Lagged Association between Climate Variables and Hospital Admissions for Pneumonia in South Africa
Abstract
1. Introduction
2. Materials and Methods
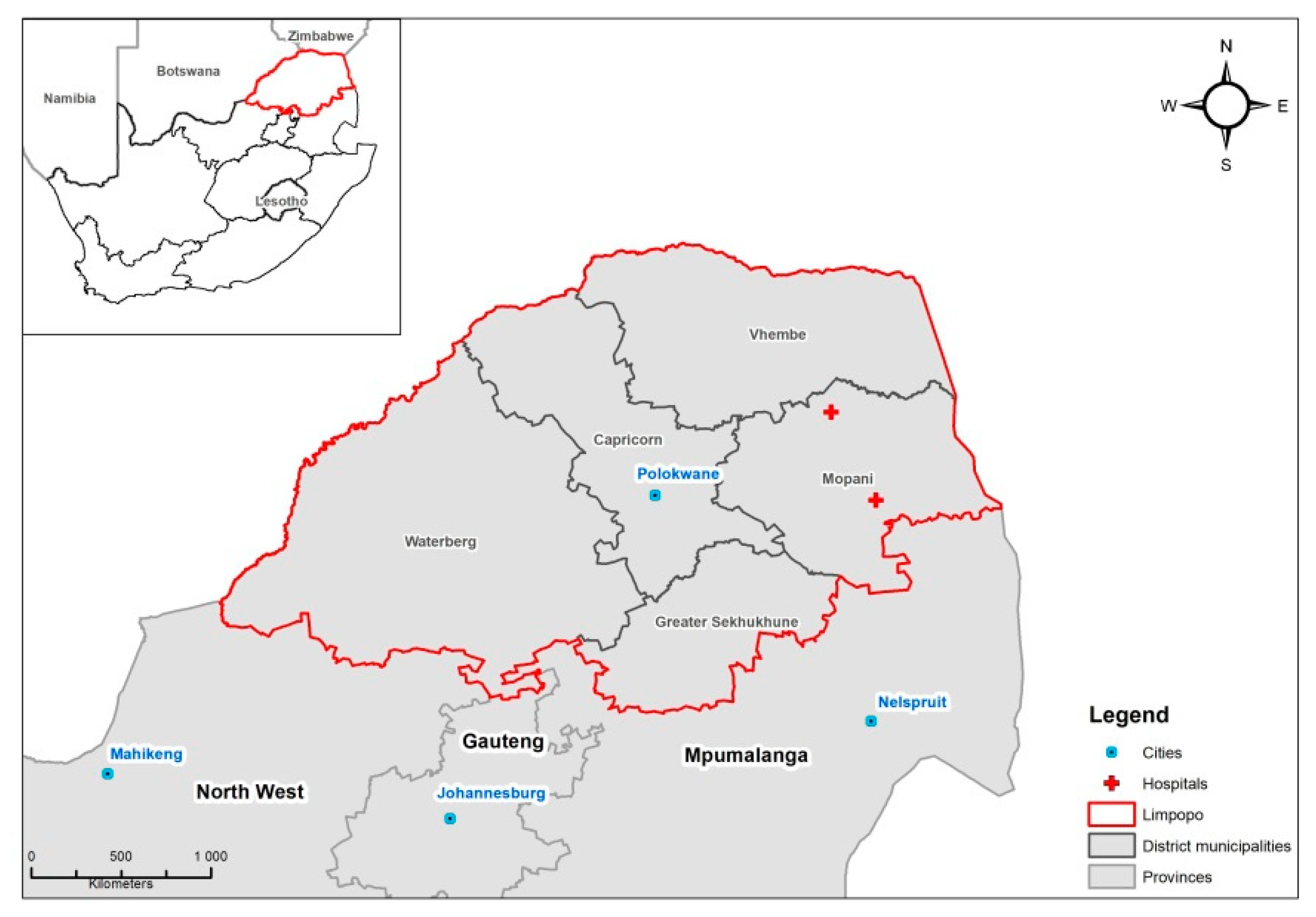
2.1. Dataset
2.2. Statistical Methodology
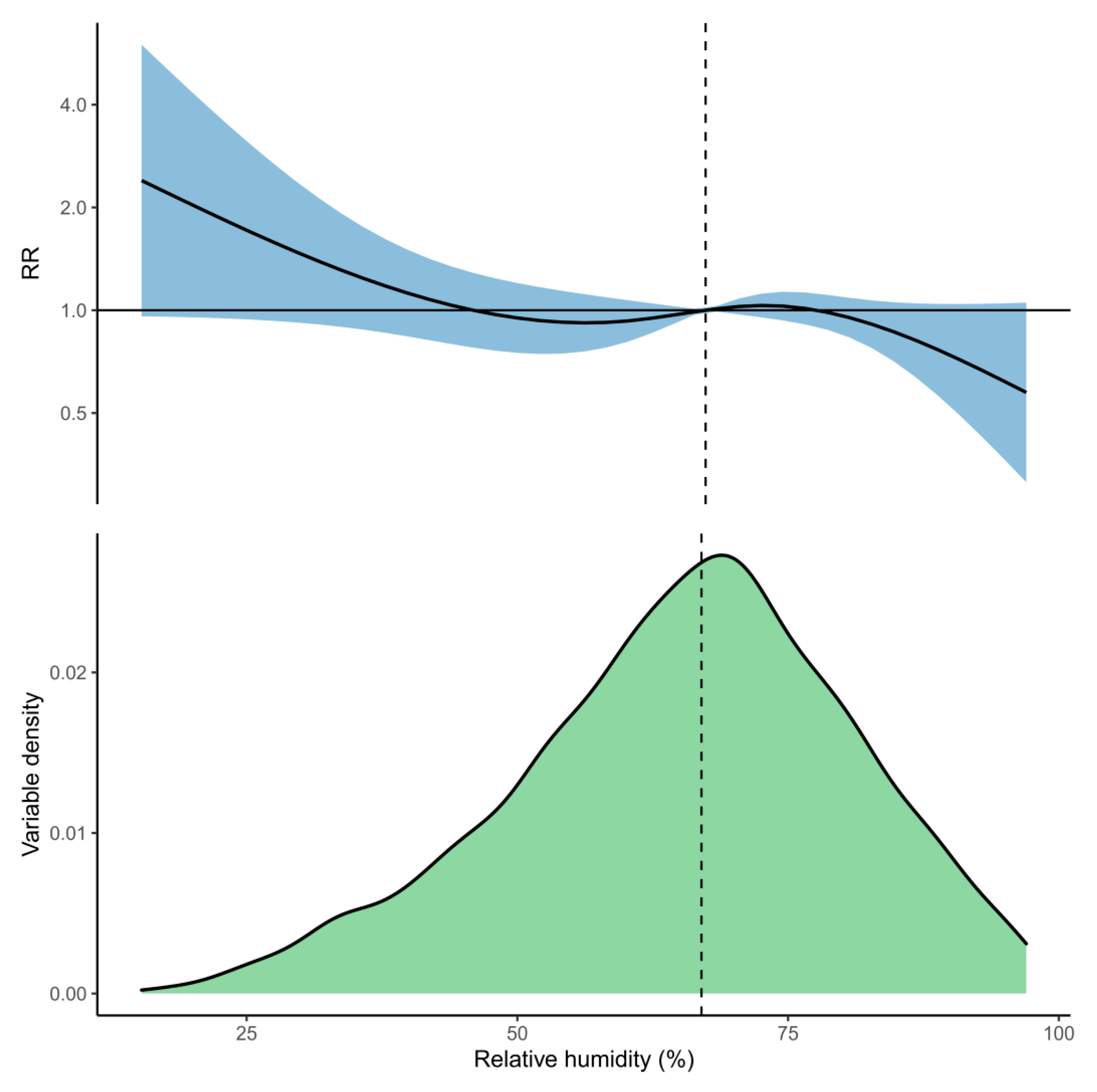
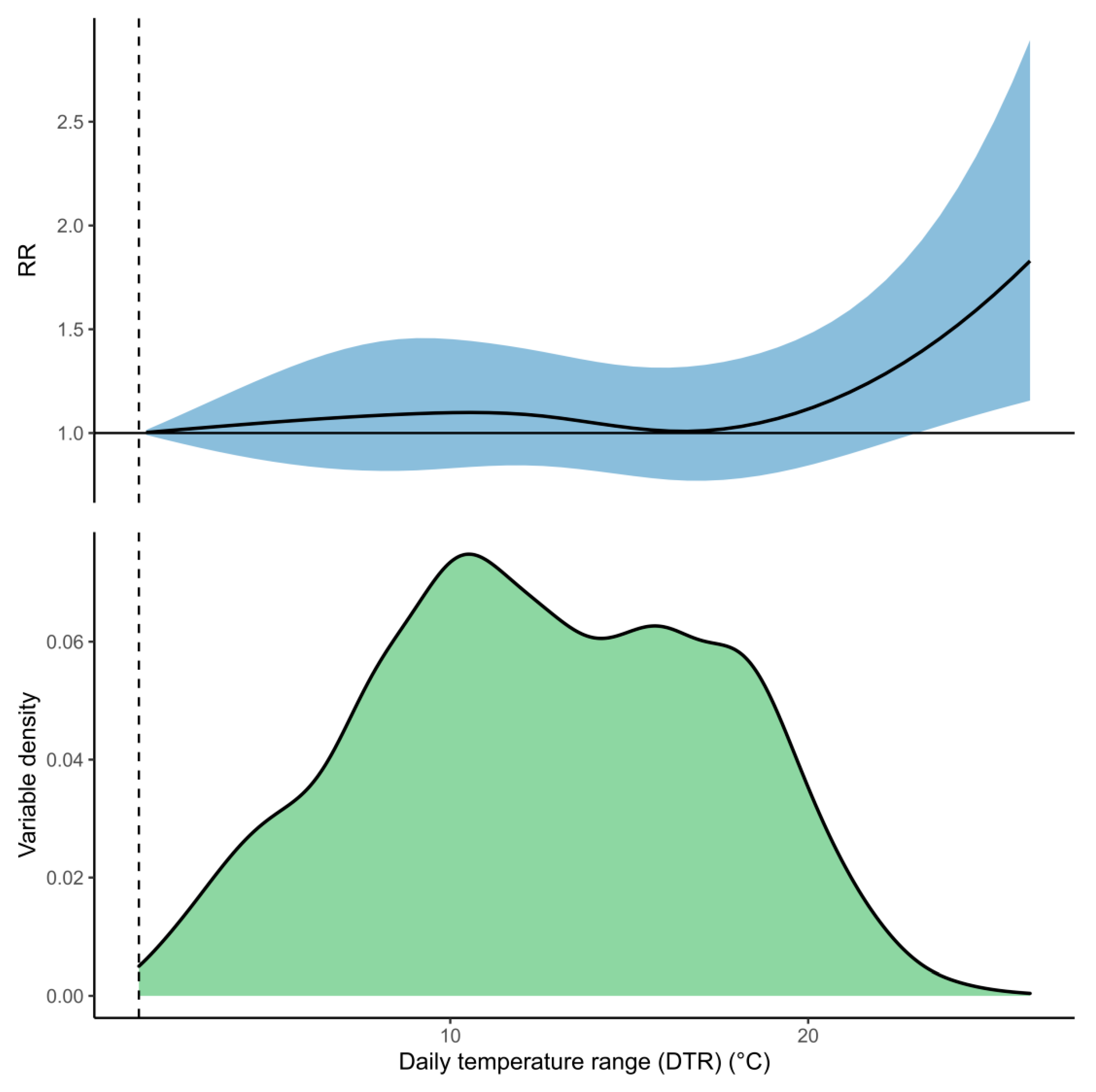
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Detailed Model Structure
Appendix B. Model Parameters
| Parameter | NegBin Estimate | NegBin SE | NegBin p | Zero Estimate | Zero SE | Zero p |
|---|---|---|---|---|---|---|
| Intercept | 1.686 | 0.793 | 0.033 | −0.547 | 0.618 | 0.376 |
| Mean Temp v1.l1 | −0.137 | 0.099 | 0.165 | - | - | - |
| Mean Temp v1.l2 | −0.015 | 0.061 | 0.81 | - | - | - |
| Mean Temp v1.l3 | 0.06 | 0.081 | 0.46 | - | - | - |
| Mean Temp v2.l1 | −0.088 | 0.092 | 0.34 | - | - | - |
| Mean Temp v2.l2 | 0.044 | 0.058 | 0.44 | - | - | - |
| Mean Temp v2.l3 | 0.009 | 0.075 | 0.899 | - | - | - |
| Mean Temp v3.l1 | −0.358 | 0.229 | 0.118 | - | - | - |
| Mean Temp v3.l2 | −0.136 | 0.153 | 0.372 | - | - | - |
| Mean Temp v3.l3 | 0.123 | 0.193 | 0.524 | - | - | - |
| Mean Temp v4.l1 | −0.026 | 0.128 | 0.84 | - | - | - |
| Mean Temp v4.l2 | −0.141 | 0.08 | 0.079 | - | - | - |
| Mean Temp v4.l3 | 0.08 | 0.104 | 0.443 | - | - | - |
| Humidity v1.l1 | 0.004 | 0.053 | 0.944 | - | - | - |
| Humidity v1.l2 | −0.031 | 0.036 | 0.403 | - | - | - |
| Humidity v1.l3 | −0.023 | 0.042 | 0.579 | - | - | - |
| Humidity v2.l1 | 0.199 | 0.184 | 0.281 | - | - | - |
| Humidity v2.l2 | −0.31 | 0.134 | 0.021 | - | - | - |
| Humidity v2.l3 | −0.218 | 0.155 | 0.159 | - | - | - |
| Humidity v3.l1 | 0.134 | 0.073 | 0.066 | - | - | - |
| Humidity v3.l2 | −0.144 | 0.049 | 0.003 | - | - | - |
| Humidity v3.l3 | 0.031 | 0.056 | 0.585 | - | - | - |
| Temp Range v1 | 0.102 | 0.126 | 0.417 | - | - | - |
| Temp Range v2 | −0.15 | 0.125 | 0.232 | - | - | - |
| Temp Range v3 | 0.395 | 0.302 | 0.191 | - | - | - |
| Temp Range v4 | 0.576 | 0.224 | 0.01 | - | - | - |
| sin(Annual) | −0.017 | 0.054 | 0.756 | - | - | - |
| cos(Annual) | −0.037 | 0.09 | 0.678 | - | - | - |
| Long-Term v1 | −0.228 | 0.116 | 0.05 | - | - | - |
| Long-Term v2 | −0.053 | 0.142 | 0.71 | - | - | - |
| Long-Term v3 | −0.147 | 0.107 | 0.169 | - | - | - |
| Long-Term v4 | −0.108 | 0.228 | 0.637 | - | - | - |
| Long-Term v5 | 0.425 | 0.104 | <0.001 | - | - | - |
| Monday | 0.18 | 0.077 | 0.02 | −0.831 | 1.41 | 0.556 |
| Tuesday | 0.108 | 0.078 | 0.168 | −2.145 | 346.547 | 0.995 |
| Wednesday | 0.108 | 0.078 | 0.167 | −0.773 | 1.233 | 0.531 |
| Thursday | 0.122 | 0.079 | 0.121 | −0.021 | 0.753 | 0.978 |
| Friday | 0.081 | 0.079 | 0.307 | −0.136 | 0.943 | 0.885 |
| Saturday | 0.099 | 0.079 | 0.209 | −0.432 | 1.156 | 0.708 |
| Lagged admissions | 0.047 | 0.006 | <0.001 | −0.575 | 0.272 | 0.034 |
References
- World Health Organisation. Pneumonia: Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/pneumonia (accessed on 20 December 2020).
- The Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of Severe Pneumonia Requiring Hospital Admission in Children without HIV Infection from Africa and Asia: The PERCH Multi-Country Case-Control Study. Available online: https://reader.elsevier.com/reader/sd/pii/S0140673619307214?token=EBEE2C363CB11520C13C0EFDA547D488075320EBAC752668B7B2BD5E102C537C6988ACEBC003D47719757DE457D45FE2 (accessed on 20 December 2020).
- World Health Organisation. Global Action Plan for Pneumonia and Diarrhoea (GAPPD) Monitoring Framework. Available online: https://www.who.int/maternal_child_adolescent/epidemiology/pneumonia-diarrhoea-monitoring/en/ (accessed on 20 December 2020).
- Simmerman, J.M.; Chittaganpitch, M.; Levy, J.; Chantra, S.; Maloney, S.; Uyeki, T.; Areerat, P.; Thamthitiwat, S.; Olsen, S.J.; Fry, A.; et al. Incidence, Seasonality and Mortality Associated with Influenza Pneumonia in Thailand: 2005–2008. PLoS ONE 2009, 4, e7776. [Google Scholar] [CrossRef]
- Oloyede, I.P.; Ijezie, E. Variations of Pneumonia in Children Admitted in the University of Uyo, Teaching Hospital, Uyo, Akwa Ibon State, Nigeria: A Retrospective 5-Year Study (2013–2017). Niger. J. Paediatr. 2020, 47, 42–47. [Google Scholar] [CrossRef]
- Lin, H.-C.; Lin, C.-C.; Chen, C.-S.; Lin, H.-C. Seasonality of Pneumonia Admissions and Its Association with Climate: An Eight-Year Nationwide Population-Based Study. Chronobiol. Int. 2009, 26, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.C.Y.; Chan, E.Y.Y.; Goggins, W.B. Short-Term Association between Meteorological Factors and Childhood Pneumonia Hospitalization in Hong Kong. Epidemiology 2019, 30, S107–S114. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, O.A.; McBryde, E.; Eisen, D.P. Epidemiological analysis of association between lagged meteorological variables and pneumonia in wet-dry tropical North Australia, 2006–2016. J. Expo. Sci. Environ. Epidemiol. 2019, 30, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Fisk, W.J. Review of some effects of climate change on indoor environmental quality and health and associated no-regrets mitigation measures. Build. Environ. 2015, 86, 70–80. [Google Scholar] [CrossRef]
- Kabir, I.; Rahman, B.; Smith, W.; Lusha, M.A.F.; Milton, A.H. Climate change and health in Bangladesh: A baseline cross-sectional survey. Glob. Health Action 2016, 9, 29609. [Google Scholar] [CrossRef] [PubMed]
- Mirsaeidi, M.; Motahari, H.; Khamesi, M.T.; Sharifi, A.; Campos, M.; Schraufnagel, D.E. Climate Change and Respiratory Infections. Ann. Am. Thorac. Soc. 2016, 13, 1223–1230. [Google Scholar] [CrossRef]
- Schweitzer, M.D.; Calzadilla, A.S.; Salamo, O.; Sharifi, A.; Kumar, N.; Holt, G.; Campos, M.; Mirsaeidi, M. Lung health in era of climate change and dust storms. Environ. Res. 2018, 163, 36–42. [Google Scholar] [CrossRef]
- National Institute for Communicable Diseases (NICD). World Pneumonia Day 2020. Available online: https://www.nicd.ac.za/world-pneumonia-day-2020/ (accessed on 21 December 2020).
- DEA (Department of Environmental Affairs). Long-Term Adaptation Scenarios Flagship Research Programme (LTAS) for South Africa. Climate Trends and Scenarios for South Africa; DEA: Pretoria, South Africa, 2013. Available online: https://www.environment.gov.za/sites/default/files/docs/summary_policymakers_bookV3.pdf (accessed on 2 June 2021).
- Engelbrecht, F.; Adegoke, J.; Bopape, M.-J.; Naidoo, M.; Garland, R.; Thatcher, M.; McGregor, J.; Katzfey, J.; Werner, M.; Ichoku, C.; et al. Projections of rapidly rising surface temperatures over Africa under low mitigation. Environ. Res. Lett. 2015, 10, 085004. [Google Scholar] [CrossRef]
- Gasparrini, A.; Armstrong, B.; Kenward, M.G. Distributed lag non-linear models. Stat. Med. 2010, 29, 2224–2234. [Google Scholar] [CrossRef]
- Ikeda, T.; Kapwata, T.; Behera, S.K.; Minakawa, N.; Hashizume, M.; Sweijd, N.; Mathee, A.; Wright, C.Y. Climatic Factors in Relation to Diarrhoea Hospital Admissions in Rural Limpopo, South Africa. Atmosphere 2019, 10, 522. [Google Scholar] [CrossRef]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Kapwata, T.; Gebreslasie, M.T.; Mathee, A.; Wright, C.Y. Current and Potential Future Seasonal Trends of Indoor Dwelling Temperature and Likely Health Risks in Rural Southern Africa. Int. J. Environ. Res. Public Health 2018, 15, 952. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, W.; Tong, S. Temperature variability and childhood pneumonia: An ecological study. Environ. Health 2014, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.-D.; Jiang, R.; Chen, X.-J.; Ye, Q. Meteorological factors on the incidence of MP and RSV pneumonia in children. PLoS ONE 2017, 12, e0173409. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.; Cho, W.; A Kim, J.; Altaluoni, A.; Hong, K.; Chun, B.C. ‘Pneumonia Weather’: Short-Term Effects of Meteorological Factors on Emergency Room Visits Due to Pneumonia in Seoul, Korea. J. Prev. Med. Public Health 2019, 52, 82–91. [Google Scholar] [CrossRef]
- Gasparrini, A. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. J. Stat. Softw. 2011, 43, 1–20. [Google Scholar] [CrossRef]
- American Lung Association. What Causes Pneumonia? Available online: https://www.lung.org/lung-health-diseases/lung-disease-lookup/pneumonia/what-causes-pneumonia (accessed on 21 December 2020).
- Imai, C.; Armstrong, B.; Chalabi, Z.; Mangtani, P.; Hashizume, M. Time series regression model for infectious disease and weather. Environ. Res. 2015, 142, 319–327. [Google Scholar] [CrossRef]
- Gasparrini, A.; Leone, M. Attributable risk from distributed lag models. BMC Med. Res. Methodol. 2014, 14, 55. [Google Scholar] [CrossRef]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza Virus Transmission Is Dependent on Relative Humidity and Temperature. PLoS Pathog. 2007, 3, e151. [Google Scholar] [CrossRef]
- Dowell, S.F.; Whitney, C.G.; Wright, C.; Rose, C.E.; Schuchat, A. Seasonal Patterns of Invasive Pneumococcal Disease. Emerg. Infect. Dis. 2003, 9, 573–579. [Google Scholar] [CrossRef]
- Lofgren, E.; Fefferman, N.H.; Naumov, Y.N.; Gorski, J.; Naumova, E.N. Influenza Seasonality: Underlying Causes and Modeling Theories. J. Virol. 2006, 81, 5429–5436. [Google Scholar] [CrossRef]
- Eccles, R.; Wilkinson, J. Exposure to cold and acute upper respiratory tract infection. Rhinol. J. 2015, 53, 99–106. [Google Scholar] [CrossRef]
- Liu, Y.; Kan, H.; Xu, J.; Rogers, D.; Peng, L.; Ye, X.; Chen, R.; Zhang, Y.; Wang, W. Temporal relationship between hospital admissions for pneumonia and weather conditions in Shanghai, China: A time-series analysis. BMJ Open 2014, 4, e004961. [Google Scholar] [CrossRef] [PubMed]
- Tellier, R. Review of Aerosol Transmission of Influenza A Virus. Emerg. Infect. Dis. 2006, 12, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Ben Hnia, N.G.; Barre, R.S.; Wexler, A.S.; Ristenpart, W.D.; Bouvier, N.M. Influenza A virus is transmissible via aerosolized fomites. Nat. Commun. 2020, 11, 4062. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Tong, S.; Bambrick, H.; Khan, A.F.; Hore, S.K.; Hu, W. Weather factors, PCV intervention and childhood pneumonia in rural Bangladesh. Int. J. Biometeorol. 2019, 64, 561–569. [Google Scholar] [CrossRef]
- Davis, R.E.; Dougherty, E.; McArthur, C.; Huang, Q.S.; Baker, M.G. Cold, dry air is associated with influenza and pneumonia mortality in Auckland, New Zealand. Influenza Other Respir. Viruses 2016, 10, 310–313. [Google Scholar] [CrossRef]




| Meteorological Exposure | Total (95%CI) | Low a (95%CI) | High b (95%CI) |
|---|---|---|---|
| Mean daily temperature | −8.4% (−25%, 6.6%) | 0.3% (−1%, 1.5%) | −3.4% (−8.2%, 0.3%) |
| +2 °C | −22.8% (−56.7%, −1.2%) | 0% (−0.2%, 0.1%) | −19.2% (−54.9%, −1.4%) |
| −2 °C | −9.3% (−27.2%, 4%) | 1.5% (−3%, 4.4%) | −0.5% (−1.4%, 0.1%) |
| Relative humidity | −2.1% (−12.9%, 7.7%) | 2.2% (−0.8%, 4.1%) | −4.4% (−11.6%, 0.6%) |
| +5% | −9.3% (−29.7%, 4.6%) | 1.2% (−0.5%, 2.4%) | −9.5% (−25.4%, 1.1%) |
| −5% | −0.6% (−13.2%, 9.4%) | 3.6% (−0.6%, 6.5%) | −1.8% (−4.8%, 0.3%) |
| DTR | 6.7% (−19.4%, 26%) | - | 1.3% (−0.6%, 2.6%) |
| +2 °C | 7.9% (−17%, 29.2%) | - | 3.8% (−0.9%, 7%) |
| −2 °C | 5.3% (−20.4%, 24.7%) | - | 0.3% (−0.2%, 0.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedder, H.; Kapwata, T.; Howard, G.; Naidoo, R.N.; Kunene, Z.; Morris, R.W.; Mathee, A.; Wright, C.Y. Lagged Association between Climate Variables and Hospital Admissions for Pneumonia in South Africa. Int. J. Environ. Res. Public Health 2021, 18, 6191. https://doi.org/10.3390/ijerph18126191
Pedder H, Kapwata T, Howard G, Naidoo RN, Kunene Z, Morris RW, Mathee A, Wright CY. Lagged Association between Climate Variables and Hospital Admissions for Pneumonia in South Africa. International Journal of Environmental Research and Public Health. 2021; 18(12):6191. https://doi.org/10.3390/ijerph18126191
Chicago/Turabian StylePedder, Hugo, Thandi Kapwata, Guy Howard, Rajen N. Naidoo, Zamantimande Kunene, Richard W. Morris, Angela Mathee, and Caradee Y. Wright. 2021. "Lagged Association between Climate Variables and Hospital Admissions for Pneumonia in South Africa" International Journal of Environmental Research and Public Health 18, no. 12: 6191. https://doi.org/10.3390/ijerph18126191
APA StylePedder, H., Kapwata, T., Howard, G., Naidoo, R. N., Kunene, Z., Morris, R. W., Mathee, A., & Wright, C. Y. (2021). Lagged Association between Climate Variables and Hospital Admissions for Pneumonia in South Africa. International Journal of Environmental Research and Public Health, 18(12), 6191. https://doi.org/10.3390/ijerph18126191








