Causal Attributions in Breast Cancer Patients Planning to Undergo Adjuvant Endocrine Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
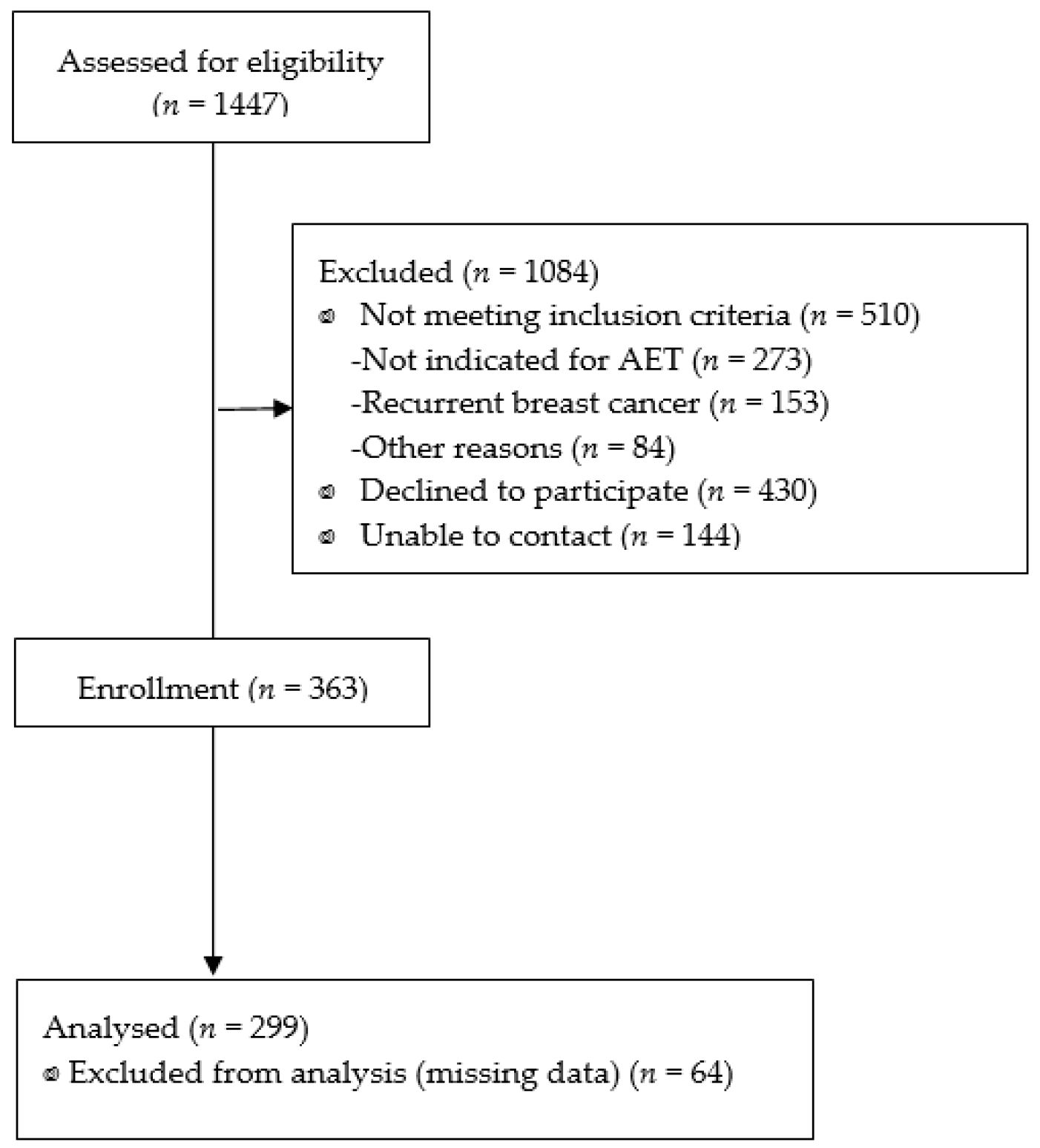
2.2. Sample and Settings
2.3. Data Collection
2.4. Data Analysis
2.5. Ethical Considerations
3. Results
3.1. Characteristics of the Patients
3.2. Causal Attributions of Breast Cancer
3.3. Associations between Demographic and Clinical Characteristics of Patients and Causal Attributions
3.4. Relationships between Causal Attributions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- 2018 Trend Analysis of Cancer Incidence in Korea. Available online: https://www.cancer.go.kr/lay1/S1T639C640/contents.do (accessed on 1 February 2021).
- Kang, S.Y.; Kim, Y.S.; Kim, Z.; Kim, H.Y.; Kim, H.J.; Park, S.; Bae, S.Y.; Yoon, K.H.; Lee, S.B.; Lee, S.K. Breast cancer statistics in Korea in 2017: Data from a breast cancer registry. J. Breast Cancer 2020, 23, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Fisher, B.; Jeong, J.-H.; Bryant, J.; Anderson, S.; Dignam, J.; Fisher, E.R.; Wolmark, N. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: Long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 2004, 364, 858–868. [Google Scholar] [CrossRef]
- Burstein, H.J.; Temin, S.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Rowden, D.; Solky, A.J. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J. Clin. Oncol. 2014, 32, 2255–2269. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause and breast cancer risk: Individual participant meta-analysis, including 118964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012, 13, 1141–1151. [Google Scholar] [CrossRef]
- Suzuki, S.; Kojima, M.; Tokudome, S.; Mori, M.; Sakauchi, F.; Wakai, K.; Fujino, Y.; Lin, Y.; Kikuchi, S.; Tamakoshi, K. Obesity/weight gain and breast cancer risk: Findings from the Japan collaborative cohort study for the evaluation of cancer risk. J. Epidemiol. 2013, 23, 139–145. [Google Scholar] [CrossRef]
- Lahmann, P.; Schulz, M.; Hoffmann, K.; Boeing, H.; Tjønneland, A.; Olsen, A.; Overvad, K.; Key, T.; Allen, N.; Khaw, K. Long-term weight change and breast cancer risk: The European prospective investigation into cancer and nutrition (EPIC). Br. J. Cancer 2005, 93, 582–2589. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.F.; Cantwell, M.M.; Cardwell, C.R.; Velentzis, L.S.; Woodside, J.V. Dietary patterns and breast cancer risk: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, M.; Ciszewski, T.; Łopacka-Szatan, K.; Miotła, P.; Starosławska, E. Breast cancer risk factors. Prz. Menopauzalny 2015, 14, 196–202. [Google Scholar] [CrossRef]
- Peuker, A.C.W.; Armiliato, M.J.; Souza, L.V.d.; Castro, E.K.d. Causal attribution among women with breast cancer. Psicol. Refl. Crít. 2016, 29, 1–6. [Google Scholar] [CrossRef]
- McKenna, M.C.; Zevon, M.A.; Corn, B.; Rounds, J. Psychosocial factors and the development of breast cancer: A meta-analysis. Health Psychol. 1999, 18, 520–531. [Google Scholar] [CrossRef]
- Karabulutlu, E.Y.; Avcı, İ.A.; Karayurt, Ö.; Gürsoy, A.; Köşgeroğlu, N.; Tuna, A.; Ersin, F.; Arıkan, F.; Karaman, S. Evaluation of Illness Perception of women with breast cancer in Turkey. Eur. J. Breast Health 2019, 15, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Fielding, R.; Soong, I.; Chan, K.K.; Tsang, J.; Lee, V.; Lee, C.; Ng, A.; Sze, W.K.; Tin, P. Illness perceptions among cancer survivors. Support. Care Cancer 2016, 24, 1295–1304. [Google Scholar] [CrossRef]
- Gonzalez, P.; Lim, J.-W.; Wang-Letzkus, M.; Flores, K.F.; Allen, K.M.; Castañeda, S.F.; Talavera, G.A. Breast cancer cause beliefs: Chinese, Korean, and Mexican American breast cancer survivors. West. J. Nurs. Res. 2015, 37, 1081–1099. [Google Scholar] [CrossRef]
- Ferrucci, L.M.; Cartmel, B.; Turkman, Y.E.; Murphy, M.E.; Smith, T.; Stein, K.D.; McCorkle, R. Causal attribution among cancer survivors of the 10 most common cancers. J. Psychosoc. Oncol. 2011, 29, 121–140. [Google Scholar] [CrossRef]
- Dieterich, M.; Stubert, J.; Reimer, T.; Erickson, N.; Berling, A. Influence of lifestyle factors on breast cancer risk. Breast Care 2014, 9, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Rotter, J.B. Generalized expectancies for internal versus external control of reinforcement. Psychol. Monogr. 1966, 80, 1–28. [Google Scholar] [CrossRef]
- Rabin, C.; Pinto, B. Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psychooncology 2006, 15, 701–712. [Google Scholar] [CrossRef]
- Dumalaon-Canaria, J.; Prichard, I.; Hutchinson, A.; Wilson, C. Fear of cancer recurrence and psychological well-being in women with breast cancer: The role of causal cancer attributions and optimism. Eur. J. Cancer Care 2018, 27, e12579. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeon, Y.-W.; Im, E.-O.; Baek, J.-M. Causal Attributions and Quality of Life of Korean Breast Cancer Survivors. Asian Nurs. Res. Korean Soc. Nurs. Sci. 2021, 15, 53–59. [Google Scholar] [CrossRef]
- Nafradi, L.; Nakamoto, K.; Schulz, P.J. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS ONE 2017, 12, e0186458. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.-J.; Jeong, D.; Song, Y.W.; Lee, S.H.; Kim, N.J.; Hahm, B.-J. A network analysis of the Brief Illness Perception Questionnaire in patients with rheumatic diseases and human immunodeficiency virus infection. Psychol. Health 2020, 35, 838–853. [Google Scholar] [CrossRef]
- Broadbent, E.; Petrie, K.J.; Main, J.; Weinman, J. The brief illness perception questionnaire. Psychosom. Res. 2006, 60, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Dumalaon-Canaria, J.A.; Hutchinson, A.D.; Prichard, I.; Wilson, C. What causes breast cancer? A systematic review of causal attributions among breast cancer survivors and how these compare to expert-endorsed risk factors. Cancer Causes Control 2014, 25, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Park, H.A.; Lee, J.Y.; On, J.; Lee, J.H.; Jung, H.; Park, S.K. 2016 Year-in-Review of Clinical and Consumer Informatics: Analysis and Visualization of Keywords and Topics. Healthc. Inform. Res. 2017, 23, 77–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Memon, N.; Xu, J.J.; Hicks, D.L.; Chen, H. Data Mining for Social Network Data; Springer: New York, NY, USA, 2010; Volume 12. [Google Scholar]
- Oba, S.; Takatsuka, N.; Nagata, C.; Nagao, Y.; Yamamoto, S.; Shibuya, C.; Kashiki, Y.; Shimizu, H. Causal attributions to epidemiological risk factors and their associations to later psychological adjustment among Japanese breast cancer patients. Support Care Cancer 2009, 17, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Baider, L.; Sarell, M. Perceptions and causal attributions of Israeli women with breast cancer concerning their illness: The effects of ethnicity and religiosity. Psychother. Psychosom. 1983, 39, 136–143. [Google Scholar] [CrossRef]
- Chiriac, V.-F.; Baban, A.; Dumitrascu, D.L. Psychological stress and breast cancer incidence: A systematic review. Clujul Med. 2018, 91, 18–26. [Google Scholar] [CrossRef]
- Santos, M.C.L.; Horta, B.L.; Amaral, J.J.F.d.; Fernandes, P.F.C.B.C.; Galvão, C.M.; Fernandes, A.F.C. Association between stress and breast cancer in women: A meta-analysis. Cad. Saude Publica 2009, 25, S453–S463. [Google Scholar] [CrossRef][Green Version]
- Duijts, S.F.; Zeegers, M.P.; Borne, B.V. The association between stressful life events and breast cancer risk: A meta-analysis. Int. J. Cancer 2003, 107, 1023–1029. [Google Scholar] [CrossRef]
- Butow, P.N.; Hiller, J.E.; Price, M.A.; Thackway, S.V.; Kricker, A.; Tennant, C.C. Epidemiological evidence for a relationship between life events, coping style, and personality factors in the development of breast cancer. J. Psychosom. Res. 2000, 49, 169–181. [Google Scholar] [CrossRef]
- Wintermantel, T.M.; Bock, D.; Fleig, V.; Greiner, E.F.; Schütz, G. The epithelial glucocorticoid receptor is required for the normal timing of cell proliferation during mammary lobuloalveolar development but is dispensable for milk production. Mol. Endocrinol. 2005, 19, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.C.; Lu, Y.S.; Cheng, A.L.; Chang, W.C.; Jeng, Y.M.; Kuo, Y.H.; Huang, C.S.; Chang, K.J.; Yao, Y.T. Differential expression of glucocorticoid receptor in human breast tissues and related neoplasms. J. Pathol. 2006, 209, 317–327. [Google Scholar] [CrossRef]
- Courtin, A.; Communal, L.; Vilasco, M.; Cimino, D.; Mourra, N.; de Bortoli, M.; Taverna, D.; Faussat, A.-M.; Chaouat, M.; Forgez, P.; et al. Glucocorticoid receptor activity discriminates between progesterone and medroxyprogesterone acetate effects in breast cells. Breast Cancer Res Treat. 2012, 131, 49–63. [Google Scholar] [CrossRef]
- Antonova, L.; Aronson, K.; Mueller, C.R. Stress and breast cancer: From epidemiology to molecular biology. Breast Cancer Res. 2011, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Panjari, M.; Davis, S.R.; Fradkin, P.; Bell, R.J. Breast cancer survivors’ beliefs about the causes of breast cancer. Psychooncology 2012, 21, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, E.S.; Lutgendorf, S.K.; Roeder, S.L. Common-sense beliefs about cancer and health practices among women completing treatment for breast cancer. Psychooncology 2011, 20, 53–61. [Google Scholar] [CrossRef]
- Bleiker, E.M.; Hendriks, J.H.; Otten, J.D.; Verbeek, A.L.; van Der Ploeg, H.M. Personality factors and breast cancer risk: A 13-year follow-up. J. Natl. Cancer Inst. 2008, 100, 213–218. [Google Scholar] [CrossRef]
- Minami, Y.; Hosokawa, T.; Nakaya, N.; Sugawara, Y.; Nishino, Y.; Kakugawa, Y.; Fukao, A.; Tsuji, I. Personality and breast cancer risk and survival: The Miyagi cohort study. Breast Cancer Res. Treat. 2015, 150, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Nishiyama, T.; Kikuchi, N.; Wang, C.; Lin, Y.; Mori, M.; Tanno, K.; Tamakoshi, A.; Kikuchi, S. The influence of personality and perceived stress on the development of breast cancer: 20-year follow-up of 29,098 Japanese women. Sci. Rep. 2016, 6, 32559. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, M.; Blanca, M.J.; Ferragut, M. Personality Profiles and Psychological Adjustment in Breast Cancer Patients. Int. J. Environ. Res. Public Health 2020, 17, 9452. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Son, B.H.; Ahn, S.H.; Lee, M.H.; Park, S.K.; Kim, S.-W. Hereditary breast cancer in Korea: A review of the literature. J. Breast Cancer 2008, 11, 1–9. [Google Scholar] [CrossRef][Green Version]
- Nindrea, R.D.; Aryandono, T.; Lazuardi, L. Breast cancer risk from modifiable and non-modifiable risk factors among women in Southeast Asia: A meta-analysis. Asian Pac. J. Cancer Prev. 2017, 18, 3201–3206. [Google Scholar] [PubMed]
- Martin, L.J.; Melnichouk, O.; Guo, H.; Chiarelli, A.M.; Hislop, T.G.; Yaffe, M.J.; Minkin, S.; Hopper, J.L.; Boyd, N.F. Family history, mammographic density, and risk of breast cancer. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 456–463. [Google Scholar] [CrossRef]
- Haber, G.; Ahmed, N.U.; Pekovic, V. Family history of cancer and its association with breast cancer risk perception and repeat mammography. Am. J. Public Health 2012, 102, 2322–2329. [Google Scholar] [CrossRef]
- Park, E.H.; Min, S.Y.; Kim, Z.; Yoon, C.S.; Jung, K.W.; Nam, S.J.; Oh, S.J.; Lee, S.; Park, B.W.; Lim, W.; et al. Basic Facts of Breast Cancer in Korea in 2014: The 10-Year Overall Survival Progress. J. Breast Cancer 2017, 20, 1–11. [Google Scholar] [CrossRef]



| Categories | Subcategories |
|---|---|
| Biological factors | Genetics/Family history |
| Poor organ function | |
| Breast condition | |
| Environmental factors | Environmental factors |
| Behavioral factors | Lifestyle in general |
| Exercise | |
| Diet | |
| Weight control | |
| Alcohol/Smoking | |
| Sleep | |
| Lack of prevention | |
| Work-related problems | |
| Reproduction-related factors | Reproductive history |
| Breastfeeding | |
| Hormones | |
| Psychological factors | Emotion |
| Personality | |
| Stress | |
| Others | Low income |
| Existential influences/Fates | |
| Health condition | |
| Restrictive clothing | |
| No causal attribution |
| Characteristics | Mean (SD 1) or n (%) | |
|---|---|---|
| Age (years) | 46.75 (8.18) | |
| ≤40 | 58 (19.4) | |
| >40 | 241 (80.6) | |
| Education level | High School or below | 114 (38.1) |
| College and above | 185 (61.9) | |
| Marital status | No | 40 (13.4) |
| Yes | 257 (86.0) | |
| No information | 2 (0.7) | |
| Employment status | No | 146 (48.8) |
| Yes | 153 (51.2) | |
| Family history of cancer | No | 169 (56.5) |
| Yes | 130 (43.5) | |
| Family history of breast cancer | No | 267 (89.3) |
| Yes | 32 (10.7) | |
| Cancer stage | In situ | 39 (13.0) |
| Invasive (Stage I, II, or III) | 260 (87.0) | |
| Type of surgery | Mastectomy | 57 (19.1) |
| Conservation | 242 (80.9) | |
| Chemotherapy | No | 199 (66.6) |
| Yes | 100 (33.4) | |
| Radiation therapy | No | 51 (17.1) |
| Yes | 248 (82.9) | |
| Menopausal status | No | 226 (75.6) |
| Yes | 73 (24.4) | |
| Causes | Age | Education Level | Marital Status | Employment Status | Family History of Cancer | Family History of Breast Cancer | Cancer Stage | Type of Surgery | Chemotherapy | Radiation Therapy | MenopausalStatus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤40 (n = 58) | >40 (n = 241) | High school or below (n = 114) | College and above (n = 185) | No (n = 40) | Yes (n = 257) | No (n = 146) | Yes (n = 153) | No (n = 169) | Yes (n = 130) | No (n = 267) | No (n = 226) | Yes (n = 73) | Invasive (n = 260) | Mastectomy (n = 57) | Conservation (n = 242) | No (n = 199) | Yes (n = 100) | No (n = 51) | Yes (n = 248) | No (n = 226) | Yes (n = 73) | |
| Stress (n = 217) | 74.1 | 69.3 | 69.3 | 70.8 | 67.5 | 70.4 | 69.9 | 70.6 | 69.8 | 70.8 | 70.8 | 65.6 | 69.2 | 70.4 | 66.7 | 71.1 | 71.4 | 68.0 | 72.5 | 69.8 | 72.6 | 63.0 |
| Diet (n = 138) | 56.9 | 42.7 | 46.5 | 44.9 | 50.0 | 45.1 | 42.5 | 48.4 | 46.2 | 44.6 | 46.8 | 34.4 | 38.5 | 46.5 | 49.1 | 44.6 | 47.7 | 41.0 | 56.9 | 43.1 | 46.5 | 42.5 |
| Exercise (n = 68) | 15.5 | 24.5 | 23.7 | 22.2 | 15.8 | 24.1 | 22.6 | 22.9 | 24.9 | 20.0 | 22.5 | 25.0 | 25.6 | 22.3 | 19.3 | 23.6 | 23.1 | 22.0 | 23.5 | 22.6 | 21.7 | 26.0 |
| Work-related problems (n = 34) | 8.6 | 12 | 9.6 | 12.4 | 12.5 | 10.9 | 8.2 | 14.4 | 13.0 | 9.2 | 12.0 | 6.3 | 7.7 | 11.9 | 8.8 | 12.0 | 9.0 | 16.0 | 7.8 | 12.1 | 10.6 | 13.7 |
| Weight control (n = 29) | 12.1 | 9.1 | 7.9 | 10.8 | 5.0 | 10.5 | 11.6 | 7.8 | 7.7 | 12.3 | 9.0 | 15.6 | 10.3 | 9.6 | 10.5 | 9.5 | 8.5 | 12.0 | 5.9 | 10.5 | 9.7 | 9.6 |
| Sleep (n = 27) | 13.8 | 7.9 | 7.0 | 10.3 | 17.5 | 7.8 | 8.9 | 9.2 | 6.5 | 12.3 | 9.0 | 9.4 | 7.7 | 9.2 | 14.0 | 7.9 | 8.5 | 10.0 | 9.8 | 8.9 | 10.6 | 4.1 |
| Alcohol/Smoking (n = 26) | 6.9 | 9.1 | 9.6 | 8.1 | 15.0 | 7.0 | 11.0 | 6.5 | 9.5 | 7.7 | 9.4 | 3.1 | 5.1 | 9.2 | 7.0 | 9.1 | 6.5 | 13.0 | 2.0 | 10.1 | 9.3 | 6.8 |
| Personality (n = 25) | 3.4 | 8.7 | 7.0 | 8.1 | 2.5 | 8.6 | 7.5 | 7.8 | 4.7 * | 11.5 * | 6.7 | 15.6 | 10.3 | 7.3 | 7.0 | 7.9 | 8.0 | 7.0 | 3.9 | 8.5 | 7.1 | 9.6 |
| Genetics/Family history (n = 21) | 6.9 | 6.6 | 4.4 | 8.6 | 5.0 | 7.4 | 7.5 | 6.5 | 3.6 ** | 11.5 ** | 4.1 *** | 31.3 *** | 7.7 | 6.9 | 7.0 | 7.0 | 6.0 | 9.0 | 5.9 | 7.3 | 8.4 | 2.7 |
| Environmental Factors (n = 20) | 3.4 | 7.1 | 5.3 | 7.0 | 2.5 | 7.0 | 2.7 * | 9.8 * | 7.7 | 4.6 | 6.4 | 6.3 | 7.7 | 6.2 | 5.3 | 6.6 | 7.5 | 4.0 | 5.9 | 6.5 | 6.2 | 6.8 |
| Lifestyle in general (n = 20) | 6.9 | 6.6 | 9.6 | 4.9 | 12.5 | 5.8 | 5.5 | 7.8 | 8.3 | 4.6 | 7.1 | 3.1 | 5.1 | 6.9 | 7.0 | 6.6 | 6.0 | 8.0 | 3.9 | 7.3 | 7.1 | 5.5 |
| Hormones (n = 18) | 5.2 | 6.2 | 3.5 | 7.6 | 7.5 | 5.8 | 7.5 | 4.6 | 4.1 | 8.5 | 6.4 | 3.1 | 12.8 | 5.0 | 3.5 | 6.6 | 7.5 | 3.0 | 2.0 | 6.9 | 5.3 | 8.2 |
| Lack of prevention (n = 17) | 5.2 | 5.4 | 5.3 | 5.4 | 2.5 | 5.8 | 5.5 | 5.2 | 5.9 | 4.6 | 6.0 | 0.0 | 5.1 | 5.4 | 5.3 | 5.4 | 6.5 | 3.0 | 5.9 | 5.2 | 3.5 * | 11.0 * |
| Breastfeeding (n = 16) | 5.2 | 5.4 | 2.6 | 7.0 | 0.0 | 6.2 | 4.8 | 5.9 | 7.1 | 3.1 | 5.6 | 3.1 | 5.1 | 5.4 | 5.3 | 5.4 | 5.0 | 6.0 | 2.0 | 6.0 | 6.2 | 2.7 |
| Reproductive history (n = 14) | 3.4 | 4.6 | 2.6 | 5.4 | 10.0 | 3.5 | 2.7 | 5.9 | 7.1 ** | 0.8 ** | 4.5 | 3.1 | 2.6 | 4.6 | 0.0 | 5.4 | 5.0 | 3.0 | 2.0 | 4.8 | 4.0 | 5.5 |
| Poor organ function (n = 7) | 1.7 | 2.5 | 1.8 | 2.7 | 0.0 | 2.7 | 2.7 | 2.0 | 3.0 | 1.5 | 2.6 | 0.0 | 2.6 | 2.3 | 1.8 | 2.5 | 3.0 | 1.0 | 2.0 | 2.4 | 2.2 | 2.7 |
| Emotion (n = 3) | 0 | 1.2 | 0.9 | 1.1 | 0.0 | 1.2 | 2.1 | 0.0 | 0.0 | 2.3 | 0.7 | 3.1 | 0.0 | 1.2 | 3.5 | 0.4 | 1.5 | 0.0 | 2.0 | 0.8 | 0.4 | 2.7 |
| Existential influences/Fates (n = 2) | 0 | 0.8 | 0.9 | 0.5 | 0.0 | 0.8 | 0.0 | 1.3 | 0.6 | 0.8 | 0.7 | 0.0 | 0.0 | 0.8 | 0.0 | 0.8 | 1.0 | 0.0 | 0.0 | 0.8 | 0.4 | 1.4 |
| Health condition (n = 2) | 0 | 0.8 | 0.9 | 0.5 | 0.0 | 0.8 | 0.7 | 0.7 | 1.2 | 0.0 | 0.7 | 0.0 | 0.0 | 0.8 | 0.0 | 0.8 | 1.0 | 0.0 | 0.0 | 0.8 | 0.9 | 0.0 |
| Breast condition (n = 1) | 1.7 | 0 | 0.0 | 0.5 | 0.0 | 0.4 | 0.7 | 0.0 | 0.6 | 0.0 | 0.4 | 0.0 | 0.0 | 0.4 | 1.8 | 0.0 | 0.0 | 1.0 | 0.0 | 0.4 | 0.4 | 0.0 |
| Restrictive clothing (n = 1) | 1.7 | 0 | 0.0 | 0.5 | 0.0 | 0.4 | 0.0 | 0.7 | 0.6 | 0.0 | 0.4 | 0.0 | 0.0 | 0.4 | 0.0 | 0.4 | 0.0 | 1.0 | 0.0 | 0.4 | 0.4 | 0.0 |
| No causal attribution (n = 1) | 0 | 0.4 | 0.0 | 0.5 | 0.0 | 0.4 | 0.7 | 0.0 | 0.6 | 0.0 | 0.4 | 0.0 | 0.0 | 0.4 | 0.0 | 0.4 | 0.0 | 1.0 | 0.0 | 0.4 | 0.0 | 1.4 |
| No Family History of Cancer (n = 169) | Family History of Cancer (n = 130) | ||||||
|---|---|---|---|---|---|---|---|
| Causes | Frequency | Causes | Degree | Causes | Frequency | Causes | Degree |
| Stress | 120 | Diet | 16 | Stress | 97 | Stress | 15 |
| Diet | 80 | Stress | 16 | Diet | 58 | Diet | 14 |
| Exercise | 42 | Exercise | 12 | Exercise | 26 | Genetics/Family history | 12 |
| Work-related problems | 22 | Breastfeeding | 10 | Personality | 17 | Exercise | 11 |
| Alcohol/Smoking | 16 | Environmental Factors | 9 | Weight control | 16 | Personality | 11 |
| Environmental Factors | 14 | Lifestyle in general | 9 | Sleep | 16 | Weight control | 11 |
| Lifestyle in general | 14 | Alcohol/Smoking | 9 | Genetics/Family history | 15 | Alcohol/Smoking | 9 |
| Weight control | 13 | Work-related problems | 9 | Work-related problems | 12 | Sleep | 8 |
| Reproductive history | 13 | Reproductive history | 9 | Hormones | 11 | Hormones | 8 |
| Breastfeeding | 12 | Weight control | 8 | Alcohol/Smoking | 10 | Work-related problems | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.K.; Min, Y.H.; Lee, M.; Lee, S.B. Causal Attributions in Breast Cancer Patients Planning to Undergo Adjuvant Endocrine Therapy. Int. J. Environ. Res. Public Health 2021, 18, 5931. https://doi.org/10.3390/ijerph18115931
Park SK, Min YH, Lee M, Lee SB. Causal Attributions in Breast Cancer Patients Planning to Undergo Adjuvant Endocrine Therapy. International Journal of Environmental Research and Public Health. 2021; 18(11):5931. https://doi.org/10.3390/ijerph18115931
Chicago/Turabian StylePark, Seul Ki, Yul Ha Min, Minsun Lee, and Sae Byul Lee. 2021. "Causal Attributions in Breast Cancer Patients Planning to Undergo Adjuvant Endocrine Therapy" International Journal of Environmental Research and Public Health 18, no. 11: 5931. https://doi.org/10.3390/ijerph18115931
APA StylePark, S. K., Min, Y. H., Lee, M., & Lee, S. B. (2021). Causal Attributions in Breast Cancer Patients Planning to Undergo Adjuvant Endocrine Therapy. International Journal of Environmental Research and Public Health, 18(11), 5931. https://doi.org/10.3390/ijerph18115931






