on AD/HD: A review and theoretical appraisal. Clin. Psychol. Rev. 2005, 25, 183–213, Erratum in 2005, 25, 533. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, E.; Bado, P.; Tripp, G.; Mattos, P.; Wickens, J.R.; Bramati, I.E.; Alsop, B.; Meireles Ferreira, F.; Lima, D.; Tovar-Moll, F.; et al. Abnormal striatal BOLD responses to reward anticipation and reward delivery in ADHD. PLoS ONE 2014, 26, e89129. [Google Scholar] [CrossRef]
- Scheres, A.; Milham, M.P.; Knutson, B.; Castellanos, F.X. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol. Psychiatry 2007, 61, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Plichta, M.M.; Scheres, A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: A meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 2014, 38, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Nigg, J.T.; Casey, B.J. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev. Psychopathol. 2005, 17, 785–806. [Google Scholar] [CrossRef]
- Slusarek, M.; Velling, S.; Bunk, D.; Eggers, C. Motivational effects on inhibitory control in children with ADHD. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 355–363. [Google Scholar] [CrossRef]
- McInerney, R.J.; Kerns, K.A. Time reproduction in children with ADHD: Motivation matters. Child Neuropsychol. 2003, 9, 91–108. [Google Scholar] [CrossRef]
- Konrad, K.; Gauggel, S.; Manz, A.; Schöll, M. Lack of inhibition: A motivational deficit in children with attention deficit/hyperactivity disorder and children with traumatic brain injury. Child Neuropsychol. 2000, 6, 286–296. [Google Scholar] [CrossRef]
- Cortese, S.; Kelly, C.; Chabernaud, C.; Proal, E.; Di Martino, A.; Milham, M.P.; Castellanos, F.X. Toward systems neuroscience of ADHD: A meta-analysis of 55 fMRI studies. Am. J. Psychiatry 2012, 169, 1038–1055. [Google Scholar] [CrossRef]
- Faraone, S.V.; Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef] [Green Version]
- Kemper, A.R.; Maslow, G.R.; Hill, S.; Namdari, B.; Allen LaPointe, N.M.; Goode, A.P.; Coeytaux, R.R.; Befus, D.; Kosinski, A.S.; Bowen, S.E.; et al. Attention Deficit Hyperactivity Disorder: Diagnosis and Treatment in Children and Adolescents; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018. [Google Scholar]
- Fried, R.; DiSalvo, M.; Kelberman, C.; Biederman, J. Can the CANTAB identify adults with attention-deficit/hyperactivity disorder? A controlled study. Appl. Neuropsychol. Adult 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brom, S.S.; Kliegel, M. Improving everyday prospective memory performance in older adults: Comparing cognitive process and strategy training. Psychol. Aging 2014, 29, 744–755. [Google Scholar] [CrossRef]
- Hering, A.; Rendell, P.G.; Rose, N.S.; Schnitzspahn, K.M.; Kliegel, M. Prospective memory training in older adults and its relevance for successful aging. Psychol. Res. 2014, 78, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Mahan, S.; Rous, R.; Adlam, A. Systematic Review of Neuropsychological Rehabilitation for Prospective Memory Deficits as a Consequence of Acquired Brain Injury. J. Int. Neuropsychol. Soc. 2017, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McCauley, S.R.; Pedroza, C.; Chapman, S.B.; Cook, L.G.; Vásquez, A.C.; Levin, H.S. Monetary incentive effects on event-based prospective memory three months after traumatic brain injury in children. J. Clin. Exp. Neuropsychol. 2011, 33, 639–646. [Google Scholar] [CrossRef] [Green Version]
- McLean, A.; Dowson, J.; Toone, B.; Young, S.; Bazanis, E.; Robbins, T.W.; Sahakian, B.J. Characteristic neurocognitive profile associated with adult attention-deficit/hyperactivity disorder. Psychol. Med. 2004, 34, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Bahçivan Saydam, R.; Ayvaşik, H.B.; Alyanak, B. Executive Functioning in Subtypes of Attention Deficit Hyperactivity Disorder. Noro Psikiyatr. Ars. 2015, 52, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.; Fogler, J.M.; Hammerness, P.G. Treatment of Attention-Deficit/Hyperactivity Disorder in Adolescents: A Systematic Review. JAMA 2016, 315, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Seymour, K.E.; Mostofsky, S.H.; Rosch, K.S. Cognitive Load Differentially Impacts Response Control in Girls and Boys with ADHD. J. Abnorm. Child Psychol. 2016, 44, 141–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Measure | ADHD N 24; 15M/9F | CON N 23; 16M/7F | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | F(1,45) | p | |
| CA | 10.42 | (1.34) | 10.15 | (1.51) | 0.44 | 0.51 |
| IQ | 108.12 | (10.05) | 112.39 | (9.15) | 2.31 | 0.14 |
| CPRS Oppositional | 63.35 | (15.45) | 46.37 | (13.96) | 13.70 | <0.001 * |
| CPRS Cognitive probl./inattention | 75.33 | (12.72) | 43.79 | (5.75) | 100.27 | <0.0001 * |
| CPRS Hyperactivity | 66.71 | (14.40) | 45.11 | (8.36) | 33.66 | <0.0001 * |
| CPRS Anxious | 54.09 | (13.78) | 45.79 | (7.15) | 5.62 | 0.02 |
| CPRS Perfectionism | 51.52 | (11.41) | 46.69 | (10.70) | 1.98 | 0.17 |
| CPRS Social problems | 57.87 | (14.17) | 46.95 | (12.01) | 7.20 | 0.01 |
| CPRS Psychosomatic | 58.65 | (16.90) | 47.68 | (7.45) | 6.87 | 0.01 |
| CPRS ADHD index | 75.00 | (12.12) | 46.47 | (9.88) | 68.90 | <0.0001 * |
| CPRS Global index- Restless-impulsive | 67.33 | (11.75) | 45.42 | (8.91) | 45.36 | <0.0001 * |
| CPRS Global index-Emotional lability | 60.04 | (16.82) | 48.26 | (11.50) | 6.71 | 0.01 |
| CPRS Global index | 69.67 | (13.78) | 45.68 | (10.36) | 39.68 | <0.0001 * |
| CPRS DSM Inattentive | 75.04 | (14.44) | 45.68 | (8.10) | 62.69 | <0.0001 * |
| CPRS DSM Hyperactive/impulsive | 64.79 | (12.60) | 46.63 | (9.19) | 27.73 | <0.0001 * |
| CPRS DSM Total | 73.46 | (13.27) | 44.37 | (7.68) | 72.00 | <0.0001 * |
| CPRS | BC | 1T | 4T | UN | RE | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ongoing | PM | Ongoing | PM | Ongoing | PM | Ongoing | PM | |||||||||||
| Acc | Speed | Acc | Speed | Acc | Speed | Acc | Speed | Acc | Speed | Acc | Speed | Acc | Speed | Acc | Speed | Acc | Speed | |
| ADHD Index | −0.19 | 0.19 | −0.38 * | 0.38 * | −0.27 | 0.17 | −0.40 ** | 0.36 * | −0.32 * | 0.28 | −0.35 * | 0.12 | −0.32 * | 0.29 | −0.21 | 0.31 * | −0.32 * | 0.34 * |
| Global index- Restless-impulsive | −0.13 | 0.09 | −0.26 | 0.30 | −0.12 | 0.09 | −0.30 * | 0.26 | −0.17 | 0.19 | −0.24 | 0.17 | −0.30 | 0.24 | −0.19 | 0.23 | −0.22 | 0.33 * |
| Global index-Emotional lability | −0.03 | 0.18 | −0.14 | 0.14 | −0.20 | −0.03 | −0.26 | 0.11 | −0.13 | 0.04 | −0.22 | 0.25 | −0.17 | 0.18 | −0.14 | 0.11 | −0.07 | 0.23 |
| Global index | −0.13 | 0.08 | −0.24 | 0.31 * | −0.18 | 0.04 | −0.34 * | 0.33 * | −0.22 | 0.20 | −0.25 | 0.21 | −0.26 | 0.24 | −0.19 | 0.24 | −0.23 | 0.26 |
| DSM Inattentive | −0.21 | 0.22 | −0.37 * | 0.40 ** | −0.26 | 0.22 | −0.40 ** | 0.39 * | −0.32 * | 0.31 * | −0.37 * | 0.11 | −0.36 * | 0.28 | −0.23 | 0.32 * | −0.32 * | 0.36 * |
| DSM Hyperactive/impulsive | −0.18 | 0.04 | −0.37 * | 0.16 | −0.23 | 0.06 | −0.52 **^ | 0.21 | −0.32 * | 0.26 | −0.28 | 0.20 | −0.35 * | 0.30 | −0.33 * | 0.26 | −0.36 * | 0.43 ** |
| DSM Total | −0.18 | 0.08 | −0.34 * | 0.28 | −0.28 | 0.12 | −0.48 ** | 0.35 * | −0.34 * | 0.36 * | −0.40 ** | 0.13 | −0.45 ** | 0.36 * | −0.30 | 0.30 | −0.32 * | 0.42 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanzo, F.; Fucà, E.; Menghini, D.; Circelli, A.R.; Carlesimo, G.A.; Costa, A.; Vicari, S. Event-Based Prospective Memory Deficit in Children with ADHD: Underlying Cognitive Factors and Association with Symptoms. Int. J. Environ. Res. Public Health 2021, 18, 5849. https://doi.org/10.3390/ijerph18115849
Costanzo F, Fucà E, Menghini D, Circelli AR, Carlesimo GA, Costa A, Vicari S. Event-Based Prospective Memory Deficit in Children with ADHD: Underlying Cognitive Factors and Association with Symptoms. International Journal of Environmental Research and Public Health. 2021; 18(11):5849. https://doi.org/10.3390/ijerph18115849
Chicago/Turabian StyleCostanzo, Floriana, Elisa Fucà, Deny Menghini, Antonella Rita Circelli, Giovanni Augusto Carlesimo, Alberto Costa, and Stefano Vicari. 2021. "Event-Based Prospective Memory Deficit in Children with ADHD: Underlying Cognitive Factors and Association with Symptoms" International Journal of Environmental Research and Public Health 18, no. 11: 5849. https://doi.org/10.3390/ijerph18115849







