Perfluoroalkyl Substances in Plasma of Smallmouth Bass from the Chesapeake Bay Watershed
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Smallmouth Bass Morphometric Characteristics
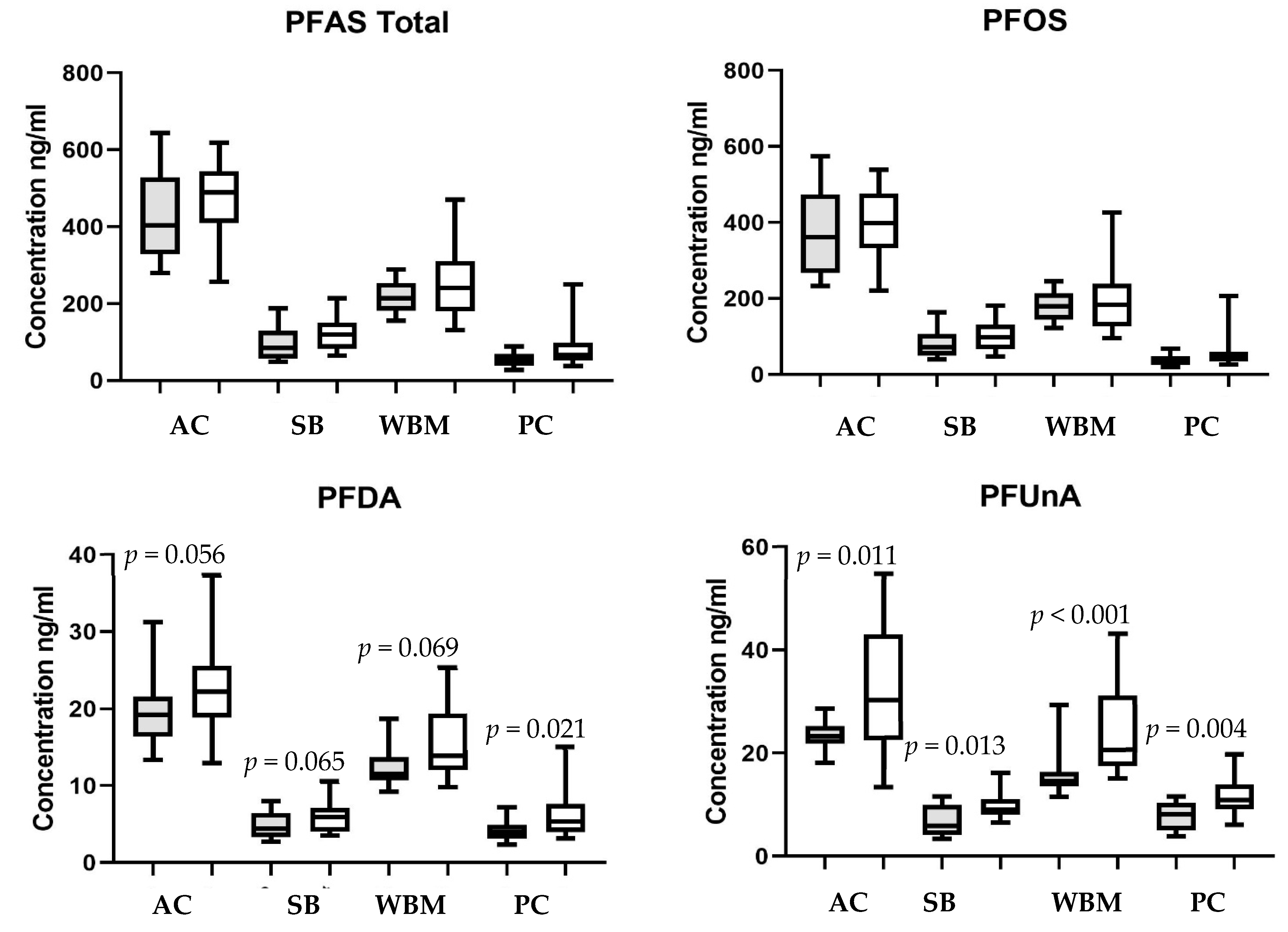
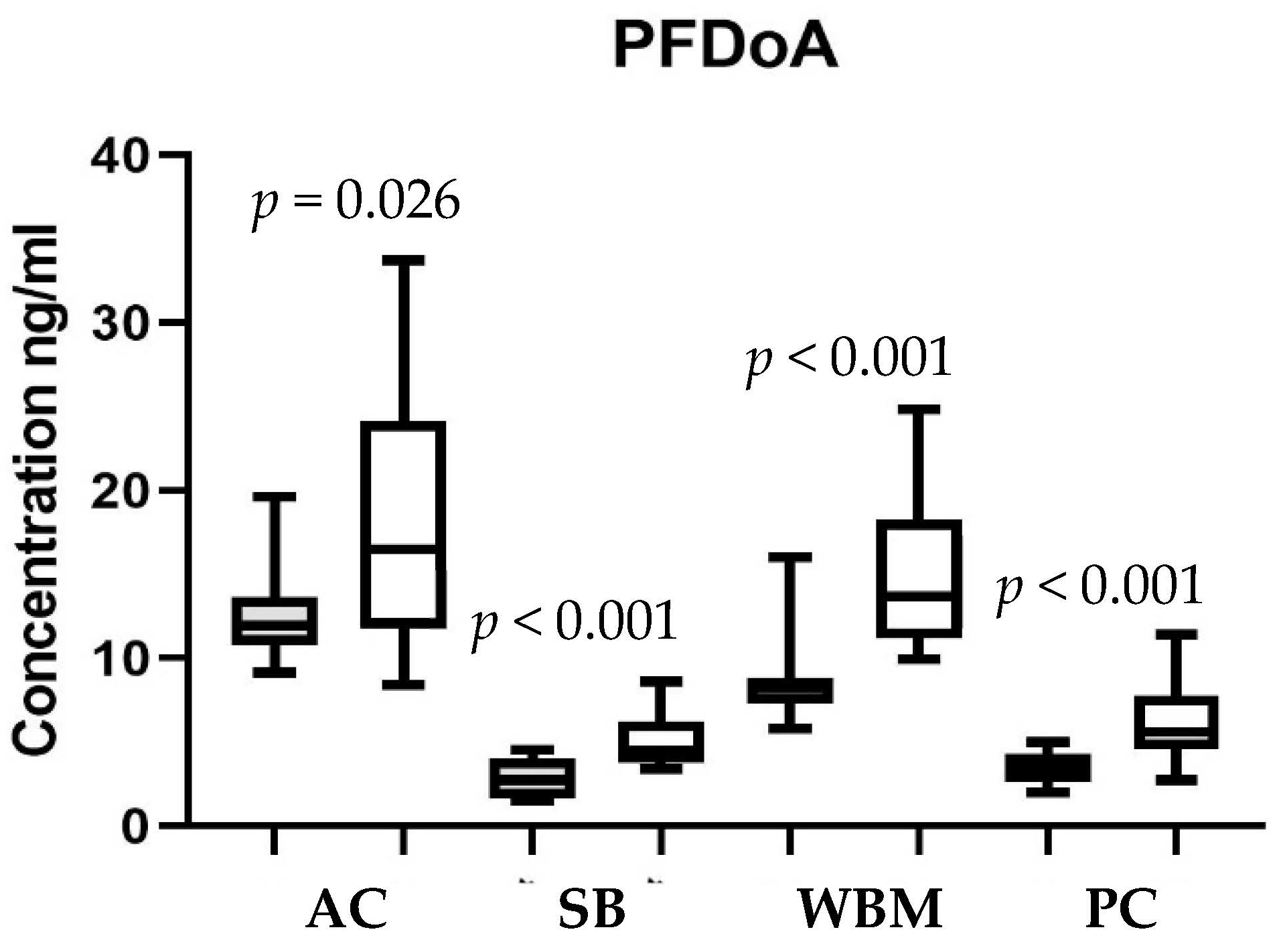
3.2. Chemical Detections and Site Comparisons
3.3. Seasonal Comparisons
3.4. Sex Comparisons
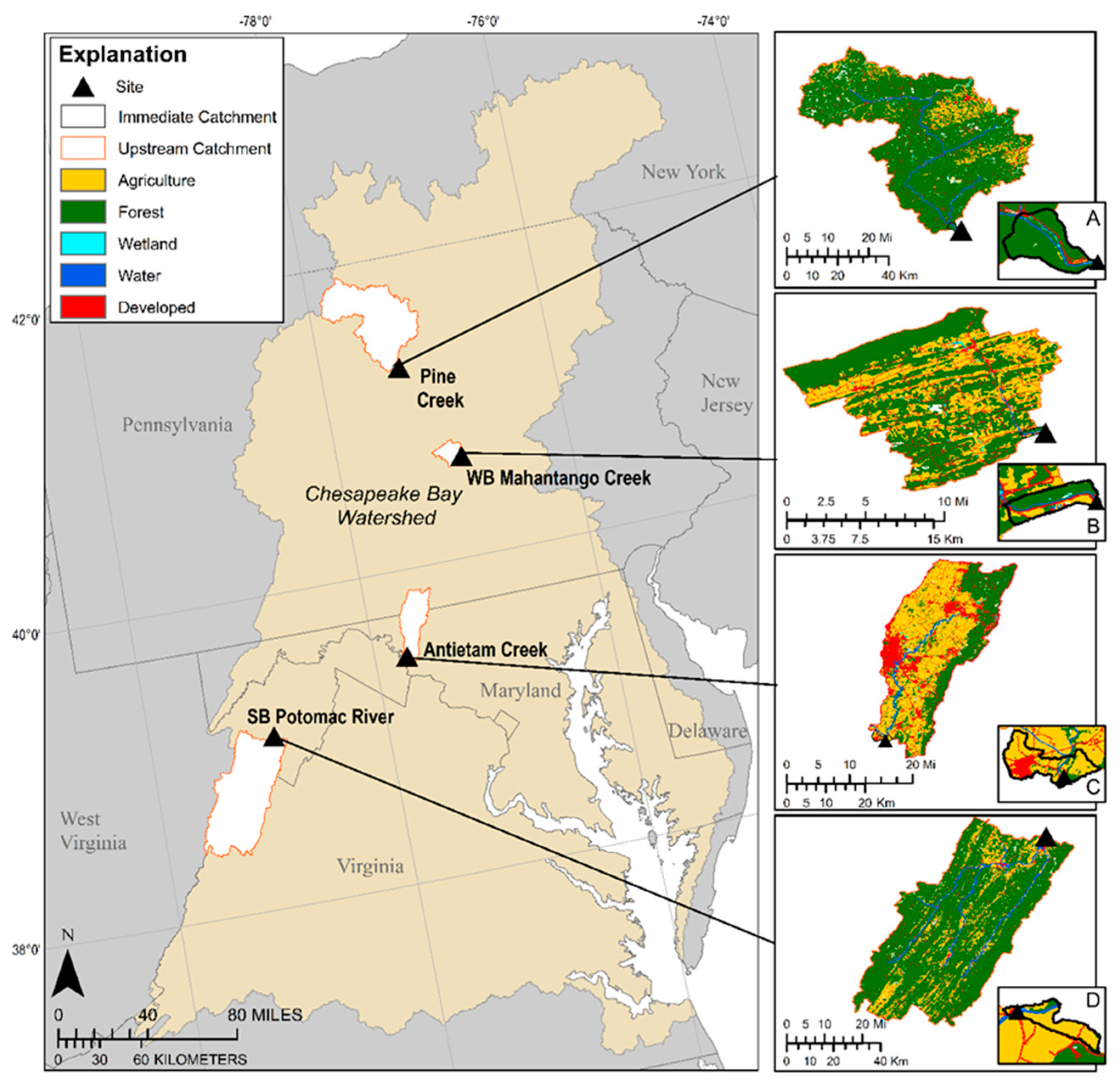
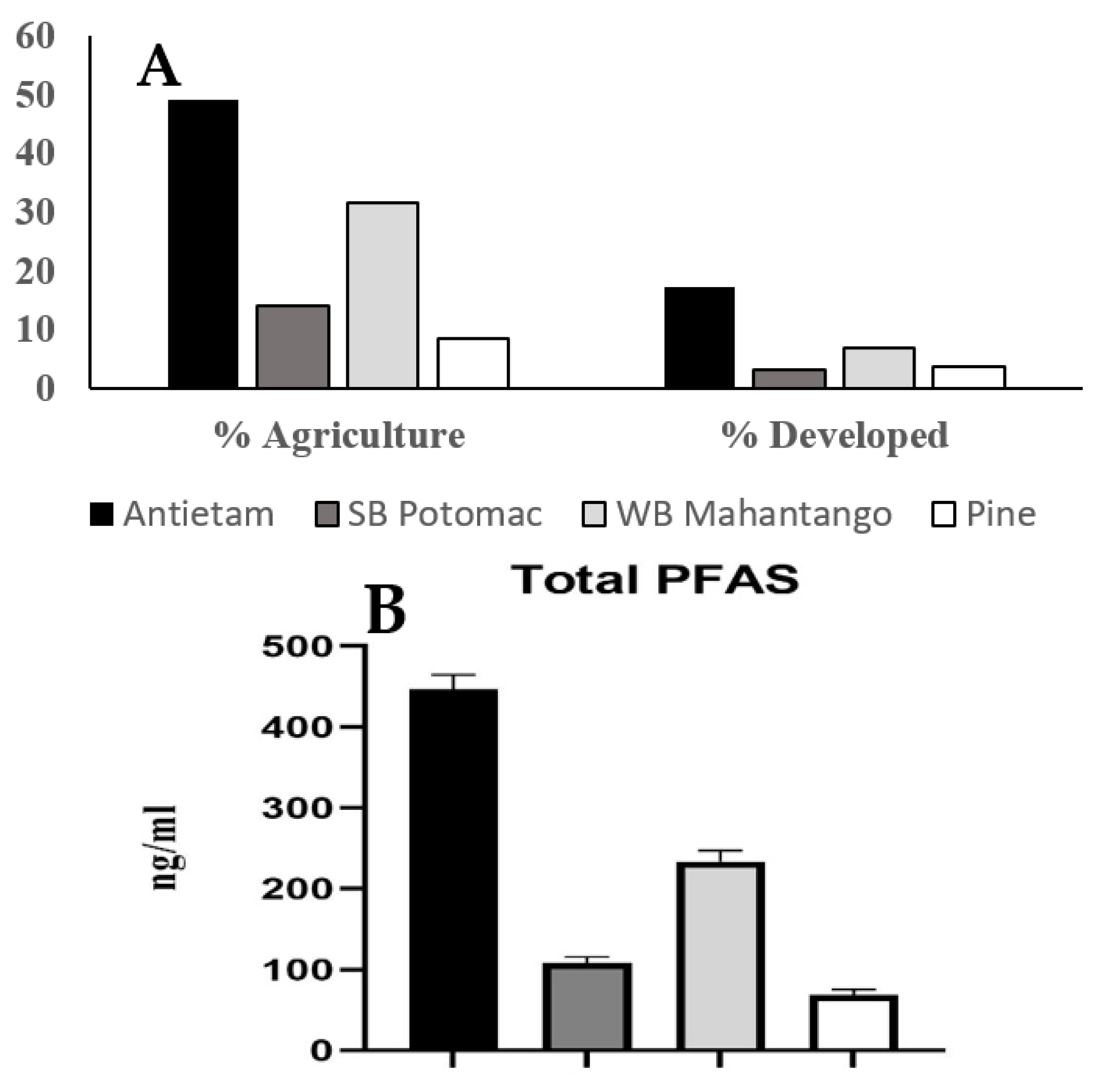
3.5. Site Land-Use Comparisons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Lanza, H.A.; Cochran, R.S.; Mudge, J.F.; Olson, A.D.; Blackwell, B.R.; Maul, J.D.; Salice, C.J.; Anderson, T.A. Temporal monitoring of perfluorooctane sulfonate accumulation in aquatic biota downstream of historic aqueous film forming foam use areas. Environ. Toxicol. Chem. 2017, 36, 2022–2029. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Becker, A.M.; Gerstmann, S.; Frank, H. Perfluorooctane surfactants in waste waters, the major source of river pollution. Chemosphere 2008, 72, 115–121. [Google Scholar] [CrossRef]
- Gómez-Canela, C.; Barth, J.A.C.; Lacorte, S. Occurrence and fate of perfluorinated compounds in sewage sludge from Spain and Germany. Environ. Sci. Pollut. Res. 2012, 19, 4109–4119. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.K.; Halden, R.U. National inventory of perfluoroalkyl substances in archived U.S. biosolids from the 2001 EPA National Sewage Sludge Survey. J. Hazard. Mater. 2013, 252–253, 413–418. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Starliper, C.E.; Iwanowicz, D.D.; Barbash, P.; Hedrick, J.D.; Reeser, S.J.; Mullican, J.E.; Zaugg, S.D.; Burkhardt, M.R.; et al. Mortality of Centrarchid Fishes in the Potomac Drainage: Survey Results and Overview of Potential Contributing Factors. J. Aquat. Anim. Heal. 2010, 22, 190–218. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Iwanowicz, D.D.; Smith, D.R.; Young, J.A.; Hedrick, J.D.; Foster, S.W.; Reeser, S.J. Inter-sex (testicular oocytes) in Smallmouth Bass Micropterus dolomieu from the Potomac River and selected nearby drainages. J. Aquat. Animal Health 2007, 19, 242–253. [Google Scholar] [CrossRef]
- Iwanowicz, L.R.; Blazer, V.S.; Guy, C.P.; Pinkney, A.E.; Mullican, J.; Alvarez, D.A. Reproductive health of bass in the Potomac, USA, drainage: Part 1. Exploring the effects of proximity to wastewater treatment plant effluent. Environ. Toxicol. Chem. 2009, 28, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.S.; Iwanowicz, D.D.; Walsh, H.L.; Sperry, A.J.; Iwanowicz, L.; Alvarez, D.A.; Brightbill, R.A.; Smith, G.; Foreman, W.T.; Manning, R. Reproductive health indicators of fishes from Pennsylvania watersheds: Association with chemicals of emerging concern. Environ. Monit. Assess. 2014, 186, 6471–6491. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Blazer, V.S.; Walsh, H.L.; Iwanowicz, L.R.; Starliper, C.; Sperry, A.J. The effects of disease-related mortal-ity of young-of-year smallmouth bass on the population characteristics in the Susquehanna River basin, Pennsylva-nia and potential implications to conservation of black bass diversity. Am. Fish. Soc. Symp. 2015, 82, 319–332. [Google Scholar]
- Walsh, H.L.; Blazer, V.S.; Smith, G.D.; Lookenbill, M.; Alvarez, D.A.; Smalling, K.L. Risk Factors Associated with Mortality of Age-0 Smallmouth Bass in the Susquehanna River Basin, Pennsylvania. J. Aquat. Anim. Heal. 2018, 30, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Starliper, C.; Blazer, V.S.; Iwanowicz, L.R.; Walsh, H.L. Bacterial isolates in diseased fishes, primarily smallmouth bass (Micropterus dolomieu), within the Chesapeake Bay drainage in 2009–2011. Proc. West Va. Acad. Sci. 2013, 85, 18–32. [Google Scholar]
- Blazer, V.S.; Young, K.T.; Smith, G.D.; Sperry, A.J.; Iwanowicz, L.R. Hyperpigmented melanistic skin lesions of smallmouth bass Micropterus dolomieu from the Chesapeake Bay watershed. Dis. Aquat. Org. 2020, 139, 199–212. [Google Scholar] [CrossRef]
- Ciparis, S.; Iwanowicz, L.; Voshell, J.R. Effects of watershed densities of animal feeding operations on nutrient concentrations and estrogenic activity in agricultural streams. Sci. Total. Environ. 2012, 414, 268–276. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.; Henderson, H.; Mazik, P.M.; Jenkins, J.A.; Alvarez, D.A.; Young, J.A. Reproductive endocrine disruption in smallmouth bass (Micropterus dolomieu) in the Potomac River basin: Spatial and temporal comparisons of biological effects. Environ. Monit. Assess. 2011, 184, 4309–4334. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Blazer, V.S.; Gray, J.L.; Focazio, M.J.; Young, J.A.; Alvarez, D.A.; Iwanowicz, L.R.; Foreman, W.T.; Fur-long, E.T.; Speiran, G.K.; et al. Chemical contami-nants in water and sediment near fish nesting sites in the Potomac River Basin: Determining potential exposures to Smallmouth Bass (Micropterus dolomieu). Sci. Total Environ. 2013, 443, 700–716. [Google Scholar] [CrossRef]
- McClure, C.M.; Smalling, K.L.; Blazer, V.S.; Sperry, A.J.; Schall, M.K.; Kolpin, D.W.; Phillips, P.J.; Hladik, M.L.; Wagner, T. Spatiotemporal variation in occurrence and co-occurrence of chemicals of pesticides, hormones, and other organic con-taminants in rivers in the Chesapeake Bay watershed. Sci. Total. Environ. 2020, 728, 138765. [Google Scholar] [CrossRef]
- Grandjean, P.; Budtz-Jørgensen, E. Immunotoxicity of perfluorinated alkylates: Calculation of benchmark doses based on serum concentrations in children. Environ. Health 2013, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Han, Z.; Liu, Y.; Wu, D.; Zhu, Z.; Lu, C. Immunotoxicology and hepatotoxicity of PFOS and PFOA in tilapia (Oreo-chromis niloticus). Chin. J. Geochem. 2012, 31, 424–430. [Google Scholar] [CrossRef]
- Han, J.; Fang, Z. Estrogenic effects, reproductive impairment and developmental toxicity in ovoviparous swordtail fish (Xiphophorus helleri) exposed to perfluorooctane sulfonate (PFOS). Aquat. Toxicol. 2010, 99, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Dai, J.; Liu, M.; Wang, J.; Xu, M.; Zha, J.; Wang, Z. Estrogen-like properties of perfluorooctanoic acid as re-vealed by expressing hepatic estrogen-responsive genes in rare minnows (Gobiocypris rurus). Environ. Toxicol. Chem. 2007, 26, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Houde, M.; Douville, M.; Despatie, S.-P.; De Silva, A.O.; Spencer, C. Induction of gene responses in St. Lawrence northern pike (Esox lucius) environmentally exposed to perfluorinated compounds. Chemosphere 2013, 92, 1195–1200. [Google Scholar] [CrossRef]
- McKay, L.; Bondelip, T.; Dewald, T.; Johnstone, J.; Moore, R.; Rea, A. NHDPlus Version 2: User Guide; U.S. Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Yang, L.; Jin, S.; Danielson, P.; Homer, C.; Gass, L.; Bender, S.M.; Case, A.; Costello, C.; Dewitz, J.; Fry, J.; et al. A new generation of the United States National Land Cover Database: Requirements, research priorities, design, and implementation strategies. ISPRS J. Photogramm. Remote. Sens. 2018, 146, 108–123. [Google Scholar] [CrossRef]
- Conder, J.M.; Hoke, R.A.; De Wolf, W.; Russell, M.H.; Buck, R.C. Are PFCAs bioaccumulative? A critical review and comparison with regulatory lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, A.R. Temporal Trends of Per- and Polyfluoroalkyl Substances in Delaware River Fish, USA. Integr. Environ. Assess. Manag. 2021, 17, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Stahl, L.L.; Snyder, B.D.; Olsen, A.R.; Kincaid, T.M.; Wathen, J.B.; McCarty, H.B. Perfluorinated compounds in fish from U.S. urban rivers and the Great Lakes. Sci. Total. Environ. 2014, 499, 185–195. [Google Scholar] [CrossRef]
- Kannan, K.; Tao, L.; Sinclair, E.; Pastva, S.D.; Jude, D.J.; Giesy, J.P. Perfluorinated Compounds in Aquatic Organisms at Various Trophic Levels in a Great Lakes Food Chain. Arch. Environ. Contam. Toxicol. 2005, 48, 559–566. [Google Scholar] [CrossRef]
- Newsted, J.L.; Holem, R.; Hohenstein, G.; Lange, C.; Ellefson, M.; Reagen, W.; Wolf, S. Spatial and temporal trends of poly- and perfluoroalkyl substances in fish fillets and water collected from pool 2 of the Upper Mississippi River. Environ. Toxicol. Chem. 2017, 36, 3138–3147. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, E.; Mayack, D.T.; Roblee, K.; Yamashita, N.; Kannan, K. Occurrence of Perfluoroalkyl Surfactants in Water, Fish, and Birds from New York State. Arch. Environ. Contam. Toxicol. 2006, 50, 398–410. [Google Scholar] [CrossRef]
- Ankley, G.T.; Kuehl, D.W.; Kahl, M.D.; Jensen, K.M.; Linnum, A.; Leino, R.L.; Villeneuve, D.A. Reproductive and developmental toxicity and bioconcentration of perfluorooctanesulfonate in a partial life-cycle test with the fathead minnow (pimephales promelas). Environ. Toxicol. Chem. 2005, 24, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Son, Y. Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA. Sci. Total. Environ. 2021, 751, 141622. [Google Scholar] [CrossRef]
- Rodriguez-Jorquera, I.A.; Colli-Dula, R.C.; Kroll, K.J.; Jayasinghe, B.S.; Marco, M.V.P.; Silva-Sanchez, C.; Toor, G.S.; Denslow, N.D. Blood Transcriptomics Analysis of Fish Exposed to Perfluoro Alkyls Substances: Assessment of a Non-Lethal Sampling Technique for Advancing Aquatic Toxicology Research. Environ. Sci. Technol. 2019, 53, 1441–1452. [Google Scholar] [CrossRef]
- Honda, M.; Muta, A.; Shimazaki, A.; Akasaka, T.; Yoshikuni, M.; Shimasaki, Y.; Oshima, Y. High concentrations of perfluorooctane sulfonate in mucus of tiger fish Takifugu rubripes: A laboratory exposure study. Environ. Sci. Pollut. Res. 2018, 25, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Mabury, S.A.; Solomon, K.R.; Muir, D.C.G. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2003, 22, 196–204. [Google Scholar] [CrossRef]
- Taniyasu, S.; Kannan, K.; Horii, Y.; Hanari, N.; Yamashita, N. A survey of perfluorooctane sulfonate and related per-fluorinated organic compounds in water, fish, birds, and humans from Japan. Environ. Sci. Technol. 2003, 37, 2634–2639. [Google Scholar] [CrossRef] [PubMed]
- Beach, S.A.; Newsted, J.L.; Coady, K.; Giesy, J.P. Ecotoxicological Evaluation of Perfluorooctanesulfonate (PFOS). Rev. Environ. Contam. Toxicol. 2006, 186, 133–174. [Google Scholar] [CrossRef]
- Li, X.; Yeung, L.W.Y.; Xu, M.; Taniyasu, S.; Lam, P.K.; Yamashita, N.; Dai, J. Perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish blood collected near the outfall of wastewater treatment plant (WWTP) in Beijing. Environ. Pollut. 2008, 156, 1298–1303. [Google Scholar] [CrossRef]
- Olson, M.H.; Young, B.P. Patterns of diet and growth in co-occurring populations of largemouth bass and small-mouth bass. Trans. Amer. Fish. Soc. 2003, 132, 1207–1213. [Google Scholar] [CrossRef]
- Guillette, T.C.; McCord, J.; Guillette, M.; Polera, M.E.; Rachels, K.T.; Morgeson, C.; Kotlarz, N.; Knappe, D.R.U.; Reading, B.J.; Strynar, M.; et al. Elevated levels of per- and polyfluoroalkyl substances in Cape Fear River Striped Bass (Morone saxatilis) are associated with biomarkers of altered immune and liver function. Environ. Int. 2020, 136, 105358. [Google Scholar] [CrossRef]
- Kovarova, J.; Marsalek, P.; Blahova, J.; Jurcikova, J.; Kasikova, B.; Svobodova, Z. Occurrence of perfluoroalkyl sub-stances in fish and water from Svitava and Svratka Rivers, Czech Republic. Bull. Environ. Contam. Toxicol. 2012, 88, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Yamashita, N.; Taniyasu, S.; Lee, K.T.; Jones, P.D.; Newsted, J.L.; Khim, J.S.; Giesy, J.P. Perfluoroalkyl Acids in Marine Organisms from Lake Shihwa, Korea. Arch. Environ. Contam. Toxicol. 2009, 57, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Sepulvado, J.G.; Blaine, A.C.; Hundal, L.S.; Higgins, C.P. Occurrence and fate of perfluorochemicals in soils fol-lowing the land application of municipal biosolids. Environ. Sci. Technol. 2011, 45, 8106–8112. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Scharf, S.; Weiss, S.; Gans, O.; Scheffknecht, C. Emissions of perfluorinated alkylated substances (PFAS) from point sources—identification of relevant branches. Water Sci. Technol. 2008, 58, 59–66. [Google Scholar] [CrossRef]
| Chemical Name | Abbreviation | Carbon Chain Length |
|---|---|---|
| Perfluoroalkyl Carboxylic Acid Compounds | ||
| Perfluorobutanoic acid | PFBA | 4 |
| Perfluoro-n-pentanoic acid | PFPeA | 5 |
| Perfluorohexanoic acid | PFHxA | 6 |
| Perfluoroheptanoic acid | PFHpA | 7 |
| Perfluorooctanoic acid | PFOA | 8 |
| Perfluorononanoic acid | PFNA | 9 |
| Perfluorodecanoic acid | PFDA | 10 |
| Perfluoroundecanoic acid | PFUnA | 11 |
| Perfluorododecanoic acid | PFDoA | 12 |
| Perfluoroalkyl Sulfonic Acid Compounds | ||
| Perfluorobutane sulfonic acid | PFBS | 4 |
| Perfluorohexane sulfonic acid | PFHxS | 6 |
| Perfluorooctane sulfonamide | PFOSA | 8 |
| Perfluorooctane sulfonic acid | PFOS | 8 |
| Site | Sample Size | Length (mm) 1 | Weight (gm) 1 | Age (Years) 1 |
|---|---|---|---|---|
| Antietam Creek | 34 | 304 ± 40 a | 375 ± 169 a | 3.9 ± 1.2 a |
| South Branch Potomac | 36 | 309 ± 57 a | 436 ± 258 a | 4.0 ± 1.5 a |
| West Branch Mahantango | 28 | 368 ± 37 b | 694 ± 259 b | 5.0 ± 1.8 b |
| Pine Creek | 32 | 280 ± 43 a | 314 ± 135 a | 4.2 ± 1.0 a,b |
| Site | Sample Size | PFOS ng/mL | PFUnA ng/mL | PFDA ng/mL | PFDoA ng/mL | Total 1 PFAS ng/mL |
|---|---|---|---|---|---|---|
| Antietam Creek | 34 | 381.4 ± 16.5 a (220–574) | 28.4 ± 1.7 a (13–55) | 21.4 ± 1.0 a (13–37) | 15.1 ± 1.1 a (8–34) | 447.0 ± 17.8 a (256–644) |
| South Branch Potomac | 36 | 90.4 ± 6.5 c (40–181) | 8.4 ± 0.5 c (3–14) | 5.3 ± 0.3 c (3–11) | 4.0 ± 0.3 c (1–9) | 108.2 ± 7.5 c (49–214) |
| West Branch Mahantango Creek | 28 | 187.1 ± 12.6 b (95–427) | 20.2 ± 1.5 b (12–43) | 14.1 ± 0.8 b (9–25) | 11.8 ± 0.9 b (6–25) | 234.0 ± 3.3 b (131–470) |
| Pine Creek | 32 | 48.4 ± 5.8 d (20–206) | 9.8 ± 0.6 c (4–20) | 5.2 ± 0.4 c (2–15) | 4.9 ± 0.5 c (2–11) | 68.6 ± 7.0 c (28–250) |
| Characteristics | Antietam Creek | South Branch Potomac River | West Branch Mahantango Creek | Pine Creek |
|---|---|---|---|---|
| Drainage Area (km2) | 5.95 | 1.04 | 1.21 | 8.49 |
| Percent Agriculture | 63.6 | 74.4 | 20.4 | 1.1 |
| Percent Forest | 14.1 | 10.4 | 66.1 | 88.2 |
| Percent Developed | 21.4 | 7.2 | 10.0 | 4.2 |
| Nutrients from Biosolids (kg) | 0.1 | 0 | 2.9 | 3.4 |
| Domestic Wastewater Treatment Plants | 0 | 0 | 0 | 0 |
| Industrial Wastewater Facilities | 0 | 0 | 0 | 0 |
| Characteristics | Antietam Creek | South Branch Potomac River | West Branch Mahantango Creek | Pine Creek |
|---|---|---|---|---|
| Drainage Area (km2) | 730 | 3151 | 218 | 2437 |
| Percent Agriculture | 49.1 | 14.0 | 31.6 | 8.5 |
| Percent Forest | 32.2 | 80.6 | 60.1 | 84.2 |
| Percent Developed | 17.3 | 3.2 | 7.0 | 3.6 |
| Nutrients from Biosolids (kg) | 18,240 | 3.8 | 799 | 1486 |
| Domestic Wastewater Treatment Plants | 27 | 13 | 1 | 7 |
| Industrial Wastewater Facilities | 51 | 77 | 9 | 12 |
| Species | Range (ng/mL) | Citation | |
|---|---|---|---|
| Smallmouth bass Micropterus dolomieu | PFOS | 20–574 | Current study |
| PFDA | 2–37 | ||
| PFUnDA | 3–55 | ||
| PFNA | BD–1.3 | ||
| Chub Leuciscus cephalus | PFOS | 38.9–57.8 | [44] |
| PFNA | 0.88–7.1 | ||
| Grey mullet Mugil cephalus | PFOS | 93 (mean) | [45] |
| PFDA | 13 (mean) | ||
| PFNA | 3.8 (mean) | ||
| PFUnDA | 26 (mean) | ||
| Rockfish Sebastes inermis | PFOS | 31 (mean) | [45] |
| PFDA | 1.9 (mean) | ||
| PFNA | 1.9 (mean) | ||
| PFUnDA | 3.6 (mean) | ||
| Crucian carp Carassius auratus | PFOS | 48.9–84.4 | [41] |
| PFDA | 9.7–25.4 | ||
| PFUnDA | 7.0–15.3 | ||
| PFOSA | 0.3–2.0 | ||
| PFNA | 0.3–1.2 | ||
| Common carp Cyprinus carpio | PFOS | 14.2–32.2 | [41] |
| PFDA | 1.4–11.7 | ||
| PFUnDA | 1.1–9.0 | ||
| PFNA | 0.1–0.7 | ||
| White semiknife carp Hemiculter leucisculus | PFOS | 8.4–11.4 | [41] |
| PFDA | 5.2–10.3 | ||
| PFUnDA | 1.7–2.5 | ||
| PFOSA | 1.0–5.8 | ||
| PFNA | 0.3–0.5 | ||
| Nile tilapia Oreochromis niloticus | PFOS | 4.8–6.7 | [41] |
| PFDA | 2.9–4.3 | ||
| PFUnDA | 1.6–1.9 | ||
| PFOSA | 0.1–2.8 | ||
| PFNA | 0.3–0.5 | ||
| Leather catfish Clarias lazera | PFOS | 7.0–25.9 | [41] |
| PFDA | 5.1–15.5 | ||
| PFUnDA | 5.4–14.1 | ||
| PFNA | 0.1–1.0 | ||
| Largemouth bass Micropterus salmoides | PFOS | 317–322 | [39] |
| Blue gill Lepomis macrochirus | PFOS | 455–834 | [39] |
| Common carp Cyprinus carpio | PFOS | 68–77 | [39] |
| Striped bass Morone saxatilis | PFOS | 4.6–977 | [43] |
| PFDA | 1.7–146 | ||
| PFNA | 0.3–11.6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blazer, V.S.; Gordon, S.E.; Walsh, H.L.; Smith, C.R. Perfluoroalkyl Substances in Plasma of Smallmouth Bass from the Chesapeake Bay Watershed. Int. J. Environ. Res. Public Health 2021, 18, 5881. https://doi.org/10.3390/ijerph18115881
Blazer VS, Gordon SE, Walsh HL, Smith CR. Perfluoroalkyl Substances in Plasma of Smallmouth Bass from the Chesapeake Bay Watershed. International Journal of Environmental Research and Public Health. 2021; 18(11):5881. https://doi.org/10.3390/ijerph18115881
Chicago/Turabian StyleBlazer, Vicki S., Stephanie E. Gordon, Heather L. Walsh, and Cheyenne R. Smith. 2021. "Perfluoroalkyl Substances in Plasma of Smallmouth Bass from the Chesapeake Bay Watershed" International Journal of Environmental Research and Public Health 18, no. 11: 5881. https://doi.org/10.3390/ijerph18115881
APA StyleBlazer, V. S., Gordon, S. E., Walsh, H. L., & Smith, C. R. (2021). Perfluoroalkyl Substances in Plasma of Smallmouth Bass from the Chesapeake Bay Watershed. International Journal of Environmental Research and Public Health, 18(11), 5881. https://doi.org/10.3390/ijerph18115881






