Differential Laboratory Diagnosis of Acute Fever in Guinea: Preparedness for the Threat of Hemorrhagic Fevers
Abstract
:1. Introduction
2. Materials and Methods
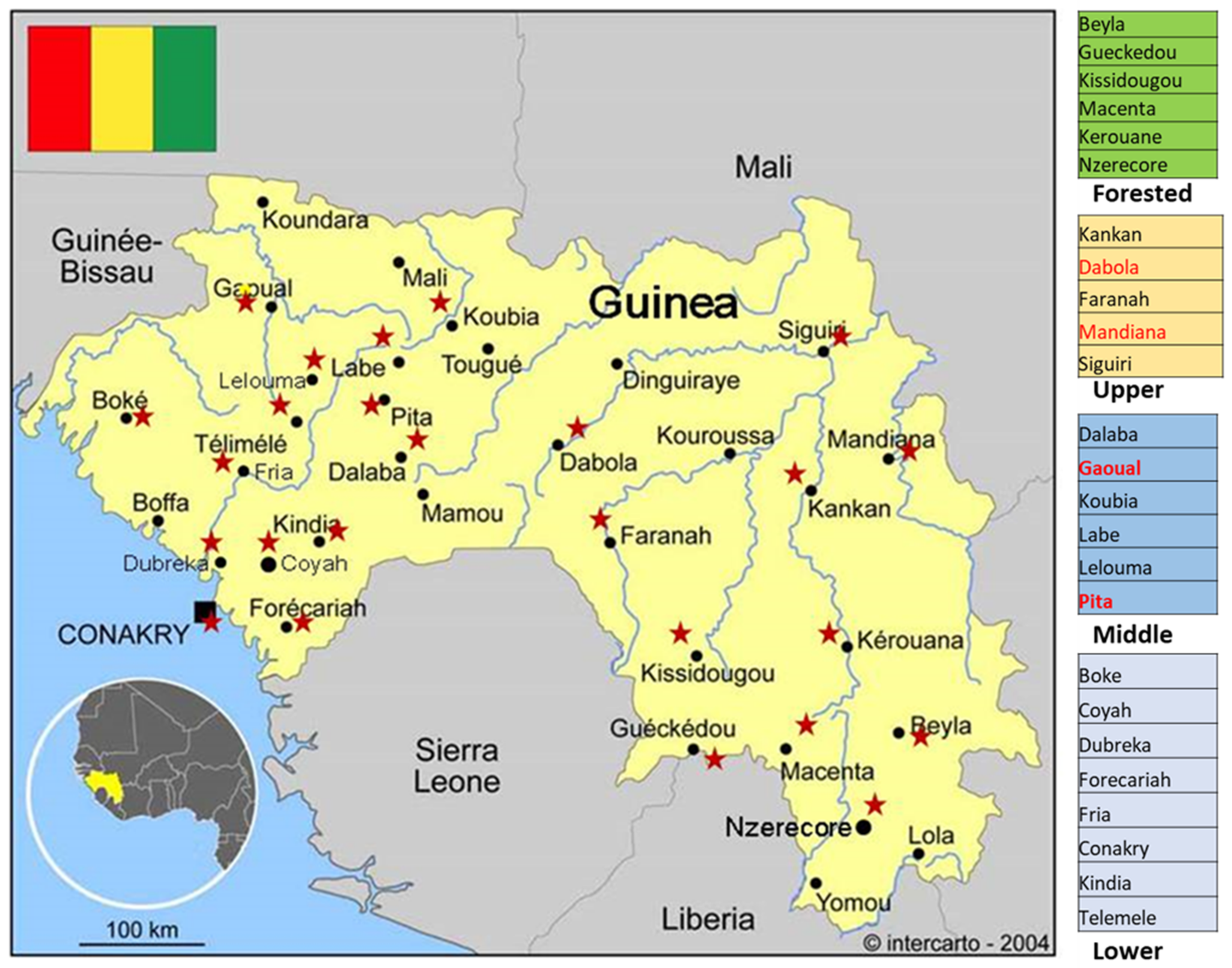
2.1. Sampling
2.2. Nucleic Acid Extraction
2.3. Molecular Analysis Using Real-Time Polymerase Chain Reaction
2.4. Serological Study Using Enzyme-Linked Immunosorbent Assay
2.5. Serological Study Using Protein Microarray
2.5.1. Design of the Planar Protein Microarray
2.5.2. Microarray Production and Processing
2.5.3. Data Quantification
2.5.4. Interpretation of Microarray Data
2.6. Statistical Analysis
3. Results
4. Discussion
“In 1978–1991, the USSR–Guinea Virological and Microbiological Laboratory functioned in Kindia, the Republic of Guinea. … About 74,000 mosquitoes, 100,000 Ixodidae ticks, 1500 wild birds, 2700 bats, 106 monkeys, 308 other mammals, and 927 blood samples collected from febrile patients were examined in 1978–1989, using inoculation of new-born white mice. As a result of this work, 127 strains of the following arboviruses were isolated: Chikungunia (one strain), Dengue 2 (four), Saboya (seven), Wesselsbron (one), Bunyamwera (four), M’Poko (five), Rift Valley Fever (six), CHF-Congo (nine), Dugbe (22), Bhanja (six), Forecariah (two), Jos (26), Abadina (15), Kindia (two), Ark 6956 (one), Fomede (two), Bluetongue (nine), Mossuril (two), AnK 6009 (one), and Kolente (two). Dengue 2, Wesselsbron, Bunyamwera, M’Poko, Kindia, and Mossuril viruses were isolated from mosquitoes. Ixodidae ticks were sources for isolation of Chikungunia, Saboya, CCHF, Dugbe, Bhanja, Forecaciah, Jos, Abadina, Kindia, Ark 6956, Fomede, Bluetongue, and Kolente viruses. Saboya, RVF, Fomede, Kolente, and AnK 6909 were isolated from bats (Chiroptera); Saboya, Abadina, and Bluetongue viruses were isolated from birds. One strain of Dugbe virus was originated from the brain of Cercopithecus patas. Bunyamwera and Abadina viruses were isolated from the blood of two febrile patients. Serological identification of many strains was kindly conducted at the Pasteur Institute, Dakar (J. P.Digoutte) and some at the YARU, USA (R. Shope)”.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/countries/gin/en/ (accessed on 16 April 2021).
- World Health Organization. Available online: http://gamapserver.who.int/gho/interactive_charts/mbd/life_expectancy/atlas.html (accessed on 16 April 2021).
- World Health Organization. Available online: http://apps.who.int/gho/data/node.country.country-GIN (accessed on 16 April 2021).
- Parpia, A.S.; Ndeffo-Mbah, M.L.; Wenzel, N.S.; Galvani, A.P. Effects of Response to 2014–2015 Ebola Outbreak on Deaths from Malaria, HIV/AIDS, and Tuberculosis, West Africa. Emerg. Infect. Dis. 2016, 3, 433–441. [Google Scholar] [CrossRef]
- Jentes, E.S.; Robinson, J.; Johnson, B.W.; Conde, I.; Sakouvougui, Y.; Iverson, J.; Beecher, S.; Bah, M.A.; Diakite, F.; Coulibaly, M.; et al. Acute Arboviral Infections in Guinea, West Africa. Am. J. Trop. Med. Hyg. 2006, 83, 388–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafri, I.; El Hamzaoui, B.; Bitam, I.; Leulmi, H.; Lalout, R.; Mediannikov, O.; Chergui, M.; Karakellah, M.; Raoult, D.; Parola, F. Detection of Relapsing Fever Borrelia spp., Bartonella spp. and Anaplasmataceae Bacteria in Argasid Ticks in Algeria. PLoS Negl. Trop. Dis. 2017, 11, e0006064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutler, S.J.; Bonilla, E.B.; Singh, R.J. Population Structure of East African Relapsing Fever Borrelia spp. Emerg. Infect. Dis. 2010, 16, 1076–1080. [Google Scholar] [CrossRef]
- Ehounoud, C.B.; Yao, K.P.; Dahmani, M.; Achi, Y.L.; Amanzougaghene, N.; N’Douba, A.K.; N’Guessan, J.D.; Raoult, D.; Fenollar, F.; Mediannikov, O. Multiple Pathogens Including Potential New Species in Tick Vectors in Côted’Ivoire. PLoS Negl. Trop. Dis. 2016, 10, e0004367. [Google Scholar] [CrossRef] [Green Version]
- Shoman, H.; Karafillakis, E.; Rawaf, S. The Link Between the West African Ebola Outbreak and Health Systems in Guinea, Liberia and Sierra Leone: A Systematic Review. Glob. Health 2017, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Bempong, N.E.; De Castañeda, R.; Schütte, S.; Bolon, I.; Keiser, O.; Escher, G.; Flahault, A. Precision Global Health—The Case of Ebola: A Scoping Review. J. Glob. Health 2019, 9, 010404. [Google Scholar] [CrossRef] [PubMed]
- Coltart, C.E.M.; Lindsey, B.; Ghinai, I.; Johnson, A.M.; Heymann, D.L. The Ebola Outbreak, 2013–2016: Old Lessons for New Epidemics. Phil. Trans. R. Soc. 2017, 372, 20160297. [Google Scholar] [CrossRef]
- Faye, O.; Boëlle, P.Y.; Heleze, E.; Faye, O.; Loucoubar, C.; Magassouba, N.; Soropogui, B.; Keita, S.; Gakou, T.; Bah el, H.I.; et al. Chains of Transmission and Control of Ebola Virus Disease in Conakry, Guinea, in 2014: An Observational Study. Lancet Infect. Dis. 2015, 15, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Dedkov, V.G.; Magassouba, N.F.; Safonova, M.V.; Deviatkin, A.A.; Dolgova, A.S.; Pyankov, O.V.; Sergeev, A.A.; Utkin, D.V.; Odinokov, G.N.; Safronov, V.A.; et al. Development and Evaluation of a Real-time RT-PCR Assay for the Detection of Ebola Virus (Zaire) During an Ebola Outbreak in Guinea in 2014–2015. J. Virol. Methods 2016, 228, 26–30. [Google Scholar] [CrossRef]
- Barzon, L. Ongoing and emerging arbovirus threats in Europe. J. Clin. Virol. 2018, 107, 38–47. [Google Scholar] [CrossRef]
- Napoli, C.; Salcuni, P.; Pompa, M.G.; Declich, S.; Rizzo, C. Estimated imported infections of Chikungunya and Dengue in Italy, 2008 to 2011. J. Travel Med. 2012, 19, 294–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortuna, C.; Remoli, M.E.; Rizzo, C.; Benedetti, E.; Fiorentini, C.; Bella, A.; Argentini, C.; Farchi, F.; Castilletti, C.; Capobianchi, M.R.; et al. Imported arboviral infections in Italy, July 2014–October 2015: A National Reference Laboratory report. BMC Infect. Dis. 2017, 17, 216. [Google Scholar] [CrossRef] [Green Version]
- Dedkov, V.G.; Magassouba, N.; Safonova, M.V.; Bodnev, S.A.; Pyankov, O.V.; Camara, J.; Sylla, B.; Agafonov, A.P.; Maleev, V.V.; Shipulin, G.A. Sensitive Multiplex Real-time RT-qPCR Assay for the Detection of Filoviruses. Health Secur. 2018, 16, 14–21. [Google Scholar] [CrossRef]
- Ölschläger, S.; Lelke, M.; Emmerich, P.; Panning, M.; Drosten, C.; Hass, M.; Asogun, D.; Ehichioya, D.; Omilabu, S.; Günther, S. Improved Detection of Lassa Virus by Reverse Transcription-PCR Targeting the 5′ Region of S RNA. J. Clin. Microbiol. 2010, 48, 2009–2013. [Google Scholar] [CrossRef] [Green Version]
- Panning, M.; Emmerich, P.; Ölschläger, S.; Bojenko, S.; Koivogui, L.; Marx, A.; Lugala, P.T.; Günther, S.; Bausch, D.G.; Drosten, C. Laboratory Diagnosis of Lassa Fever, Liberia. Emerg. Infect. Dis. 2010, 16, 1041–1043. [Google Scholar] [CrossRef]
- World Health Organization. Manual for the Monitoring of Yellow Fever Virus Infection. 2004. Available online: https://apps.who.int/iris/bitstream/handle/10665/68715/WHO_IVB_04.08.pdf?sequence=1 (accessed on 16 April 2021).
- Vladyko, A.S.; Scheslenok, E.P.; Fomina, E.G.; Semizhon, P.A.; Ignatyev, G.M.; Shkolina, T.V.; Krasko, A.G.; Semenov, S.F.; Vinokurova, N.V. Detection and Antigenic Characteristics of the Recombinant Nucleocapsid Proteins of Lassa and Marburg Viruses. Probl. Virol. 2012, 57, 41–44. Available online: https://cyberleninka.ru/article/n/poluchenie-i-antigennaya-harakteristika-rekombinantnyh-nukleokapsidnyh-belkov-virusov-lassa-i-marburg/viewer (accessed on 16 April 2021). (In Russian).
- Stukolova, O.; Karan, L.; Magassouba, N.F.; Goptar, I.; Sudina, A.; Dedkov, V.; Dolgova, A.; Cherkashina, A.; Boiro, M.Y.; Shipulin, G. Detection of IgM and IgG in the Sera Samples from Patients with Unspecified Fever Collected in Republic of Guinea, Using the Developed Microarray Based on the Recombinant Antigens of Arboviruses (ZIKV, DV, CHIKV, CCFV, RVFV). Int. J. Infect. Dis. 2018, 73, 168. [Google Scholar] [CrossRef]
- Tchekanova, T.A.; Markelov, M.L.; Karan, L.S.; Ushakova, M.A.; Pudova, E.A.; Romashkina, A.S.; Kirdiyashkina, N.P.; Manzeniyuk, I.N.; Sajin, A.I.; Snarskaya, E.S.; et al. The New Possibilities in Serological Diagnostic of Ixodes Mite-borne Borreliosis Using Immunochip. Klin. Lab. Diagn. 2013, 12, 51–56. Available online: https://cyberleninka.ru/article/n/novye-vozmozhnosti-v-serologicheskoy-diagnostike-ikso-dovyh-kleschevyh-borreliozov-s-ispolzovaniem-immunochipa/viewer (accessed on 16 April 2021). (In Russian).
- Koetsveld, J.; Kolyasnikova, N.M.; Wagemakers, A.; Stukolova, O.A.; Hoornstra, D.; Sarksyan, D.S.; Toporkova, M.G.; Henningsson, A.J.; Hvidsten, D.; Ang, W.; et al. Serodiagnosis of Borrelia miyamotoi Disease by Measuring Antibodies against GlpQ and Variable Major Proteins. Clin. Microbiol. Infect. 2018, 24, 1338.e1–1338.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardizzoni, A.; Cappuccini, B.; Baschieri, M.C.; Orsi, C.F.; Rumpianesi, F.; Peppoloni, S.; Carmelli, C.; Meacci, M.; Crisanti, A.; Steensgaard, P.; et al. A Protein Microarray for Serological Evaluation of Antibody Response in Vertically Transmitted Infectioons. Eur. J. Microbiol. Infect. Dis. 2009, 28, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Rubina, A.Y.; Dementieva, E.I.; Stomakhin, A.A.; Darii, E.L.; Pan’kov, S.V.; Barsky, V.E.; Ivanov, S.M.; Konovalova, E.V.; Mirzabekov, A.D. Hydrogel-Based Protein Microchips: Manufacturing, Properties, and Applications. Biotechniques 2003, 34, 1008–1022. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.S.; White, A.M.; Varnum, S.M.; Anderson, K.K.; Zangar, R.C. Evaluating concentration estimation errors in ELISA microarray experiments. BMC Bioinform. 2005, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Molochkov, A.V.; Tchekanova, T.A.; Markelov, M.L.; Karan, L.S.; Snarskaya, E.S.; Romashkina, A.S. Detection of Borrelia Infection by an Innovation Test System (Immunochip) in Patients with Scleroatrophic Lessons of the Skin. Ross. Zhurnal Kozhnykh Vener. Bolezn. 2012, 2, 32–35. Available online: https://elibrary.ru/download/elibrary_18052578_32377450.pdf (accessed on 16 April 2021). (In Russian).
- Stukolova, O.; Koetsveld, J.; Kolyasnikova, N.; Sarksyan, D.; Toporkova, M.; Karan, L.; Dolgova, A.; Markelov, M.; Hovius, J.; Shipulin, G.; et al. Antibody Response in Borrelia miyamotoi Infection Studied by Protein Microarray. Int. J. Infect. Dis. 2019, 79, 18. [Google Scholar] [CrossRef] [Green Version]
- Stukolova, O.; Goptar, I.; Karan, L.; Kotenev, E.; Sudina, A.; Polugalova, M.; Kulichenko, A.; Boiro, M.Y.; Shipulin, G. Development and Validation of Microarray-based Serological Assay for Crimean-Congo Hemorrhagic Fver (CCF) and Determination of the Prevalence of CCFV in Guinea. Int. J. Infect. Dis. 2019, 79, 14. [Google Scholar] [CrossRef] [Green Version]
- Dolgova, A.; Stukolova, O.; Karan, L.; Grigoreva, I.; Shipulin, G.; Maleev, V. Development of the Microarray Technology for Investigation of Antibody Responses During Zika virus Infection and of anti-Flaviviruses IgM/IgG Cross-reactivity. Int. J. Infect. Dis. 2018, 73, 168. [Google Scholar] [CrossRef]
- Cherkashina, A.S.; Vaskina, M.I.; Bulanenko, V.A.; Soloveva, E.D.; Sudina, A.E.; Stukolova, O.A.; Karan, L.S. Obtaining recombinant antigens for the diagnosis of West Nile fever. In Molecular Diagnostics and Biosafety-2020: Collection of Materials, Moscow, March 19–20, 2020; Akimkin, V.G., Tvorogova, M.G., Eds.; FBIS CRIE of FSSCRPHW: Moscow, Russia, 2020; p. 102. ISBN 978-5-9900432-8-2. Available online: http://www.crie.ru/pdf/news/mdb2020-thezis-preview.pdf (accessed on 16 April 2021). (In Russian)
- Newcombe, R.G. Two-Sided Confidence Intervals for the Single Proportion: Comparison of Seven Methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]
- Brown, L.D.; Cat, T.T.; DasGupta, A. Interval Estimation for a proportion. Stat. Sci. 2001, 16, 101–117. Available online: www.jstor.org/stable/2676784 (accessed on 16 April 2021). [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the p Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safronetz, D.; Sacko, M.; Sogoba, N.; Rosenke, K.; Martellaro, C.; Traoré, S.; Cissé, I.; Maiga, O.; Boisen, M.; Nelson, D.; et al. Vectorborne Infections, Mali. Emerg. Infect. Dis. 2016, 22, 340–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mari Saez, A.; Cherif Haidara, M.; Camara, A.; Kourouma, F.; Sage, M.; Magassouba, N.; Fichet-Calvet, E. Rodent control to fight Lassa fever: Evaluation and lessons learned from a 4-year study in Upper Guinea. PLoS Negl. Trop. Dis. 2018, 12, e0006829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoepp, R.J.; Rossi, C.A.; Khan, S.H.; Goba, A.; Fair, J.N. Undiagnosed acute viral febrile illnesses, Sierra Leone. Emerg. Infect. Dis. 2014, 20, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Yellow Fever in Africa and Central and South America, 2008–2009. Wkly. Epidemiol. Rec. 2011, 86, 25–36. Available online: https://www.who.int/wer/2011/wer8604.pdf?ua=1 (accessed on 16 April 2021).
- Hwang, J.; Ryu, H.S.; Kim, H.; Lee, S.A. The first reported case of West Nile encephalitis in Korea. J. Korean Med. Sci. 2015, 30, 343–345. [Google Scholar] [CrossRef] [Green Version]
- Herrera, B.B.; Chang, C.A.; Hamel, D.J.; Mboup, S.; Ndiaye, D.; Imade, G.; Okpokwu, J.; Agbaji, O.; Bei, A.K.; Kanki, P.J. Continued Transmission of Zika Virus in Humans in West Africa, 1992–2016. J. Infect. Dis. 2017, 215, 1546–1550. [Google Scholar] [CrossRef]
- Fritzell, C.; Rousset, D.; Adde, A.; Kazanji, M.; van Kerkhove, M.D.; Flamand, C. Current challenges and implications for dengue, chikungunya and Zika seroprevalence studies worldwide: A scoping review. PLoS Negl. Trop. Dis. 2018, 12, e0006533. [Google Scholar] [CrossRef] [Green Version]
- Sergeeva, E.I.; Ternovoi, V.A.; Chausov, E.V.; Berillo, S.A.; Demina, O.K.; Shikov, A.N.; Plasunova, I.V.; Kartashov, M.J.; Agafonov, A.P. Imported cases of dengue fever in Russia during 2010–2013. Asian Pac. J. Trop. Med. 2015, 8, 90–93. [Google Scholar] [CrossRef]
- Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing. On the State of Sanitary and Epidemiological Well-Being of the Population of the Russian Federation in 2018: State Report; Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing: Moscow, Russia, 2019; ISBN 978-5-7508-1681-1. Available online: https://www.rospotrebnadzor.ru/upload/iblock/798/gosudarstvennyy-doklad-o-sostoyanii-sanitarno_epidemiologicheskogo-blagopoluchiya-naseleniya-v-rossiyskoy-federatsii-v-2018-godu.pdf (accessed on 27 May 2021). (In Russian)
- Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing. On the State of Sanitary and Epidemiological Well-Being of the Population of the Russian Federation in 2019: State Report; Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing: Moscow, Russia, 2020; ISBN 978-5-7508-1742-9. Available online: https://www.rospotrebnadzor.ru/upload/iblock/8e4/gosdoklad-za-2019_seb_29_05.pdf (accessed on 27 May 2021). (In Russian)
- Sokhna, C.; Mediannikov, O.; Fenollar, F.; Bassene, H.; Diatta, G.; Tall, A.; Trape, J.F.; Drancourt, M.; Raoult, D. Point-of-care laboratory of pathogen diagnosis in rural Senegal. PLoS Negl. Trop. Dis. 2013, 7, e1999. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Lebuisson, A.; Menager, C.; Moulin, F.; Dupouy Camet, J. Fever in a 7-year-old girl returning from Mali. Clin. Infect. Dis. 2008, 47, 1490–1491. [Google Scholar] [CrossRef]
- Million, M.; Cazorla, C.; Doudier, B.; La Scola, B.; Parola, P.; Drancourt, M.; Brouqui, P. Molecular identification of Borrelia crocidurae in a patient returning from Senegal. BMJ Case Rep. 2009, 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tordini, G.; Giaccherini, R.; Corbisiero, R.; Zanelli, G. Relapsing fever in a traveller from Senegal: Determination of Borrelia species using molecular methods. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 992–994. [Google Scholar] [CrossRef] [Green Version]
- Cherry, C.C.; Denison, A.M.; Kato, C.Y.; Thornton, K.; Paddock, C.D. Diagnosis of spotted fever group rickettsioses in U.S. travelers returning from Africa, 2007–2016. Am. J. Trop. Med. Hyg. 2018, 99, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ángel-Moreno, A.; Bolaños, M.; Santana, E.; Pérez-Arellano, J.L. Tifus murino importado de Senegal en un inmigrante viajero. Enferm. Infecc. Microbiol. Clínica 2006, 24, 406–407. [Google Scholar] [CrossRef]
- Martell, H.J.; Masterson, S.G.; McGreig, J.E.; Michaelis, M.; Wass, M.N. Is the bombali virus pathogenic in humans? Bioinformatics 2019, 35, 3553–3558. [Google Scholar] [CrossRef]
- Butenko, A.M. Arbovirus circulation in the Republic of Guinea. Meditsinskaia Parazitol. Parazit. Bolezn. 1996, 2, 40–45. (In Russian) [Google Scholar]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- MacLeod, I.J.; Rowley, C.F.; Essex, M. PANDAA intentionally violates conventional qPCR design to enable durable, mismatch-agnostic detection of highly polymorphic pathogens. Commun. Biol. 2021, 4, 227. [Google Scholar] [CrossRef]
- Pigott, D.M.; Deshpande, A.; Letourneau, I.; Morozoff, C.; Reiner, R.C., Jr.; Kraemer, M.U.G.; Brent, S.E.; Bogoch, I.I.; Khan, K.; Biehl, M.H.; et al. Local, national, and regional viral haemorrhagic fever pandemic potential in Africa: A multistage analysis. Lancet 2017, 390, 2662–2672. [Google Scholar] [CrossRef] [Green Version]

| Infections and Methods * | YFD | CCHFD | LFD | DFD | ZIKFD | EVD | MFD | WNFD | RVFD | CHIKF | SFG Rick. | Bor. spp. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polymerase chain reaction | PCR Amplisens | PCR Ölschläger | PCR Amplisens | PCR Amplisens | PCR Amplisens | PCR Amplisens | ||||||
| ELISA | MAC-ELISA IgM capture assay | ReLASV® Pan-Lassa IgG/IgM ELISA | ||||||||||
| Protein microarray, IgM | Array | Array | Array | Array | Array | Array | Array | Array | Array | Array |
| Antigens Used in Microarray | IgM Antibody to: | IgM Antibody to: | IgM Antibody to: | Conclusion * |
|---|---|---|---|---|
| Bor. spp. antigens | OspC alone or with any other antigen | two of three (p41, p17, VlsE) | GlpQ and at least one antigen in a set (p39, p41, VlsE, Vsp1, Vlp5, Vlp15/16, Vlp18) | any of the options on the left: Bor. spp. IgM present; none of the options on the left: Bor. spp. IgM absent |
| SFG rickettsia antigens | OmpA | OmpB | any of the options on the left: SFG rickettsia IgM present; none of the options on the left: SFG rickettsia IgM absent | |
| ZEBOV antigen | NP | if present—ZEBOV IgM present; if absent—ZEBOV IgM absent | ||
| MARV antigen | NP | if present—MARV IgM present; if absent—MARV IgM absent | ||
| CCHFV antigens | NP and/or NPsh | any number of G-antigens and L-protein | any of the options on the left: CCHFV IgM present; none of the options on the left: CCHFV IgM absent | |
| WNFV antigen | NS1 | if present—WNFV IgM present; if absent—WNFV IgM absent | ||
| DENV antigens | any NS1 | if present DENV IgM present; if absent—DENV IgM absent | ||
| ZIKV antigen | NS1 | if present—ZIKV IgM present; if absent—ZIKV IgM absent | ||
| unspecified flaviviruses | any DENV E | ZIKV E | any of the options on the left: IgM to unspecified flaviviruses present | |
| CHIKV antigens | E1 | E2 | any of the options on the left: CHIKV IgM present; none of the options on the left: CHIKV IgM absent | |
| RVFV antigens | NP | NPsh | any of the options on the left: RVFV IgM present; none of the options on the left: RVFV IgM absent |
| Pathogen | Number of Samples from PCR-Confirmed Patients | Sensitivity, %, and 95% Confidence Interval (In Parentheses) | Number of Samples from Healthy Donors | Specificity, %, and 95% Confidence Interval (In Parentheses) |
|---|---|---|---|---|
| Borrelia spp.(acute) | 132 | 66 (57.5–73.4) | 300 | 97 (94.4–98.4) |
| SFG rickettsia | 100 | 72 (62.5–80.0) | 200 | 98 (95.0–99.2) |
| DENV | 60 | 72 (59.2–81.5) | 100 | 98 (93.0–99.5) |
| ZIKV | 30 | 83 (66.4–92.7) | 100 | 98 (93.0–99.5) |
| CHIKV | 4 | 75 (30.1–95.4) | 100 | 98 (93.0–99.5) |
| CCHFV | 20 | 85 (64.0–94.8) | 100 | 98 (93.0–99.5) |
| WNFV | 12 | 67 (39.1–86.2) | 100 | 98 (93.0–99.5) |
| RVFV | not available | - | 100 | 98 (93.0–99.5) |
| ZEBOV | 3 | 100 (43.8–100) | 100 | 98 (93.0–99.5) |
| MARV | not available | - | 100 | 98 (93.0–99.5) |
| Part of Guinea | Total Number of Samples | Males | Females | YFD | CCHFD | LFD | DFD | ZIKFD | SFG Rick. | Bor. spp. | Bor. spp. + SFG Rick. | DFD + CHIKFD | RVFD + Bor. spp. | WNFD+ Bor. spp. + SFG Rick. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forested | 24 | 15 | 9 | 1 | 0 | 0 | 0 | 0 | 3 | 3 | 1 | 0 | 0 | 0 |
| Upper | 23 | 11 | 12 | 1 | 0 | 4 | 0 | 1 | 2 | 1 | 3 | 0 | 0 | 2 |
| Middle | 32 | 17 | 15 | 5 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 2 | 0 |
| Lower | 85 | 48 | 37 | 13 | 0 | 1 | 0 | 1 | 3 | 8 | 4 | 1 | 0 | 0 |
| Total | 164 | 91 | 73 | 20 | 1 | 7 | 1 | 2 | 8 | 13 | 9 | 2 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedkov, V.G.; Magassouba, N.; Stukolova, O.A.; Savina, V.A.; Camara, J.; Soropogui, B.; Safonova, M.V.; Semizhon, P.; Platonov, A.E. Differential Laboratory Diagnosis of Acute Fever in Guinea: Preparedness for the Threat of Hemorrhagic Fevers. Int. J. Environ. Res. Public Health 2021, 18, 6022. https://doi.org/10.3390/ijerph18116022
Dedkov VG, Magassouba N, Stukolova OA, Savina VA, Camara J, Soropogui B, Safonova MV, Semizhon P, Platonov AE. Differential Laboratory Diagnosis of Acute Fever in Guinea: Preparedness for the Threat of Hemorrhagic Fevers. International Journal of Environmental Research and Public Health. 2021; 18(11):6022. https://doi.org/10.3390/ijerph18116022
Chicago/Turabian StyleDedkov, Vladimir G., N’Faly Magassouba, Olga A. Stukolova, Victoria A. Savina, Jakob Camara, Barrè Soropogui, Marina V. Safonova, Pavel Semizhon, and Alexander E. Platonov. 2021. "Differential Laboratory Diagnosis of Acute Fever in Guinea: Preparedness for the Threat of Hemorrhagic Fevers" International Journal of Environmental Research and Public Health 18, no. 11: 6022. https://doi.org/10.3390/ijerph18116022
APA StyleDedkov, V. G., Magassouba, N., Stukolova, O. A., Savina, V. A., Camara, J., Soropogui, B., Safonova, M. V., Semizhon, P., & Platonov, A. E. (2021). Differential Laboratory Diagnosis of Acute Fever in Guinea: Preparedness for the Threat of Hemorrhagic Fevers. International Journal of Environmental Research and Public Health, 18(11), 6022. https://doi.org/10.3390/ijerph18116022






