Cytogenetic, Serum Liver Enzymes and Liver Cell Pathology of the Hampala Barb Fish (Hampala macrolepidota) Affected by Toxic Elements in the Contaminated Nam Kok River near the Sepon Gold-Copper Mine, Lao PDR
Abstract
:1. Introduction
2. Materials and Methods
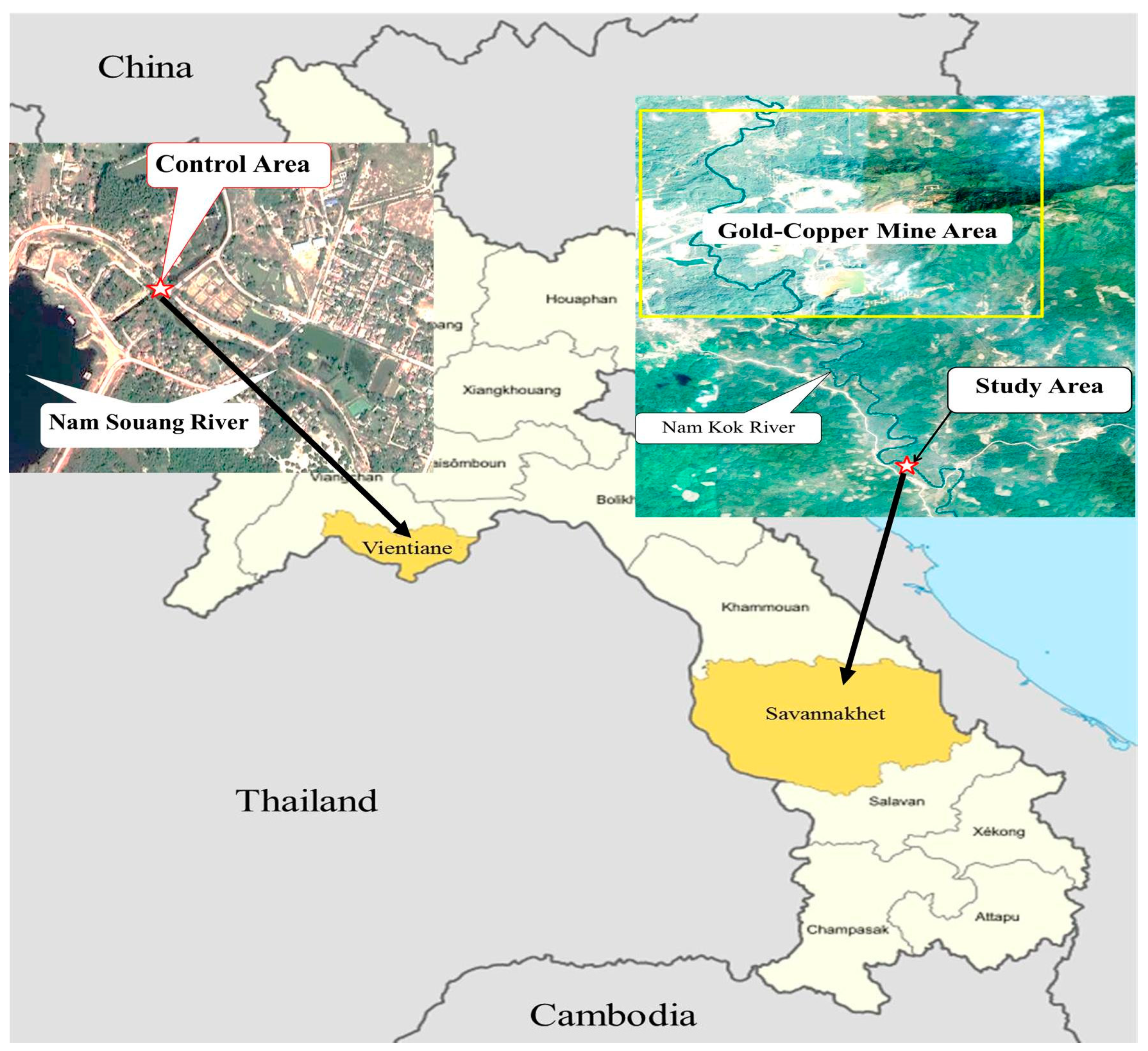
2.1. Study Area
2.2. Water Quality Parameters
2.3. Sample Collection
2.4. Measurements of the Potentially Toxic Element Concentrations
2.4.1. Water Samples
2.4.2. Sediment Samples
2.4.3. Fish Samples
2.4.4. Quality Control and Quality Assurance
2.5. Chromosome Preparation and Assessment
2.6. Serum Liver Enzymes
2.7. Liver Histopathology Study
2.8. Statistical Analysis
3. Results
3.1. Parameters of Water Quality
3.2. Potentially Toxic Element Concentrations in Water Samples
3.3. Potentially Toxic Element Concentrations in Sediment Samples
3.4. Potentially Toxic Element Concentrations in H. macrolepidota Samples
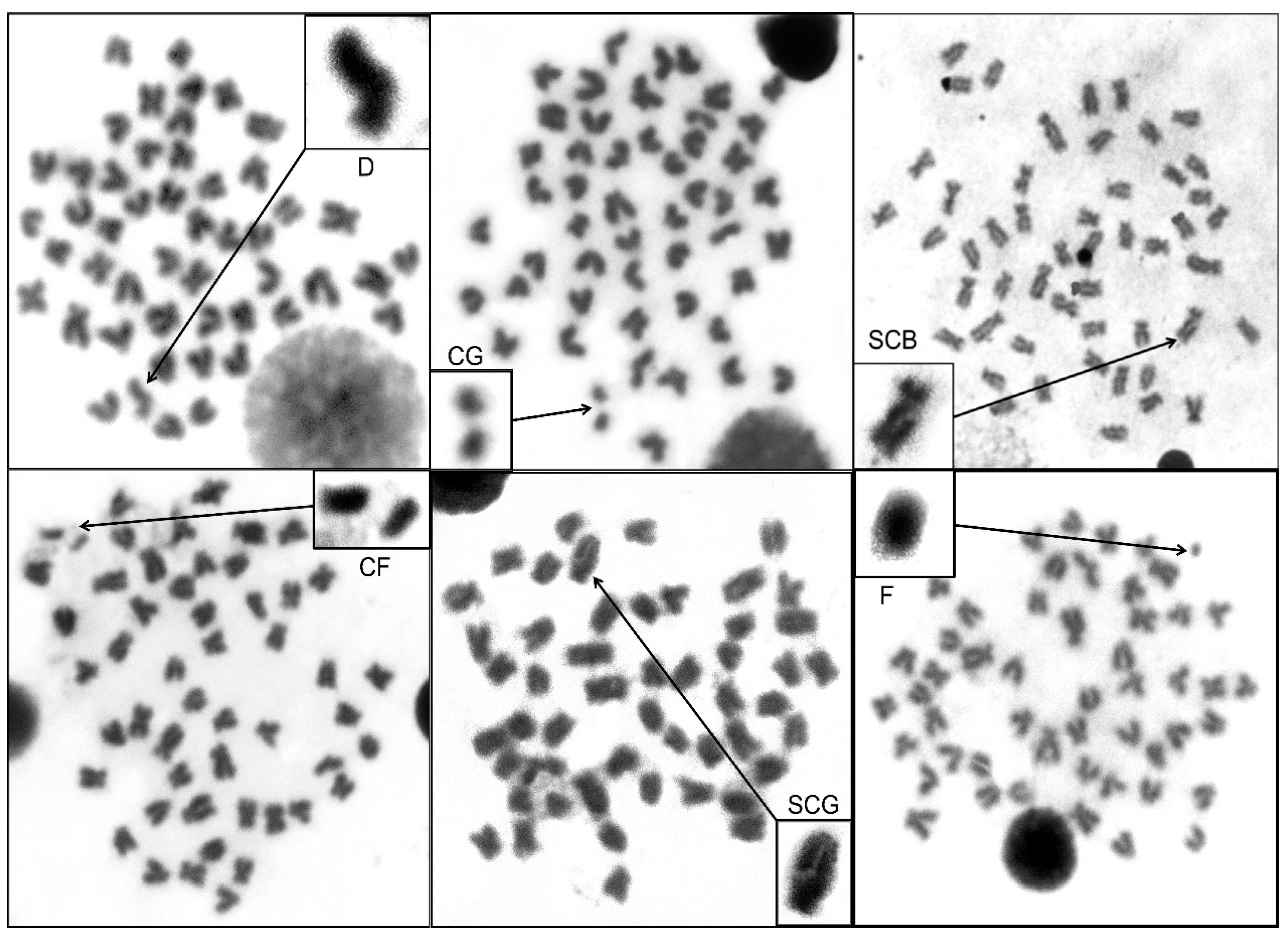
3.5. Chromosome Sssessment in H. macrolepidota
3.6. Serum Liver Enzymes
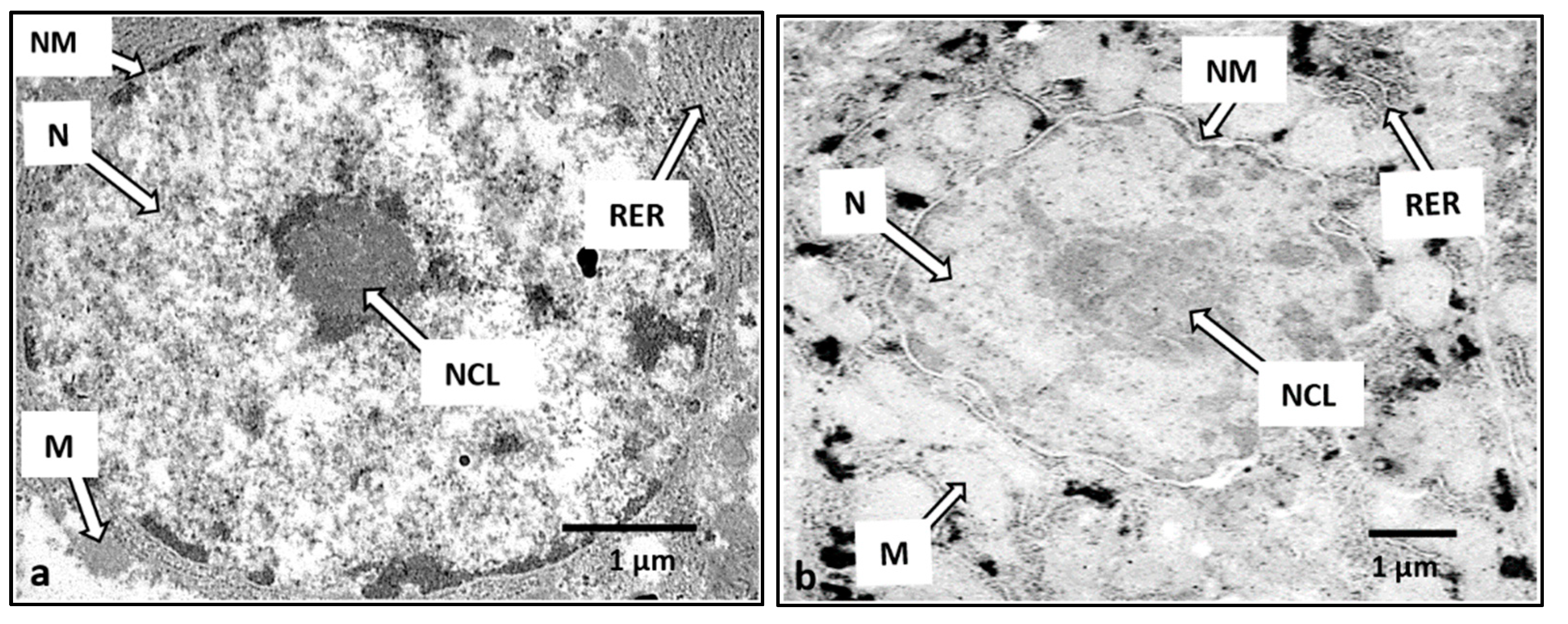
3.7. Liver Histopathology
4. Discussion
4.1. Water Quality Parameters
4.2. Potentially Toxic Element Concentrations in Water, Sediment and H. macrolepidota
4.3. Chromosome Study in H. macrolepidota
4.4. Serum Biohemistry Study in H. macrolepidota
4.5. Histopathology Study in H. macrolepidota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Bank. Lao PDR Investment Climate Assessment: Policies to Promote Growth in the Non-Resource Sectors; Report No 64800-LA; Poverty Reduction and Economic Management Sector Department, East Asia and Pacific Region, The International Bank for Reconstruction and Development/The World Bank: Vientiane, Laos, 2010. [Google Scholar]
- Ngangnouvong, I. Mining in Laos, Economic Growth, and Price Fluctuation. In Proceedings of the United Nations Conference on Trade and Development, The 11th Multi-Year Expert Meeting on Commodities and Development, Geneva, Switzerland, 15–16 April 2019. [Google Scholar]
- International Council on Mining and Metals (ICMM). Utilizing Mining and Mineral Resources to Foster the Sustainable Development of the Lao PDR; National Economic Research Institute (NERI), National University of Laos (NUOL): Vientiane, Laos, 2011. [Google Scholar]
- Houngaloune, S.; Inthavongsa, I. Trends of Gold Mining Industry in Lao PDR. In Proceedings of the 13th International Conference on Mining, Materials and Petroleum Engineering (CMMP2019), Krabi, Thailand, 13–14 June 2019. [Google Scholar]
- Phonvisay, S. An introduction to the Fisheries of Lao PDR; Mekong Development Series; Mekong River Commission: Phnom Penh, Cambodia, 2013; No. 6; ISSN 1680-4023. [Google Scholar]
- Wells-Dang, A.; Soe, K.N.; Inthakoun, L.; Tola, P.; Socheat, P.; Van, T.T.; Chabada, A.; Youttananukorn, W. A political economy of environmental impact assessment in the Mekong region. Water Altern. 2016, 9, 33–55. [Google Scholar]
- Global Green Growth Institute (GGGI). Green Growth Potential Assessment Lao PDR Country Report; Global Green Growth Institute: Seoul, Korea, 2017. [Google Scholar]
- Chandanshive, N.E. The seasonal fluctuation of physico-chemical parameters of river Mula-Mutha at Pune, India and their impact of fish biodiversity. Res. J. Anim. Vet. Fish. Sci. 2013, 1, 11–16. [Google Scholar]
- Govind, P.; Madhuri, S. Heavy metals causing toxicity in animals and fishes. Res. J. Anim. Vet. Fish. Sci. 2014, 2, 17–23. [Google Scholar]
- Danita B’ey, T. Effects of Chronic, Sublethal Ferric Iron Exposure on the Critical Swim Speed of Rainbow Trout (Oncorhynchus mykiss) and Critical Thermal Maximum of Cutthroat Trout (Oncorhynchus clarkii). Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2015. [Google Scholar]
- Shibukawa, K.; Musikasinthorn, P.; Grudpan, C.; So, N.; Tran, D.; Praxaysombath, B. Fishes of the Indochinese Mekong; Nagao Natural Environment Foundation, Member from Cambodia, Lao PDR, Thailand and Vietnam, Nha Xuat BAN Dai CAN THO: Cantho City, Vietnam, 2012. [Google Scholar]
- Shazili, N.A.M.; Yunus, K.; Ahmad, A.S.; Abdullah, N.; Rashid, M.K.A. Heavy metal pollution status in the Malaysian aquatic environment. Aquat. Ecosyst. Health Manag. 2006, 9, 137–145. [Google Scholar] [CrossRef]
- Ünlü, S.; Topçuoğlu, S.; Alpar, B.; Kırbaşoğlu, Ç.; Yılmaz, Y.Z. Heavy metal pollution in surface sediment and mussel samples in the Gulf of Gemlik. Environ. Monit. Assess. 2008, 144, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Soulivongsa, L.; Tengjaroenkul, B.; Neeratanaphan, L. Effects of contamination by heavy metals and metalloids on chromosomes, serum biochemistry and histopathology of the bonylip barb fish near Sepon gold-copper mine, Lao PDR. Int. J. Env. Res. Public Health 2020, 17, 9492. [Google Scholar] [CrossRef]
- Neeratanaphan, L.; Khamlerd, C.; Chowrong, S.; Intamat, S.; Sriuttha, M.; Tengjaroenkul, B. Cytotoxic assessment of flying barb fish (Esomus metallicus) from a gold mine area with heavy metal contamination. Int. J. Environ. Stud. 2017, 74, 613–624. [Google Scholar] [CrossRef]
- Tengjaroenkul, B.; Intamat, S.; Boonmee, S.; Neeratanaphan, L. Chromosomal aberration assessment of silver rasbora fish (Rasbora tornieri) living near gold mine area with heavy metal contamination. Hum. Ecol. Risk Assess. 2017, 23, 1140–1152. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Waste-Water; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2012. [Google Scholar]
- Chand, V.; Prasad, S. ICP-OES assessment of heavy metal contamination in tropical marine sediments: A comparative study of two digestion techniques. Microchem. J. 2013, 111, 53–61. [Google Scholar] [CrossRef]
- Rooney, D.E. Human Cytogenetics Constitutional Analysis: A Practical Approach; Oxford University Press: London, UK, 2001. [Google Scholar]
- Maneechot, N.; Supiwong, W.; Jumrusthanasan, S.; Siripiyasing, P.; Pinthong, K.; Tanomtong, A. Chromosomal characteristics of the royal knifefish, Chitala blanci (Osteoglossiformes, Notopteridae) by conventional and Ag-NOR staining techniques. Cytologia 2015, 80, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Reynders, H.; Campenhout, K.V.; Bervoets, L.; Coen, W.M.D.; Blust, R. Dynamics of cadmium accumulation and effects in common carp (Cyprinus carpio) during simultaneous exposure to water and food (Tubifex tubifex). Environ. Toxicol. Chem. 2006, 25, 1558–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giari, L.; Manera, M.; Simoni, E.; Dezfuli, B.S. Cellular alterations in different organs of European sea bass Dicentrarchus labrax (L.) exposed to cadmium. Chemosphere 2007, 67, 1171–1181. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Arsenic in Drinking-Water. In Background Document for Preparation of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Thailand Pollution Control Department (TPCD). Surface Water Quality Standard, Notification of the National Environmental Board, No. 8; Ministry of Natural Resource and Environment: Bangkok, Thailand, 1994. [Google Scholar]
- Food and Agriculture Organization (FAO). Water Quality for Agriculture; FAO Irrigation and Drainage Paper 29, Rev. 1; Food and Agriculture Organization of the United Nations: Rome, Italy, 1994. [Google Scholar]
- Food and Agriculture Organization; World Health Organization. National Research Council Recommended Dietary. In Allowances, 10th ed.; National Academy Press: Washington, DC, USA, 1989. [Google Scholar]
- United States-Environmental Protection Agency (US-EPA). Water Quality Standard for Surface Water; US-EPA: Washington, DC, USA, 2002. [Google Scholar]
- United Nations Environment Programme Global Environment Monitoring System (UNEPGEMS). Water Quality for Ecosystem and Human Health, 2nd ed.; United Nations Environment Programme Global Environment Monitoring System (GEMS)/Water Programme: Burlington, ON, Canada, 2008. [Google Scholar]
- Awashthi, S.K. Prevention of Food Adulteration Act No. 37 of 1954; Central and State Rules as Amended for 1999; Ashoka Law House: New Delhi, India, 2000. [Google Scholar]
- Thailand Pollution Control Department (TPCD). Soil Quality Standard for Residential and Agricultural Use According; Notification of the National Environment Board No. 25; Ministry of Natural Resource and Environment: Bangkok, Thailand, 2004. [Google Scholar]
- Thailand Pollution Control Department (TPCD). Criteria for Sediment Quality Standard in Surface Water Source Net. No.2; Ministry of Natural Resource and Environment: Bangkok, Thailand, 2018. [Google Scholar]
- European Union (EU). Heavy Metals in Wastes; European Commission on Environment: Copenhagen, Denmark, 2002. [Google Scholar]
- Australia and New Zealand Standards. Contaminants and Natural Toxicants, Code—Standard 1.4.1; Federal Register of Legislative Instruments F2011C00542; Standards Australia: Canberra, Australia, 2011. [Google Scholar]
- European Commission (EC). Commission Regulation (EC) No 78/2005 Amending Regulation (EC) No 466/2001 as Regards Heavy Metals; L 16/43–45; Official Journal of the European Union: Luxembourg, 2005. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:016:0043:0045:EN:PDF (accessed on 29 May 2021).
- World Health Organization (WHO). Heavy Metals Environmental Aspects; Environment Health Criteria; World Health Organization: Geneva, Switzerland, 1989. [Google Scholar]
- European Commission (EC). Commission Regulation (EC) No 466/2001 of 8 March 2001 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Comission: Luxembourg, 2001. [Google Scholar]
- Thailand Pollution Control Department (TPCD). Water Quality Standards. Notification in Ministry of Public Health, No.98; Pollution Control Department: Bangkok, Thailand, 2001. [Google Scholar]
- Boyd, C.E. Water Quality Management for Pond Fish Culture; Elsevier Scientific Publishing Co.: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Pruszynski, T. Effects of feeding on ammonium excretion and growth of the African catfish (Clarias gariepinus) fry. Czech J. Anim. Sci. 2003, 48, 106–112. [Google Scholar]
- Tram, N.D.Q.; Ngoan, L.D.; Ogle, B. Effect of Processing Pig Manure through a Biodigester as Fertilizer for Fish Ponds on Water Quality and Growth Performance of Three Fish Species. Available online: https://www.researchgate.net/publication/242358467 (accessed on 26 August 2020).
- Akinwole, A.O.; Faturoti, E.O. Biological performance of African catfish (Clarias gariepinus) cultured in recirculating system in Ibadan. Aquac. Eng. 2007, 36, 18–23. [Google Scholar] [CrossRef]
- Culioli, J.L.; Calendini, S.; Mori, C.; Orsini, A. Arsenic accumulation in a freshwater fish living in a contaminated river of Corsica, France. Ecotoxicol. Environ. Saf. 2009, 72, 1440–1445. [Google Scholar] [CrossRef]
- Nguyen; Nhi, H.Y. Utilization of Earthworms (Perionyx excavatus) as a Protein Source for Growing Fingerling Marble Baby (Oxyeleotris marmoratus) and Catfish (Pangasius hypophthalmusi). Master’s Thesis, Swedish University of Agriculture Sciences, Uppsala, Sweden, 2010. [Google Scholar]
- Intamat, S.; Phoonaploy, U.; Sriuttha, M.; Tengjaroenkul, B.; Neeratanaphan, L. Heavy metal accumulation in aquatic animals around the gold mine area of Loei province, Thailand. Hum. Ecol. Risk Assess. 2016, 22, 1418–1432. [Google Scholar] [CrossRef]
- Khamlerd, C.; Tengjaroenkul, B.; Neeratanaphan, L. Abnormal chromosome assessment of snakehead fish (Channa striata) affected by heavy metals from a reservoir near an industrial factory. Int. J. Environ. Stud. 2019, 76, 648–662. [Google Scholar] [CrossRef]
- Khammanichanh, A. Cytotoxic Assessment of Heavy Metal Concentration of Nile Tilapia (Oreochromis nioticus) from Domestic Wastewater Canal in Mung District of Maha Sarakham Province of Thailand. Master’s Thesis, Khon Kaen University, Khon Kaen, Thailand, 2016. [Google Scholar]
- Boonmee, S.; Thitiyan, T.; Tanomtong, A.; Tengjaroenkul, B.; Neeratanaphan, L. Cytotoxicity in the frog (Fejervarya limnocharis) after acute cadmium exposure in vivo. Int. J. Environ. Stud. 2018, 75, 978–989. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, N.; Liu, B.; Zhou, L.; Wang, J.; Wanga, C.; Dai, B.; Xiong, W. Metal concentrations and risk assessment in water, sediment and economic fish species with various habitat preferences and trophic guilds from Lake Caizi, Southeast China. Ecotoxicol. Environ. Saf. 2018, 157, 1–8. [Google Scholar] [CrossRef]
- Sriuttha, M.; Khammanichanh, A.; Patawang, I.; Tanomtong, A.; Tengjaroenkul, B.; Neeratanaphan, L. Cytotoxic sssessment of Nile tilapai (Oreochromis niloticus) from a domestic wastewater canal with heavy metal contamination. Cytologia 2017, 82, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Keshavarzi, B.; Hassanaghaei, M.; Moore, F.; Mehr, M.R.; Soltanian, S.; Lahijanzadeh, A.R.; Sorooshian, A. Heavy metal contamination and health risk assessment in three commercial fish species in the Persian Gulf. Mar. Pollut. Bull. 2018, 129, 245–252. [Google Scholar] [CrossRef]
- Neeratanaphan, L.; Kanjanakunti, A.; Intamat, S.; Tengjaroenkul, B. Analysis of chromosome abnormalities in the Asian swamp eel (Monopterus albus) affected by arsenic contamination near a gold mine area. Int. J. Environ. Stud. 2020, 77, 815–829. [Google Scholar] [CrossRef]
- Phoonaploy, U.; Intamat, S.; Tengjaroenkul, B.; Sriuttha, M.; Tanamtong, A.; Neeratanaphan, L. Evaluation of abnormal chromosomes in rice field frogs (Fejervarya limnocharis) from reservoirs affected by leachate with cadmium, chromium and lead contamination. EnvironmentAsia 2016, 9, 26–38. [Google Scholar]
- Tengjaroenkul, B.; Intamat, S.; Thanomsangad, P.; Phoonaploy, U.; Neeratanaphan, L. Cytotoxic effect of sodium arsenite on Nile tilapia (Oreochromis niloticus) in vivo. Int. J. Environ. Stud. 2018, 75, 580–591. [Google Scholar] [CrossRef]
- Silbergeld, E.K. Facilitative mechanisms of lead as a carcinogen. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 533, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Sordo, M.; Herrera, L.A.; Wegman, P.O.; Rojas, E. Cytotoxic and genotoxic effects of As, MMA, and DMA on leukocytes and stimulated human lymphocytes. Teratog. Carcinog. Mutagen. 2001, 21, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.E.; Razo, L.M.D.; Sanchez, J.L.M. Induction of DNA damage by free radicals generated either by organic or inorganic arsenic (AsIII, MMAIII, and DMAIII) in cultures of B and T lymphocytes. Biol. Trace Elem. Res. 2005, 108, 115–126. [Google Scholar] [CrossRef]
- Waalkes, M.P. Cadmium carcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 533, 107–120. [Google Scholar] [CrossRef]
- Castano, A.; Becerril, C. In vitro assessment of DNA damage after short- and long-term exposure to benzo(a)pyrene using RAPD and the RTG-2 fish cell line. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 552, 141–151. [Google Scholar] [CrossRef]
- Suhartono, E.; Yunanto, A.; Firdaus, R.T. Chronic cadmium hepatooxidative in rats: Treatment with haruan fish (Channa striata) extract. APCBEE Procedia 2013, 5, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Buschini, A.; Pinelli, S.; Pellacni, C.; Giordani, F.; Ferrari, M.B.; Bisceglie, F.; Giannetto, M.; Pelosi, M.; Tarasconi, P. Synthesis, characterization and deepening in the comprehension of the biological action mechanisms of a new nickel complex with antiproliferative activity. J. Inorg. Biochem. 2009, 103, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Q.; Arita, A.; Sun, H.; Costa, M. Effects of nickel, chromate, and arsenite on histone 3 lysine methylation. Toxicol. Appl. Pharmacol. 2009, 236, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M.V.R.; Acharya, Y.; Naik, J.K.; Fatteh, S.; Fateh, A.S.; Pawar, A.C. Study of heavy metals in abnormal growth and development using an alternate animal model: Heterometrus fulvipes. Int. J. Life Sci. Sci. Res. 2017, 3, 1441–1450. [Google Scholar] [CrossRef]
- Asgah, N.A.A.; Warith, A.W.A.A.; Younis, E.S.M.; Allam, H.Y. Haematological and biochemical parameters and tissue accumulations of cadmium in Oreochromis niloticus exposed to various concentrations of cadmium chloride. Saudi J. Biol. Sci. 2015, 22, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Naga, E.H.A.E.; Moselhy, K.M.E.; Hamed, M.A. Toxicity of cadmium and copper and their effect on some biochemical parameters of marine fish, Mugil sheheli. Egypt. J. Aquat. Res. 2005, 31, 60–71. [Google Scholar]
- Javed, M.; Usmani, N. Stress response of biomolecules (carbohydrate, protein and lipid profiles) in fish Channa punctatus inhabiting river polluted by Thermal Power Plant effluent. Saudi J. Biol. Sci. 2015, 22, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Ahmed, K.; Akhand, A.A.; Hasan, M.; Islam, M.; Hasan, A. Toxicity of arsenic (sodium arsenite) to fresh water spotted snakehead Channa punctatus (Bloch) on cellular death and DNA content. J. Agric. Environ. Sci. 2008, 4, 18–22. [Google Scholar]
- Ahmed, M.K.; Mamun, M.H.A.; Hossain, M.A.; Parvin, M.A.E.; Akter, M.S.; Khan, M.S.; Islam, M.M. Assessing the genotoxic potentials of arsenic in tilapia (Oreochromis mossambicus) using alkaline comet assay and micronucleus test. Chemosphere 2011, 84, 143–149. [Google Scholar] [CrossRef]
- Vera-Candioti, J.; Soloneski, S.; Larramendy, M.L. Acute toxicity of chromium on Cnesterodon decemmaculatus (pisces: Poeciliidae) (La toxicidad aguda del cromo en Cnesterodon decemmaculatus (pisces: Poeciliidae)). Theoria 2011, 20, 81–88. [Google Scholar]
- Ahmed, M.K.; Mamun, M.H.A.; Parvin, E.; Akter, M.S.; Khan, M.S. Arsenic induced toxicity and histopathological changes in gill and liver tissue of freshwater fish, tilapia (Oreochromis mossambicus). Exp. Toxicol. Pathol. 2013, 65, 903–909. [Google Scholar] [CrossRef]
- Dyk, J.C.; Pieterse, G.M.; Vuren, J.H.J. Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol. Environ. Saf. 2007, 66, 432–440. [Google Scholar] [PubMed]
- Mishra, A.K.; Mohanty, B. Chronic exposure to sublethal hexavalent chromium affects organ histopathology and serum cortisol profile of a teleost, Channa punctatus (Bloch). Sci. Total Environ. 2009, 407, 5031–5038. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, R.; Narayanan, M. Heavy metal induced histopathological alterations in selected organs of the Cyprinus carpio L. (Common Carp). Int. J. Environ. Res. 2009, 3, 95–100. [Google Scholar]

| Water Quality Parameters | Analytical Methods |
|---|---|
| Dissolved oxygen | DO meter, Model 966, Mettler Toledo |
| pH | pH meter, Model EcoScan pH 5 |
| Temperature | Eutech Thermometer |
| Total hardness, Carbonate hardness | Test kits, Chulalongkorn University, Thailand |
| Electrical conductivity | EC meter, Mettler Toledo |
| Nitrite, Nitrate, Ammonia | Titration methods |
| Samples | Concentrations | p-Value | |
|---|---|---|---|
| Nam Kok River (Study Area) | Nam Souang River (Control Area) | ||
| Temperature (°C) | 23.65 ± 0.46 | 26.20 ± 0.67 | 0.009 * |
| DO (mg/L) | 12.69 ± 0.77 | 7.96 ± 0.57 | 0.009 * |
| pH | 8.08 ± 0.21 | 7.65 ± 0.34 | 0.007 * |
| TH (mg/L) | 114.00 ± 5.48 | 94.00 ± 4.35 | 0.215 |
| CH (mg/L) | 11.00 ± 5.48 | 3.77 ± 0.48 | 0.154 |
| Nitrite (mg/L) | 0.03 ± 0.02 | 0.05 ± 0.02 | 0.118 |
| Nitrate (mg/L) | 7.43 ± 1.85 | 8.83 ± 3.67 | 0.062 |
| Ammonia (mg/L) | 0.02 ± 0.001 | 0.10 ± 0.01 | 0.065 |
| EC (μs/cm) | 443.40 ± 12.67 | 251.13 ± 6.24 | 0.207 |
| Potentially Toxic Elements | Concentration in Water (mg/L) | p-Value | Standard | |
|---|---|---|---|---|
| Nam Kok River (Study Area) | Nam Souang River (Control Area) | |||
| As | 0.005 ± 0.002 | 0.001 ± 0.000 | 0.008 * | 0.01 a |
| Ba | 5.581 ± 0.774 | 1.918 ± 1.281 | 0.009 * | 0.05 g |
| Cd | 0.002 ± 0.001 | 0.001 ± 0.001 | 0.142 | 0.01 b |
| Cr | 0.017 ± 0.007 | 0.018 ± 0.015 | 0.465 | 0.05 d |
| Cu | 0.031 ± 0.006 | 0.010 ± 0.005 | 0.009 * | - |
| Fe | 0.638 ± 0.126 ** | 0.910 ± 0.265 ** | 0.076 | 0.30 e |
| Mn | 0.109 ± 0.039 ** | 0.112 ± 0.016 ** | 0.347 | 0.05 f |
| Ni | 0.351 ± 0.436 ** | 0.017 ± 0.009 | 0.009 * | 0.02 d |
| Pb | 0.038 ± 0.030 | 0.023 ± 0.019 | 0.347 | 0.05 b |
| Se | 0.007 ± 0.002 | 0.003 ± 0.003 | 0.047 * | - |
| Zn | 3.526 ± 0.615 ** | 1.797 ± 0.978 ** | 0.028 * | 0.20 c |
| Potentially Toxic Elements | Concentration in Sediment (mg/kg) | p-Value | Standard | |
|---|---|---|---|---|
| Nam Kok River (Study Area) | Nam Souang River (Control Area) | |||
| As | 14.05 ± 7.30 ** | 0.03 ± 0.02 | 0.009 * | 3.90 a |
| Ba | 429.59 ± 15.58 | 12.42 ± 16.07 | 0.009 * | - |
| Cd | 2.35 ± 0.49 ** | 0.073 ± 0.06 | 0.009 * | 0.16 b |
| Cr | 18.08 ± 5.96 | 0.48 ±0.22 | 0.009 * | 45.50 b |
| Cu | 47.89 ± 16.55 ** | 0.42 ± 0.28 | 0.009 * | 21.50 b |
| Fe | 13,198.38 ± 1861.08 | 214.94 ± 59.49 | 0.000 * | - |
| Mn | 231.97 ± 95.91 | 2.95 ± 1.06 | 0.009 * | 1800 a |
| Ni | 37.25 ± 8.06 | 1.89 ± 1.79 | 0.009 * | 75.00 c |
| Pb | 50.50 ± 14.73 | 1.44 ± 0.93 | 0.009 * | 300.00 c |
| Se | ND | 0.03 ± 0.03 | - | - |
| Zn | 343.45 ± 28.46 ** | 9.63 ± 8.23 | 0.009 * | 300.00 c |
| Potentially Toxic Elements | Concentration (mg/kg) | p-Value | Standard | |
|---|---|---|---|---|
| Nam Kok River (Study Area) | Nam Souang River (Control Area) | |||
| As | 0.30 ± 0.10 | 0.17 ± 0.04 | 0.047 * | 2.00 a |
| Ba | 0.89 ± 0.41 | 1.96 ± 0.52 | 0.016 * | - |
| Cd | 0.21 ± 0.09 ** | 0.02 ± 0.001 | 0.009 * | 0.05 b |
| Cr | 3.58 ± 0.54 ** | 1.67 ± 0.23 | 0.009 * | 2.00 c |
| Cu | 2.20 ± 1.08 | 1.32 ± 0.22 | 0.028 * | 10.00 c |
| Fe | 44.74 ± 19.21 | 39.33 ± 8.84 | 0.917 | - |
| Mn | 4.19 ± 0.81 ** | 2.14 ± 0.55 ** | 0.009 * | 1.00 c |
| Ni | 1.70 ± 0.24 | 0.97 ± 0.11 | 0.009 * | - |
| Pb | ND | 0.04 ± 0.04 | - | 0.20 d |
| Se | 1.21 ± 0.76 | 0.28 ± 0.08 | 0.009 * | - |
| Zn | 79.25 ± 2.86 | 62.22 ± 15.95 | 0.047 * | - |
| H. macrolepidota | Number of Chromosome Abnormalities | Total Number of CA | Cell Number with CA | Percentage of CA | |||||
|---|---|---|---|---|---|---|---|---|---|
| F | SCG | CG | D | SCB | CF | ||||
| Study area | 0(1.5) | 0(1.5) | 21(20) | 5(2.5) | 0(0.5) | 2(2.5) | 30(19.5) | 15(4) | 30(8) |
| Total/Average * | 3 | 3 | 127 | 22 | 1 | 15 | 171 | 73 | 29.20 * |
| Control area | 0(0) | 0(0) | 6(2) | 1(0.5) | 0(0) | 0(1) | 8(2.5) | 6(3.5) | 12(7) |
| Total/Average * | 0 | 0 | 30 | 6 | 0 | 3 | 39 | 27 | 10.80 * |
| p-value | 0.009 ** | 0.009 ** | 0.009 ** | ||||||
| Parameter | Nam Kok River (Study Area) (n = 9) | Nam Souang River (Control Area) (n = 9) |
|---|---|---|
| AST (IU/L) | 66.34 ± 13.67 * | 32.67 ± 4.34 * |
| ALT (IU/L) | 63.38 ± 7.73 * | 28.67 ± 2.27 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soulivongsa, L.; Tengjaroenkul, B.; Patawang, I.; Neeratanaphan, L. Cytogenetic, Serum Liver Enzymes and Liver Cell Pathology of the Hampala Barb Fish (Hampala macrolepidota) Affected by Toxic Elements in the Contaminated Nam Kok River near the Sepon Gold-Copper Mine, Lao PDR. Int. J. Environ. Res. Public Health 2021, 18, 5854. https://doi.org/10.3390/ijerph18115854
Soulivongsa L, Tengjaroenkul B, Patawang I, Neeratanaphan L. Cytogenetic, Serum Liver Enzymes and Liver Cell Pathology of the Hampala Barb Fish (Hampala macrolepidota) Affected by Toxic Elements in the Contaminated Nam Kok River near the Sepon Gold-Copper Mine, Lao PDR. International Journal of Environmental Research and Public Health. 2021; 18(11):5854. https://doi.org/10.3390/ijerph18115854
Chicago/Turabian StyleSoulivongsa, Latsamy, Bundit Tengjaroenkul, Isara Patawang, and Lamyai Neeratanaphan. 2021. "Cytogenetic, Serum Liver Enzymes and Liver Cell Pathology of the Hampala Barb Fish (Hampala macrolepidota) Affected by Toxic Elements in the Contaminated Nam Kok River near the Sepon Gold-Copper Mine, Lao PDR" International Journal of Environmental Research and Public Health 18, no. 11: 5854. https://doi.org/10.3390/ijerph18115854





