The Role of Nutrition in the Prevention and Management of Bronchopulmonary Dysplasia: A Literature Review and Clinical Approach
Abstract
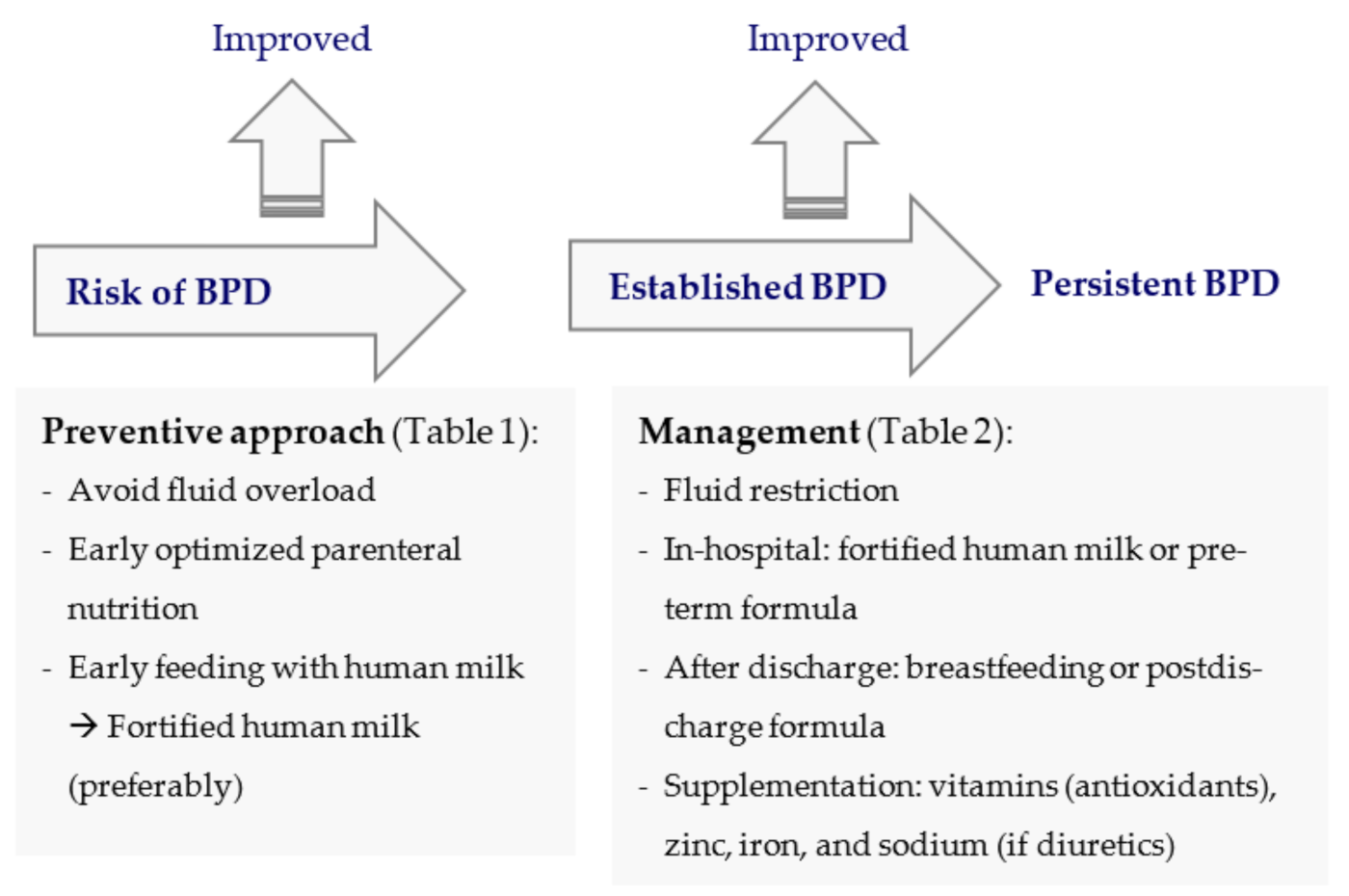
:1. Introduction
2. Aim
3. Literature Search
4. Fluid Management in Infants at Risk for and with Established BPD
5. Nutritional Requirements in Infants with BPD
6. Functional Nutrients with Potential Beneficial Effects on BPD
6.1. Polyunsaturated Fatty Acids
6.2. Amino Acids
6.3. Vitamins
6.4. Trace Elements
7. Nutritional Approach in the Prevention and Management of BPD
7.1. Parenteral Nutrition
7.2. Enteral Nutrition While in the Hospital
7.2.1. Enteral Nutrition Recommendations
7.2.2. Type of Feedings
Human Milk
Preterm Formulas
7.2.3. Timing of Initiation
7.2.4. Feeding Methods
7.2.5. Tracheostomy
7.3. Nutrition after Discharge
8. Monitoring
8.1. While in the Hospital
8.2. After Discharge
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPD | bronchopulmonary dysplasia |
| BUN | blood urea nitrogen |
| DHA | docosahexaenoic acid |
| DHM | donor human milk |
| ELBW | extremely low birth weight |
| ESPGHAN | European Society for Paediatric Gastroenterology Hepatology and Nutrition |
| GER | gastroesophageal reflux |
| HM | human milk |
| IVFEs | intravenous fat emulsions |
| LA | linoleic acid |
| MCT | medium-chain triglycerides |
| MOM | mother’s own milk |
| PMA | postmenstrual age |
| VLBW | very low birth weight |
References
- Bancalari, E.; Jain, D. Bronchopulmonary Dysplasia: 50 Years after the Original Description. Neonatology 2019, 115, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Northway, W.H.; Rosan, R.C.; Porter, D.Y. Pulmonary Disease Following Respirator Therapy of Hyaline-Membrane Disease. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H. The new bronchopulmonary dysplasia. Curr. Opin. Pediatr. 2011, 23, 167–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, H.; Rocha, G.; Vasconcellos, G.; Proença, E.; Carreira, M.; Sossai, M.; Morais, B.; Martins, I.; Rodrigues, T.; Severo, M. Bronchopulmonary dysplasia: Clinical practices in five Portuguese neonatal intensive care units. Rev. Port. Pneumol. 2010, 16, 273–286. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, T.; Radajewski, S.; Chao, C.-M.; Bellusci, S.; Ehrhardt, H. Pathogenesis of bronchopulmonary dysplasia: When inflammation meets organ development. Mol. Cell. Pediatr. 2016, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lignelli, E.; Palumbo, F.; Myti, D.; Morty, R.E. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L832–L887. [Google Scholar] [CrossRef]
- Álvarez-Fuente, M.; Moreno, L.; Mitchell, J.A.; Reiss, I.K.; Lopez, P.; Elorza, D.; Duijts, L.; Avila-Alvarez, A.; Arruza, L.; Orellana, M.R.; et al. Preventing bronchopulmonary dysplasia: New tools for an old challenge. Pediatr. Res. 2018, 85, 432–441. [Google Scholar] [CrossRef]
- Muehlbacher, T.; Bassler, D.; Bryant, M. Evidence for the Management of Bronchopulmonary Dysplasia in Very Preterm Infants. Children 2021, 8, 298. [Google Scholar] [CrossRef]
- Poindexter, B.B.; Martin, C.R. Impact of Nutrition on Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 797–806. [Google Scholar] [CrossRef]
- Hwang, J.S.; Rehan, V.K. Recent Advances in Bronchopulmonary Dysplasia: Pathophysiology, Prevention, and Treatment. Lung 2018, 196, 129–138. [Google Scholar] [CrossRef]
- Bose, C.; Van Marter, L.J.; Laughon, M.; O’Shea, T.M.; Allred, E.N.; Karna, P.; Ehrenkranz, R.A.; Boggess, K.; Leviton, A. For the Extremely Low Gestational Age Newborn Study Investigators Fetal Growth Restriction and Chronic Lung Disease Among Infants Born Before the 28th Week of Gestation. Am. Acad. Pediatr. 2009, 124, e450–e458. [Google Scholar] [CrossRef] [Green Version]
- Soudée, S.; Vuillemin, L.; Alberti, C.; Mohamed, D.; Becquet, O.; Farnoux, C.; Biran, V.; Baud, O. Fetal Growth Restriction Is Worse than Extreme Prematurity for the Developing Lung. Neonatology 2014, 106, 304–310. [Google Scholar] [CrossRef]
- Klinger, G.; Sokolover, N.; Boyko, V.; Sirota, L.; Lerner-Geva, L.; Reichman, B. Perinatal risk factors for bronchopulmonary dysplasia in a national cohort of very-low-birthweight infants. Am. J. Obstet. Gynecol. 2013, 208, e111–e119. [Google Scholar] [CrossRef]
- Reiss, I.; Landmann, E.; Heckmann, M.; Misselwitz, B.; Gortner, L. Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch. Gynecol. Obstet. 2003, 269, 40–44. [Google Scholar] [CrossRef]
- Rocha, G.; De Lima, F.F.; Machado, A.P.; Guimarães, H.; Proença, E.; Carvalho, C.; Martins, L.; Martins, T.; Freitas, A.; Dias, C.; et al. Small for gestational age very preterm infants present a higher risk of developing bronchopulmonary dysplasia. J. Neonatal-Perinatal Med. 2020, 12, 419–427. [Google Scholar] [CrossRef]
- Groh-Wargo, S.; Sapsford, A. Enteral Nutrition Support of the Preterm Infant in the Neonatal Intensive Care Unit. Nutr. Clin. Pract. 2009, 24, 363–376. [Google Scholar] [CrossRef]
- Milanesi, B.G.; Lima, P.A.; Villela, L.D.; Martins, A.S.; Gomes-Junior, S.C.S.; Moreira, M.E.L.; Méio, M.D.B.B. Assessment of early nutritional intake in preterm infants with bronchopulmonary dysplasia: A cohort study. Eur. J. Nucl. Med. Mol. Imaging 2021, 180, 1423–1430. [Google Scholar] [CrossRef]
- Al-Jebawi, Y.; Agarwal, N.; Wargo, S.G.; Shekhawat, P.; Mhanna, M. Low caloric intake and high fluid intake during the first week of life are associated with the severity of bronchopulmonary dysplasia in extremely low birth weight infants. J. Neonatal-Perinatal Med. 2020, 13, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Y.R.; Brozanski, B.; Farrow, K.N.; Zaniletti, I.; Padula, M.A.; Asselin, J.M.; Durand, D.J.; Short, B.L.; Pallotto, E.K.; Dykes, F.D.; et al. Postnatal Weight Gain in Preterm Infants with Severe Bronchopulmonary Dysplasia. Am. J. Perinatol. 2013, 31, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malikiwi, A.I.; Lee, Y.-M.; Davies-Tuck, M.; Wong, F.Y. Postnatal nutritional deficit is an independent predictor of bronchopulmonary dysplasia among extremely premature infants born at or less than 28 weeks gestation. Early Hum. Dev. 2019, 131, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.C.; Rocha, G.; Flor-De-Lima, F.; Guimarães, H. Extrauterine growth restriction at discharge in very low birth weight infants: A retrospective study in a level III neonatal intensive care unit. Minerva Pediatr. 2018, 23. [Google Scholar] [CrossRef]
- Bauer, K.; Versmold, H. Postnatal weight loss in preterm neonates less than 1500 g is due to isotonic dehydration of the extracellular volume. Acta Paediatr. Scand Suppl. 1989, 360, 37–42. [Google Scholar] [CrossRef]
- Stephens, B.E.; Gargus, R.A.; Walden, R.V.; Mance, M.; Nye, J.; McKinley, L.; Tucker, R.; Vohr, B.R. Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J. Perinatol. 2008, 28, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Biniwale, M.A.; Ehrenkranz, R.A. The Role of Nutrition in the Prevention and Management of Bronchopulmonary Dysplasia. Semin. Perinatol. 2006, 30, 200–208. [Google Scholar] [CrossRef]
- Wemhöner, A.; Ortner, D.; Tschirch, E.; Strasak, A.; Rüdiger, M. Nutrition of preterm infants in relation to bronchopulmonary dysplasia. BMC Pulm. Med. 2011, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Oh, W.; Poindexter, B.B.; Perritt, R.; Lemons, J.A.; Bauer, C.R.; Ehrenkranz, R.A.; Stoll, B.J.; Poole, K.; Wright, L.L. Neonatal Research Network. Association between Fluid Intake and Weight Loss during the First Ten Days of Life and Risk of Bronchopulmonary Dysplasia in Extremely Low Birth Weight Infants. J. Pediatr. 2005, 147, 786–790. [Google Scholar] [CrossRef]
- Bertrand, O.B.J.; Battisti, J.-M.B.O. Lung Compliance and Airways Resistance in Healthy Neonates. Pediatr. Ther. 2012, 2, 114. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.M.-H.; Chung, C.-H.; Chen, F.-S.; Chen, C.-C.; Huang, H.-C.; Chung, M.-Y. Severe Bronchopulmonary Dysplasia is Associated with Higher Fluid Intake in Very Low-Birth-Weight Infants: A Retrospective Study. Am. J. Perinatol. 2014, 30, 155–162. [Google Scholar] [CrossRef]
- Bell, E.F.; Acarregui, M.J. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2014, 2014, CD000503. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Lin, X.Z.; Chang, Y.M.; Liu, X.H.; Tong, X.M.; Ding, G.F.; Nutritional Committee of Neonatology Branch of Chinese Medical Doctor Association; Editorial Committee of Chinese Journal of Contemporary Pediatrics. Expert consensus on nutritional management of preterm infants with bronchopulmonary dysplasia. Chin. J. Contemp. Paediatr. 2020, 22, 805–814. [Google Scholar] [CrossRef]
- Barrington, K.J.; Fortin-Pellerin, E.; Pennaforte, T. Fluid restriction for treatment of preterm infants with chronic lung disease. Cochrane Database Syst. Rev. 2017, 2, CD005389. [Google Scholar] [CrossRef]
- Principi, N.; Di Pietro, G.M.; Esposito, S. Bronchopulmonary dysplasia: Clinical aspects and preventive and therapeutic strategies. J. Transl. Med. 2018, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, J.C. Servo-control for maintaining abdominal skin temperature at 36C in low birth weight infants. Cochrane Database Syst. Rev. 2002, 1, CD001074. [Google Scholar] [CrossRef]
- Glass, L.; Valdez, A. Preterm Infant Incubator Humidity Levels: A systematic review. Adv. Neonatal Care 2020. Publish Ah. [Google Scholar] [CrossRef]
- Sinclair, L.; Crisp, J.; Sinn, J. Variability in incubator humidity practices in the management of preterm infants. J. Paediatr. Child Health 2009, 45, 535–540. [Google Scholar] [CrossRef]
- Restrepo, R.D.; Walsh, B.K.; Toussaint, M.; Guillet, M.-C.; Paternotte, S.; Soudon, P.; Haan, J. Humidification During Invasive and Noninvasive Mechanical Ventilation: 2012. Respir. Care 2012, 57, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Stokowski, L.A. Fundamentals of phototherapy for neonatal jaundice. Adv. Neonatal Care 2006, 6, 303–312. [Google Scholar] [CrossRef]
- Uberos, J.; Lardón-Fernández, M.; Machado-Casas, I.; Molina-Oya, M.; Narbona-López, E. Nutrition in extremely low birth weight infants: Impact on bronchopulmonary dysplasia. Minerva Paediatr. 2016, 68, 419–426. [Google Scholar]
- Klevebro, S.; Westin, V.; Sjöström, E.S.; Norman, M.; Domellöf, M.; Bonamy, A.-K.E.; Hallberg, B. Early energy and protein intakes and associations with growth, BPD, and ROP in extremely preterm infants. Clin. Nutr. 2019, 38, 1289–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theile, A.R.; Radmacher, P.G.; Anschutz, T.W.; Davis, D.W.; Adamkin, D.H. Nutritional strategies and growth in extremely low birth weight infants with bronchopulmonary dysplasia over the past 10 years. J. Perinatol. 2011, 32, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianni, M.L.; Roggero, P.; Colnaghi, M.R.; Piemontese, P.; Amato, O.; Orsi, A.; Morlacchi, L.; Mosca, F. The role of nutrition in promoting growth in pre-term infants with bronchopulmonary dysplasia: A prospective non-randomised interventional cohort study. BMC Pediatr. 2014, 14, 235. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.; Zwerdling, R.; Ehrenkranz, R.; Gaultier, C.; Geggel, R.; Greenough, A.; Kleinman, R.; Klijanowicz, A.; Martinez, F.; Ozdemir, A.; et al. Statement on the Care of the Child with Chronic Lung Disease of Infancy and Childhood. Am. J. Respir. Crit. Care Med. 2003, 168, 356–396. [Google Scholar] [CrossRef] [Green Version]
- Pereira, G.R.; Baumgart, S.; Bennett, M.J.; Stallings, V.A.; Georgieff, M.K.; Hamosh, M.; Ellis, L. Use of high-fat formula for premature infants with bronchopulmonary dysplasia: Metabolic, pulmonary, and nutritional studies. J. Pediatr. 1994, 124, 605–611. [Google Scholar] [CrossRef]
- Kashyap, S.; Towers, H.M.; Sahni, R.; Ohira-Kist, K.; Abildskov, K.; Schulze, K.F. Effects of quality of energy on substrate oxidation in enterally fed, low-birth-weight infants. Am. J. Clin. Nutr. 2001, 74, 374–380. [Google Scholar] [CrossRef]
- Fenton, T.R.; Anderson, D.; Groh-Wargo, S.; Hoyos, A.; Ehrenkranz, R.A.; Senterre, T. An Attempt to Standardize the Calculation of Growth Velocity of Preterm Infants—Evaluation of Practical Bedside Methods. J. Pediatr. 2018, 196, 77–83. [Google Scholar] [CrossRef]
- Fink, N.H.; Collins, C.T.; Gibson, R.; Makrides, M.; Penttila, I.A. Targeting inflammation in the preterm infant: The role of the omega-3 fatty acid docosahexaenoic acid. J. Nutr. Intermed. Metab. 2016, 5, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Manley, B.J.; Makrides, M.; Collins, C.T.; McPhee, A.J.; Gibson, R.A.; Ryan, P.; Sullivan, T.R.; Davis, P.G. For the DINO Steering Committee High-Dose Docosahexaenoic Acid Supplementation of Preterm Infants: Respiratory and Allergy Outcomes. Am. Acad. Pediatr. 2011, 128, e71–e77. [Google Scholar] [CrossRef]
- Falciglia, H.S.; Johnson, J.R.; Sullivan, J.; Hall, C.F.; Miller, J.D.; Riechmann, G.C.; Falciglia, G.A. Role of Antioxidant Nutrients and Lipid Peroxidation in Premature Infants with Respiratory Distress Syndrome and Bronchopulmonary Dysplasia. Am. J. Perinatol. 2003, 20, 097–108. [Google Scholar] [CrossRef]
- Martin, C.R.; DaSilva, D.A.; Cluette-Brown, J.E.; DiMonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased Postnatal Docosahexaenoic and Arachidonic Acid Blood Levels in Premature Infants are Associated with Neonatal Morbidities. J. Pediatr. 2011, 159, 743–749.e2. [Google Scholar] [CrossRef] [Green Version]
- Marc, I.; Piedboeuf, B.; Lacaze-Masmonteil, T.; Fraser, W.; Mâsse, B.; Mohamed, I.; Qureshi, M.; Afifi, J.; Lemyre, B.; Caouette, G.; et al. Effect of maternal docosahexaenoic acid supplementation on bronchopulmonary dysplasia-free survival in breastfed preterm infants: A randomized clinical trial. JAMA 2020, 324, 157–167. [Google Scholar] [CrossRef]
- Collins, C.T.; Makrides, M.; McPhee, A.J.; Sullivan, T.; Davis, P.G.; Thio, M.; Simmer, K.; Rajadurai, V.S.; Travadi, J.; Berry, M.J.; et al. Docosahexaenoic Acid and Bronchopulmonary Dysplasia in Preterm Infants. N. Engl. J. Med. 2017, 376, 1245–1255. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, B.; Cui, Q.; Chen, C. Omega-3 Long-chain Polyunsaturated Fatty Acids for Bronchopulmonary Dysplasia: A Meta-analysis. J. Pediatr. 2019, 144, e20190181. [Google Scholar] [CrossRef]
- Tanaka, K.; Tanaka, S.; Shah, N.; Ota, E.; Namba, F. Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants: A systematic review and meta-analysis. J. Matern. Neonatal Med. 2020, 1–9. [Google Scholar] [CrossRef]
- Dani, C.; Poggi, C. Nutrition and bronchopulmonary dysplasia. J. Matern. Neonatal Med. 2012, 25 (Suppl. 3), 37–40. [Google Scholar] [CrossRef]
- Moe-Byrne, T.; E Wagner, J.V.; McGuire, W.; Moe-Byrne, T. Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2012, 1, CD001457. [Google Scholar] [CrossRef] [Green Version]
- Ahola, T.; Lapatto, R.; O Raivio, K.; Selander, B.; Stigson, L.; Jonsson, B.; Jonsbo, F.; Esberg, G.; Stövring, S.; Kjartansson, S.; et al. N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: A randomized controlled trial. J. Pediatr. 2003, 143, 713–719. [Google Scholar] [CrossRef] [Green Version]
- Mactier, H. Vitamin A and preterm infants: What we know, what we don’t know, and what we need to know. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F103–F108. [Google Scholar] [CrossRef]
- Mank, E.; Naninck, E.F.G.; Limpens, J.; Van Toledo, L.; Van Goudoever, J.B.; Akker, C.H.P.V.D. Enteral Bioactive Factor Supplementation in Preterm Infants: A Systematic Review. Nutrients 2020, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.E.; Wright, L.L.; Oh, W.; Kennedy, K.A.; Mele, L.; Ehrenkranz, R.A.; Stoll, B.J.; Lemons, J.A.; Stevenson, D.K.; Bauer, C.R.; et al. Vitamin A Supplementation for Extremely-Low-Birth-Weight Infants. N. Engl. J. Med. 1999, 340, 1962–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambalavanan, N.; Tyson, J.E.; Kennedy, K.A.; Hansen, N.I.; Vohr, B.R.; Wright, L.L.; Carlo, W.A. National Institute of Child Health and Human Development Neonatal Research Network. Vitamin A supplementation for extremely low birth weight infants: Outcome at 18 to 22 months. J. Pediatr. 2005, 115, e249–e254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, E.; Bose, C.; Snidow, T.; Ransom, L.; Young, T.; Bose, G.; Stiles, A. Trial of vitamin A supplementation in very low birth weight infants at risk for bronchopulmonary dysplasia. J. Pediatr. 1992, 121, 420–427. [Google Scholar] [CrossRef]
- Kennedy, K.A.; Stoll, B.J.; Ehrenkranz, R.A.; Oh, W.; Wright, L.L.; Stevenson, D.K.; Lemons, J.A.; Sowell, A.; Mele, L.; Tyson, J.E.; et al. The NICHD Neonatal Research Network. Vitamin A to prevent bronchopulmonary dysplasia in very-low-birth-weight infants: Has the dose been too low? Early Hum. Dev. 1997, 49, 19–31. [Google Scholar] [CrossRef]
- Araki, S.; Kato, S.; Namba, F.; Ota, E. Vitamin A to prevent bronchopulmonary dysplasia in extremely low birth weight infants: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0207730. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [Green Version]
- Ehrenkranz, R.A.; Ablow, R.C.; Warshaw, J.B. Effect of vitamin E on the development of oxygen-induced lung injury in neonates. Ann. N. Y. Acad. Sci. 1982, 393, 452–465. [Google Scholar] [CrossRef]
- Ohlsson, A. Inositol for respiratory distress syndrome in preterm infants. Cochrane Database Syst. Rev. 2003, 4, CD000366. [Google Scholar] [CrossRef]
- Bocca, B.; Ciccarelli, S.; Agostino, R.; Alimonti, A. Trace elements, oxidative status and antioxidant capacity as biomarkers in very low birth weight infants. Environ. Res. 2017, 156, 705–713. [Google Scholar] [CrossRef]
- Loui, A.; Raab, A.; Maier, R.F.; Brätter, P.; Obladen, M. Trace elements and antioxidant enzymes in extremely low birthweight infants. J. Trace Elements Med. Biol. 2010, 24, 111–118. [Google Scholar] [CrossRef]
- Vázquez-Gomis, R.; Bosch-Gimenez, V.; Juste-Ruiz, M.; Vázquez-Gomis, C.; Izquierdo-Fos, I.; Pastor-Rosado, J. Zinc concentration in preterm newborns at term age, a prospective observational study. BMJ Paediatr. Open 2019, 3, e000527. [Google Scholar] [CrossRef] [Green Version]
- Terrin, G.; Canani, R.B.; Passariello, A.; Messina, F.; Conti, M.G.; Caoci, S.; Smaldore, A.; Bertino, E.; De Curtis, M. Zinc supplementation reduces morbidity and mortality in very-low-birth-weight preterm neonates: A hospital-based randomized, placebo-controlled trial in an industrialized country. Am. J. Clin. Nutr. 2013, 98, 1468–1474. [Google Scholar] [CrossRef]
- A Darlow, B.; Austin, N. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst. Rev. 2003, 4, CD003312. [Google Scholar] [CrossRef]
- Pereira-da-Silva, L.; Pissarra, S.; Alexandrino, A.M.; Malheiro, L.; Macedo, I.; Cardoso, M.; Vieira-da-Silva, P.; Frutuoso, S.P.; Lau, H.; Soares, T. On behalf of the Portuguese Neonatal Society Guidelines for Neonatal Parenteral Nutrition: 2019 Update by the Portuguese Neonatal Society. Part I. General Aspects, Energy, and Macronutrients. Port. J. Pediatr. 2019, 50, 209–219. [Google Scholar] [CrossRef]
- Pereira-da-Silva, L.; Pissarra, S.; Alexandrino, A.M.; Malheiro, L.; Macedo, I.; Cardoso, M.; Vieira-da-Silva, P.; Frutuoso, S.P.; Lau, H.; Soares, T. On behalf of the Portuguese Neonatal Society Guidelines for Neonatal Parenteral Nutrition: 2019 Update by the Portuguese Neonatal Society. Part II. Micronutrients, Ready-to-use Solutions and Particular Conditions. Port. J. Pediatr. 2019, 50, 220–231. [Google Scholar] [CrossRef]
- Gaio, P.; Verlato, G.; Daverio, M.; Cavicchiolo, M.E.; Nardo, D.; Pasinato, A.; de Terlizzi, F.; Baraldi, E. Incidence of metabolic bone disease in preterm infants of birth weight <1250 g and in those suffering from bronchopulmonary dysplasia. Clin. Nutr. ESPEN 2018, 23, 234–239. [Google Scholar] [CrossRef]
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Energy. Clin. Nutr. 2018, 37, 2309–2314. [Google Scholar] [CrossRef]
- Van Goudoever, J.B.; Carnielli, V.; Darmaun, D.; De Pipaon, M.S.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Decsi, T.; Domellöf, M.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Amino acids. Clin. Nutr. 2018, 37, 2315–2323. [Google Scholar] [CrossRef] [Green Version]
- Lapillonne, A.; Mis, N.F.; Goulet, O.; van den Akker, C.H.V.D.; Wu, J.; Koletzko, B.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Lipids. Clin. Nutr. 2018, 37, 2324–2336. [Google Scholar] [CrossRef]
- Fan, X.; Tang, Y.; Tang, J.; Chen, J.; Shi, J.; Wang, H.; Xia, B.; Qu, Y.; Mu, D. New-generation intravenous fat emulsions and bronchopulmonary dysplasia in preterm infants: A systematic review and meta-analysis. J. Perinatol. 2020, 40, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Salama, G.S.A.; Kaabneh, M.A.; Almasaeed, M.N.; Alquran, M.I. Intravenous Lipids for Preterm Infants: A Review. Clin. Med. Insights: Pediatr. 2015, 9, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.; Premkumar, M.; Burrin, D.G. Emerging Clinical Benefits of New-Generation Fat Emulsions in Preterm Neonates. Nutr. Clin. Pract. 2017, 32, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; D’Amato, E.; Natale, M.P.; Grano, M.; Chiarito, M.; Brunetti, G.; D’Amato, G. Metabolic Bone Disease of Prematurity: Diagnosis and Management. Front. Pediatr. 2019, 7, 143. [Google Scholar] [CrossRef]
- Carlson, S.J. Current Nutrition Management of Infants with Chronic Lung Disease. Nutr. Clin. Pract. 2004, 19, 581–586. [Google Scholar] [CrossRef]
- Mihatsch, W.; Fewtrell, M.; Goulet, O.; Molgaard, C.; Picaud, J.-C.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Calcium, phosphorus and magnesium. Clin. Nutr. 2018, 37, 2360–2365. [Google Scholar] [CrossRef] [Green Version]
- Mackay, M.; Jackson, D.; Eggert, L.; Fitzgerald, K.; Cash, J. Practice-Based Validation of Calcium and Phosphorus Solubility Limits for Pediatric Parenteral Nutrition Solutions. Nutr. Clin. Pract. 2011, 26, 708–713. [Google Scholar] [CrossRef]
- Watrobska-Swietlikowska, D. Compatibility of Maximum Inorganic and Organic Calcium and Phosphate Content in Neonatal Parenteral Solutions. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mulla, S.; Stirling, S.; Cowey, S.; Close, R.; Pullan, S.; Howe, R.; Radbone, L.; Clarke, P. Severe hypercalcaemia and hypophosphataemia with an optimised preterm parenteral nutrition formulation in two epochs of differing phosphate supplementation. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F451–F455. [Google Scholar] [CrossRef] [Green Version]
- Bronsky, J.; Campoy, C.; Braegger, C.; Cai, W.; Carnielli, V.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.; Fewtrell, M.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Vitamins. Clin. Nutr. 2018, 37, 2366–2378. [Google Scholar] [CrossRef] [Green Version]
- Domellöf, M.; Szitanyi, P.; Simchowitz, V.; Franz, A.; Mimouni, F.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Iron and trace minerals. Clin. Nutr. 2018, 37, 2354–2359. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Buonocore, G.; Carnielli, V.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral Nutrient Supply for Preterm Infants: Commentary FROM the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Denne, S.C. Energy Expenditure in Infants with Pulmonary Insufficiency: Is There Evidence for Increased Energy Needs? J. Nutr. 2001, 131, 935S–937S. [Google Scholar] [CrossRef] [Green Version]
- El Koofy, N.M.; Rady, H.I.; Abdallah, S.M.; Bazaraa, H.M.; Rabie, W.A.; El-Ayadi, A.A. The effect of high fat dietary modification and nutritional status on the outcome of critically ill ventilated children: Single-center study. Korean J. Pediatr. 2019, 62, 344–352. [Google Scholar] [CrossRef]
- White, A.M.; Liu, P.; Yee, K.; Waber, B.; Monk, H.M.; Zhang, H.; Jensen, E.A. Determinants of Severe Metabolic Bone Disease in Very Low-Birth-Weight Infants with Severe Bronchopulmonary Dysplasia Admitted to a Tertiary Referral Center. Am. J. Perinatol. 2015, 33, 107–113. [Google Scholar] [CrossRef]
- Hicks, P.D.; Rogers, S.P.; Hawthorne, K.M.; Chen, Z.; Abrams, S.A. Calcium Absorption in Very Low Birth Weight Infants with and without Bronchopulmonary Dysplasia. J. Pediatr. 2011, 158, 885–890.e1. [Google Scholar] [CrossRef]
- Abrams, S.A.; The Committee on Nutrition; Bhatia, J.J.S.; Corkins, M.R.; De Ferranti, S.D.; Golden, N.H.; Silverstein, J. Calcium and Vitamin D Requirements of Enterally Fed Preterm Infants. Am. Acad. Pediatr. 2013, 131, e1676–e1683. [Google Scholar] [CrossRef] [Green Version]
- Mimouni, F.B.; Mandel, D.; Lubetzky, R.; Senterre, T. Calcium, Phosphorus, Magnesium and Vitamin D Requirements of the Preterm Infant. World Rev. Nutr. Diet. 2014, 110, 140–151. [Google Scholar] [CrossRef]
- Duan, J.; Kong, X.; Li, Q.; Hua, S.; Zhang, S.; Zhang, X.; Feng, Z. Association between anemia and bronchopulmonary dysplasia in preterm infants. Sci. Rep. 2016, 6, 22717. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Georgieff, M.K. Iron Therapy for Preterm Infants. Clin. Perinatol. 2009, 36, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Singh, B.; Chessell, L.; Wilson, J.; Janes, M.; McDonald, K.; Shahid, S.; Gardner, V.A.; Hjartarson, A.; Purcha, M.; et al. Guidelines for Feeding Very Low Birth Weight Infants. Nutrients 2015, 7, 423–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Jeong, S.-A.; Cho, J.Y.; Seo, J.-H.; Lim, J.Y.; Woo, H.O.; Youn, H.-S.; Park, C.-H. Risk Factors and Effects of Severe Late-Onset Hyponatremia on Long-Term Growth of Prematurely Born Infants. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Tang, J.; Shi, J.; Qu, Y.; Xiong, T.; Mu, D. Human milk as a protective factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 104, F128–F136. [Google Scholar] [CrossRef] [PubMed]
- Friel, J.K.; Martin, S.M.; Langdon, M.; Herzberg, G.R.; Buettner, G.R. Milk from Mothers of Both Premature and Full-Term Infants Provides Better Antioxidant Protection than Does Infant Formula. Pediatr. Res. 2002, 51, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Santaella, M.; Mira-Pascual, L.; Martínez-Arias, E.; Khodayar-Pardo, P.; Ros, G.; Martínez-Costa, C. Longitudinal Study of Cytokine Expression, Lipid Profile and Neuronal Growth Factors in Human Breast Milk from Term and Preterm Deliveries. Nutrients 2015, 7, 8577–8591. [Google Scholar] [CrossRef]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Villamor, E. Mother’s Own Milk and Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019, 7, 224. [Google Scholar] [CrossRef]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.W.; Villamor, E. Donor Human Milk Protects against Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A Systematic Review and Meta-Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef] [Green Version]
- Arslanoglu, S.; Boquien, C.-Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef]
- Radmacher, P.G.; Adamkin, D.H. Fortification of human milk for preterm infants. Semin. Fetal Neonatal Med. 2017, 22, 30–35. [Google Scholar] [CrossRef]
- Macedo, I.; Pereira-Da-Silva, L.; Cardoso, M. The fortification method relying on assumed human milk composition overestimates the actual energy and macronutrient intakes in very preterm infants. Matern. Health Neonatol. Perinatol. 2018, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Da-Silva, L.; Cardoso, M.; Macedo, I. Associations of Measured Protein and Energy Intakes with Growth and Adiposity in Human Milk-Fed Preterm Infants at Term Postmenstrual Age: A Cohort Study. Am. J. Perinatol. 2018, 35, 882–891. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Ziegler, E.E. Adjustable fortification of human milk fed to preterm infants: Does it make a difference? J. Perinatol. 2006, 26, 614–621. [Google Scholar] [CrossRef] [Green Version]
- Morlacchi, L.; Mallardi, D.; Giannì, M.L.; Roggero, P.; Amato, O.; Piemontese, P.; Consonni, D.; Mosca, F. Is targeted fortification of human breast milk an optimal nutrition strategy for preterm infants? An interventional study. J. Transl. Med. 2016, 14, 195. [Google Scholar] [CrossRef] [Green Version]
- Rochow, N.; Fusch, G.; Ali, A.; Bhatia, A.; So, H.Y.; Iskander, R.; Chessell, L.; el Helou, S.; Fusch, C. Individualized target fortification of breast milk with protein, carbohydrates, and fat for preterm infants: A double-blind randomized controlled trial. Clin. Nutr. 2021, 40, 54–63. [Google Scholar] [CrossRef]
- Cardoso, M.; Virella, D.; Macedo, I.; Silva, D.; Pereira-Da-Silva, L. Customized Human Milk Fortification Based on Measured Human Milk Composition to Improve the Quality of Growth in Very Preterm Infants: A Mixed-Cohort Study Protocol. Int. J. Environ. Res. Public Health 2021, 18, 823. [Google Scholar] [CrossRef]
- McLeod, G.; Sherriff, J.; Hartmann, P.E.; Nathan, E.; Geddes, D.; Simmer, K. Comparing different methods of human breast milk fortification using measured v. assumed macronutrient composition to target reference growth: A randomised controlled trial. Br. J. Nutr. 2015, 115, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Shaikhkhalil, A.K.; Curtiss, J.; Puthoff, T.D.; Valentine, C.J. Enteral Zinc Supplementation and Growth in Extremely-Low-Birth-Weight Infants with Chronic Lung Disease. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, H.; Rocha, G.; Guedes, M.B.; Guerra, P.; Silva, A.I.; Pissarra, S. Nutrition of preterm infants with bronchopulmonary dysplasia after hospital discharge—Part I. J. Pediatr. Neonatal Individ. Med. 2014, 3, e030117. [Google Scholar] [CrossRef]
- Bott, L.; Béghin, L.; Devos, P.; Pierrat, V.; Matran, R.; Gottrand, F. Nutritional Status at 2 Years in Former Infants with Bronchopulmonary Dysplasia Influences Nutrition and Pulmonary Outcomes During Childhood. Pediatr. Res. 2006, 60, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Hay, W.W.; Hendrickson, K.C. Preterm formula use in the preterm very low birth weight infant. Semin. Fetal Neonatal Med. 2017, 22, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Brunton, J.A.; Saigal, S.; Atkinson, S.A. Growth and body composition in infants with bronchopulmonary dysplasia up to 3 months corrected age: A randomized trial of a high-energy nutrient-enriched formula fed after hospital discharge. J. Pediatr. 1998, 133, 340–345. [Google Scholar] [CrossRef]
- Romera, G.; Figueras, J.; Rodríguez-Miguélez, J.M.; Ortega, J.; Jiménez, R. Energy Intake, Metabolic Balance and Growth in Preterm Infants Fed Formulas with Different Nonprotein Energy Supplements. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Da-Silva, L.; Dias, M.P.G.; Virella, D.; Moreira, A.C.; Serelha, M. Osmolality of preterm formulas supplemented with nonprotein energy supplements. Eur. J. Clin. Nutr. 2007, 62, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Moltu, S.J.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; de Koning, B.; Lapillonne, A.; Norsa, L.; Verduci, E.; et al. ESPGHAN Committee on Nutrition. Nutritional management of the critically ill neonate: A Position Paper of the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021. Publish Ah. [Google Scholar] [CrossRef]
- Konnikova, Y.; Zaman, M.M.; Makda, M.; D’Onofrio, D.; Freedman, S.D.; Martin, C.R. Late Enteral Feedings Are Associated with Intestinal Inflammation and Adverse Neonatal Outcomes. PLoS ONE 2015, 10, e0132924. [Google Scholar] [CrossRef] [Green Version]
- Ambalavanan, N.; Carlo, W.A.; D’Angio, C.T.; McDonald, S.; Das, A.; Schendel, D.; Thorsen, P.; Higgins, R.D. For the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Cytokines Associated with Bronchopulmonary Dysplasia or Death in Extremely Low Birth Weight Infants. Am. Acad. Pediatr. 2009, 123, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Jadcherla, S.R.; Gupta, A.; Fernandez, S.; Nelin, L.D.; Castile, R.; Gest, A.L.; Welty, S. Spatiotemporal Characteristics of Acid Refluxate and Relationship to Symptoms in Premature and Term Infants with Chronic Lung Disease. Am. J. Gastroenterol. 2008, 103, 720–728. [Google Scholar] [CrossRef]
- Wang, L.-J.; Hu, Y.; Wang, W.; Zhang, C.-Y.; Bai, Y.-Z.; Zhang, S.-C. Gastroesophageal reflux poses a potential risk for late complications of bronchopulmonary dysplasia: A prospective cohort study. Chest 2020, 158, 1596–1605. [Google Scholar] [CrossRef]
- Nobile, S.; Noviello, C.; Cobellis, G.; Carnielli, V.P. Are Infants with Bronchopulmonary Dysplasia Prone to Gastroesophageal Reflux? A Prospective Observational Study with Esophageal pH-Impedance Monitoring. J. Pediatr. 2015, 167, 279–285.e1. [Google Scholar] [CrossRef]
- Malcolm, W.F.; Smith, P.B.; Mears, S.; Goldberg, R.N.; Cotten, C.M. Transpyloric tube feeding in very low birthweight infants with suspected gastroesophageal reflux: Impact on apnea and bradycardia. J. Perinatol. 2009, 29, 372–375. [Google Scholar] [CrossRef] [Green Version]
- A Jensen, E.; Zhang, H.; Feng, R.; Dysart, K.; Nilan, K.; A Munson, D.; Kirpalani, H. Individualising care in severe bronchopulmonary dysplasia: A series of N-of-1 trials comparing transpyloric and gastric feeding. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 105, 399–404. [Google Scholar] [CrossRef]
- Reynolds, E.W.; Grider, D.; Caldwell, R.; Capilouto, G.; Patwardhan, A.; Charnigo, R. Effects of Bronchopulmonary Dysplasia on Swallow: Breath Interaction and Phase of Respiration with Swallow During Non-nutritive Suck. S. Pac. J. Nat. Appl. Sci. 2018, 4, 531. [Google Scholar]
- Luo, J.; Shepard, S.; Rn, K.N.; Wood, A.; Monk, H.M.; Jensen, E.A.; Harrington, A.T.; Maschhoff, K.; Kirpalani, H.; Feng, Z.; et al. Improved growth and developmental activity post tracheostomy in preterm infants with severe BPD. Pediatr. Pulmonol. 2018, 53, 1237–1244. [Google Scholar] [CrossRef]
- Guimarães, H.; Rocha, G.; Guedes, M.B.; Guerra, P.; Silva, A.I.; Pissarra, S. Nutrition of preterm infants with bronchopulmonary dysplasia after hospital discharge—Part II. J. Pediatr. Neonatal Individ. Med. 2014, 3, e030116. [Google Scholar] [CrossRef]
- A Morgan, J.; Young, L.; McCormick, F.M.; McGuire, W. Promoting growth for preterm infants following hospital discharge. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 97, F295–F298. [Google Scholar] [CrossRef]
- Marino, L.V.; Fudge, C.; Pearson, F.; Johnson, M.J. Home use of breast milk fortifier to promote postdischarge growth and breast feeding in preterm infants: A quality improvement project. Arch. Dis. Child. 2019, 104, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Young, T.E. Nutritional support and bronchopulmonary dysplasia. J. Perinatol. 2007, 27, S75–S78. [Google Scholar] [CrossRef] [Green Version]
- Villa, E.; Barachetti, R.; Barbarini, M. Nutritional management of preterm newborn after hospital discharge: Energy and nutrients. La Pediatr. Medica Chir. 2017, 39, 170. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Da-Silva, L.; Virella, D.; Fusch, C. Nutritional Assessment in Preterm Infants: A Practical Approach in the NICU. Nutrients 2019, 11, 1999. [Google Scholar] [CrossRef] [Green Version]
- Landau-Crangle, E.; Rochow, N.; Fenton, T.R.; Liu, K.; Ali, A.; So, H.Y.; Fusch, G.; Marrin, M.L.; Fusch, C. Individualized Postnatal Growth Trajectories for Preterm Infants. J. Parenter. Enter. Nutr. 2018, 42, 1084–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Da-Silva, L.; Virella, D. Is intrauterine growth appropriate to monitor postnatal growth of preterm neonates? BMC Pediatr. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochow, N.; Raja, P.; Liu, K.; Fenton, T.; Landau-Crangle, E.; Göttler, S.; Jahn, A.; Lee, S.; Seigel, S.; Campbell, D.; et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr. Res. 2016, 79, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Pereira-da-Silva, L.; Virella, D.; Frutuoso, S.; Cunha, M.; Rocha, G.; Pissarra, S. Recommendation of charts and reference values for assessing growth of preterm infants: Update by the Portuguese Neonatal Society. Port. J. Pediatr. 2020, 51, 73–77. [Google Scholar] [CrossRef]
- Fenton, T.; Senterre, T.; Griffin, I.J. Time interval for preterm infant weight gain velocity calculation precision. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 104, F218–F219. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.-M.; Murphy, B.P.; Kiely, M.E. Optimising preterm nutrition: Present and future. Proc. Nutr. Soc. 2016, 75, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.J.; Wiskin, A.E.; Pearson, F.; Beattie, R.M.; Leaf, A.A. How to use: Nutritional assessment in neonates. Arch. Dis. Child. Educ. Pract. Ed. 2014, 100, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Visser, F.; Sprij, A.J.; Brus, F. The validity of biochemical markers in metabolic bone disease in preterm infants: A systematic review. Acta Paediatr. 2012, 101, 562–568. [Google Scholar] [CrossRef]
- Villar, J.; Giuliani, F.; Bhutta, Z.A.; Bertino, E.; Ohuma, E.O.; Ismail, L.C.; Barros, F.C.; Altman, D.G.; Victora, C.; Noble, J.A.; et al. Postnatal growth standards for preterm infants: The Preterm Postnatal Follow-up Study of the INTERGROWTH-21 st Project. Lancet Glob. Health 2015, 3, e681–e691. [Google Scholar] [CrossRef] [Green Version]
| Intervention | Reference | |
|---|---|---|
| Avoid excessive fluid intake |
| [32,33,34] |
| Provide adequate incubator humidity |
| [37] |
| Maintain adequate temperature |
| [38,39] |
| Optimize early parenteral energy intake |
| [44,78] |
| Optimize early parenteral amino acid intake |
| [79] |
| Optimize early parenteral fat intake |
| [80] |
| Provide adequate intravenous glucose |
| [33] |
| Optimize early parenteral calcium and phosphorus intake |
| [86] |
| Provide adequate intravenous lipid soluble vitamins |
| [90] |
| Provide adequate intravenous trace elements |
| [91] |
| Initiate early enteral feeding |
| [101,106,125] |
| Intervention | Reference | |
|---|---|---|
| Fluid restriction | Less than 150 mL/kg/day Ideally, up to 135 mL/kg/day | [32,33,34] |
| Optimize enteral energy intake | Ideally, 120–150 kcal/kg/day | [33,44] |
| Optimize enteral protein intake |
| [92] |
| Optimize enteral lipid intake |
| [92] |
| Optimize enteral calcium and phosphate intake |
| [92] [97] |
| Optimize sodium intake if diuretics are used |
| [12] |
| Optimize enteral vitamin A intake | 400–1000 µg/kg/day or 1320–3300 IU/kg/day | [92] |
| Optimize enteral vitamin E (α-tocopherol) intake | 2.2–11 mg/kg/day | [92] |
| Supplemental iron | 4 mg/kg/day, from 4–8 postnatal weeks up to 12 months of life | [33] |
| Parameter | Reference | |
|---|---|---|
| Body weight (daily) |
| [141] [146] |
| Body length (weekly) | Body length velocity: 0.9–1.1 cm/week | [147] |
| Head circumference (weekly) | Head circumference velocity: 0.9–1.0 cm/week | [147] |
| Monitoring iron status | Complete blood count with reticulocyte count, and serum ferritin levels | [140] |
| Monitoring protein nutrition | Blood urea nitrogen (BUN) | [113] |
| Monitoring early metabolic bone disease | Serum phosphorus and alkaline phosphate levels | [149] |
| Monitoring electrolyte balance (diuretics use) | Serum electrolytes | [27] |
| Parameter | Reference | |
|---|---|---|
| Body weight, length, and head circumference | Intergrowth-21st standards: monitoring up to 64 weeks postmenstrual age, for infants born at >26 and <37 weeks of gestation | [150] |
| Monitoring iron status | Complete blood count with reticulocyte count, and serum ferritin levels | [140] |
| Monitoring protein nutrition | Blood urea nitrogen (BUN) | [113] |
| Monitoring metabolic bone disease | Serum phosphorus and alkaline phosphate levels | [149] |
| Monitoring electrolyte balance (if diuretic use) | Serum electrolytes | [27] |
| Monitoring vitamins and trace elements (if deficiency suspicion) | Serum levels of vitamin A, 25-hydroxy vitamin D, zinc, and selenium | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, G.; Guimarães, H.; Pereira-da-Silva, L. The Role of Nutrition in the Prevention and Management of Bronchopulmonary Dysplasia: A Literature Review and Clinical Approach. Int. J. Environ. Res. Public Health 2021, 18, 6245. https://doi.org/10.3390/ijerph18126245
Rocha G, Guimarães H, Pereira-da-Silva L. The Role of Nutrition in the Prevention and Management of Bronchopulmonary Dysplasia: A Literature Review and Clinical Approach. International Journal of Environmental Research and Public Health. 2021; 18(12):6245. https://doi.org/10.3390/ijerph18126245
Chicago/Turabian StyleRocha, Gustavo, Hercília Guimarães, and Luís Pereira-da-Silva. 2021. "The Role of Nutrition in the Prevention and Management of Bronchopulmonary Dysplasia: A Literature Review and Clinical Approach" International Journal of Environmental Research and Public Health 18, no. 12: 6245. https://doi.org/10.3390/ijerph18126245







