Lack of Zika Virus and Other Recognized Flaviviruses among the Mosquito Vectors during and Post the Hajj Mass Gathering
Abstract
:1. Introduction
2. Methods
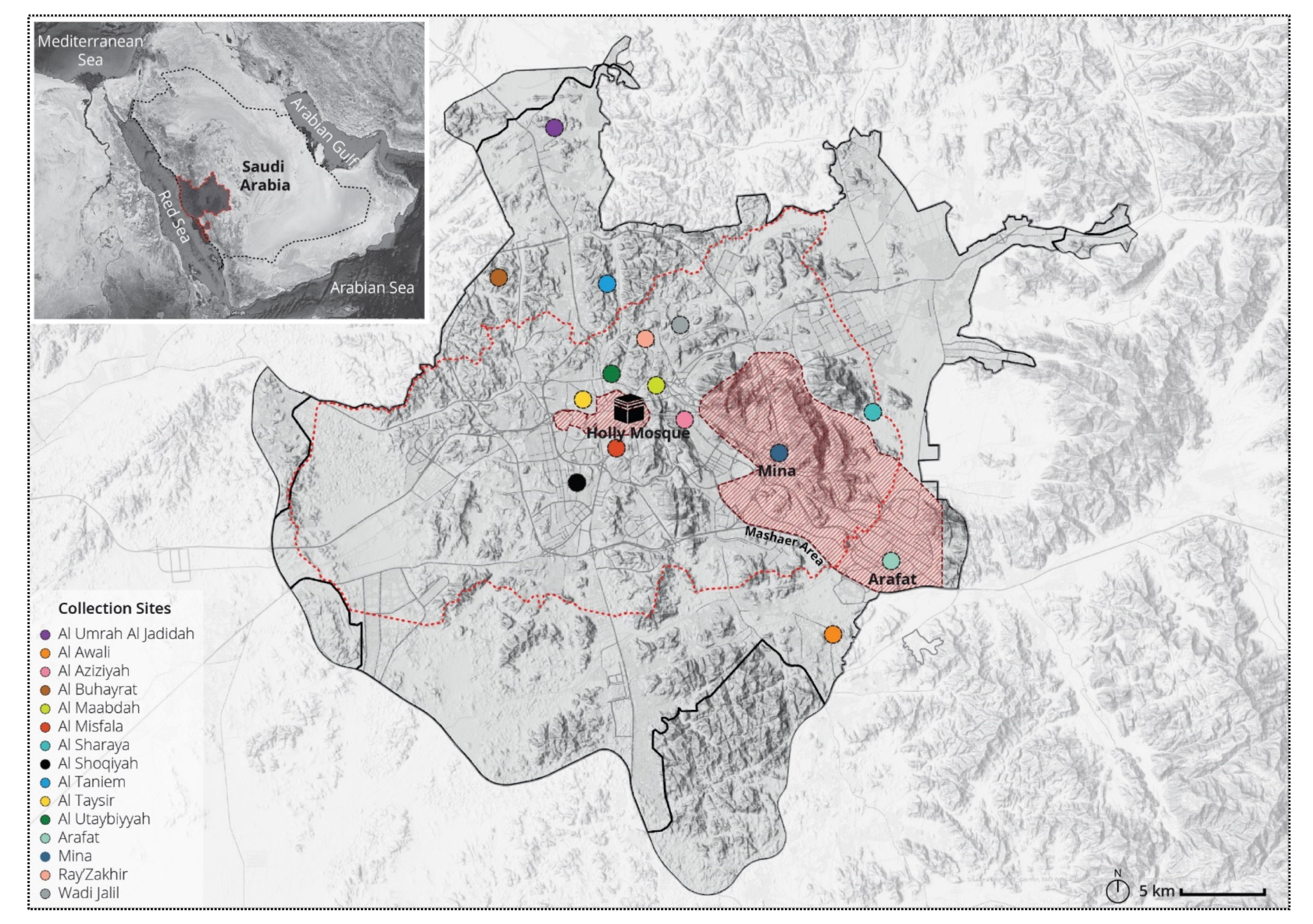
2.1. Study Area
2.2. Mosquito Samples Collection
2.3. Nucleic Acid Extraction from Mosquito Samples
2.4. Virus Detection and Identification in Mosquito Pools Homogenates
2.5. Confirmation That Mosquitoes Were Infected by Mosquito Flavivirus
2.6. Institutional Review Board Statement
3. Results
3.1. Mosquito Samples Collected
3.2. Flaviviruses Detection in the Mosquito Pools Homogenates
3.3. Identification of Flaviviruses
3.4. Confirmation That Mosquitoes Were Infected by a Mosquito Flavivirus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindenbach, B.D.; Thiel, H.J.; Rice, C.M. Flaviviridae: The viruses and their replication. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2007; pp. 1101–1152. [Google Scholar]
- Gubler, D.J.; Kuno, G.; Markoff, L. Flaviviruses. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2007; pp. 1153–1252. [Google Scholar]
- Daep, C.A.; Munoz-Jordan, J.L.; Eugenin, E.A. Flaviviruses, an expanding threat in public health: Focus on dengue, West Nile, and Japanese encephalitis virus. J. Neurovirol. 2014, 20, 539–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarner, J.; Hale, G.L. Four human diseases with significant public health impact caused by mosquito-borne flaviviruses: West Nile, Zika, dengue and yellow fever. Semin. Diagn. Pathol. 2019, 36, 170–176. [Google Scholar] [CrossRef]
- Khater, E.I.; Sowilem, M.M.; Sallam, M.F.; Alahmed, A.M. Ecology and habitat characterization of mosquitoes in Saudi Arabia. Trop. Med. 2013, 30, 409–427. [Google Scholar]
- Alahmed, A.M.; Munawar, K.; Khalil, S.M.S.; Harbach, R.E. Assessment and an updated list of the mosquitoes of Saudi Arabia. Parasites Vectors 2019, 12, 356. [Google Scholar] [CrossRef]
- Alhaeli, A.; Bahkali, S.; Ali, A.; Househ, M.S.; El-Metwally, A.A. The epidemiology of Dengue fever in Saudi Arabia: A systematic review. J. Infect. Public Health 2016, 9, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Almutairi, M.M.; Alsalem, W.S.; Hassanain, M.; Hotez, P.J. Hajj, Umrah, and the neglected tropical diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006539. [Google Scholar] [CrossRef] [PubMed]
- Elachola, H.; Gozzer, E.; Zhuo, J.; Memish, Z.A. A crucial time for public health preparedness: Zika virus and the 2016 Olympics, Umrah, and Hajj. Lancet 2016, 387, 630–632. [Google Scholar] [CrossRef] [Green Version]
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef]
- Masmejan, S.; Musso, D.; Vouga, M.; Pomar, L.; Dashraath, P.; Stojanov, M.; Panchaud, A.; Baud, D. Zika Virus. Pathogens 2020, 9, 898. [Google Scholar] [CrossRef]
- World Health Organization. In Proceedings of the WHO Statement on the First Meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika Virus and Observed Increase in Neurological Disorders and Neonatal Malformations, Teleconference, 1 February 2016.
- Coombes, R. Call to cancel 2016 Olympics because of Zika risk is not backed by WHO guidance. BMJ 2016, 353, i2899. [Google Scholar] [CrossRef] [Green Version]
- Lewnard, J.A.; Gonsalves, G.; Ko, A.I. Low Risk of International Zika Virus Spread due to the 2016 Olympics in Brazil. Ann. Intern. Med. 2016, 165, 286–287. [Google Scholar] [CrossRef] [Green Version]
- Petersen, E.; Wilson, M.E.; Touch, S.; McCloskey, B.; Mwaba, P.; Bates, M.; Dar, O.; Mattes, F.; Kidd, M.; Ippolito, G.; et al. Rapid Spread of Zika Virus in The Americas--Implications for Public Health Preparedness for Mass Gatherings at the 2016 Brazil Olympic Games. Int. J. Infect. Dis. 2016, 44, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.K. Zika virus: Epidemiology, current phobia and preparedness for upcoming mass gatherings, with examples from World Olympics and Pilgrimage. Pak. J. Med. Sci. 2016, 32, 1038–1043. [Google Scholar] [CrossRef]
- Moureau, G.; Temmam, S.; Gonzalez, J.P.; Charrel, R.N.; Grard, G.; de Lamballerie, X. A real-time RT-PCR method for the universal detection and identification of flaviviruses. Vector Borne Zoonotic Dis. 2007, 7, 467–477. [Google Scholar] [CrossRef]
- Ahmad, K. More deaths from Rift Valley fever in Saudi Arabia and Yemen. Lancet 2000, 356, 1422. [Google Scholar] [CrossRef]
- Hussain, R.; Alomar, I.; Memish, Z.A. Chikungunya virus: Emergence of an arthritic arbovirus in Jeddah, Saudi Arabia. East. Mediterr. Health J. 2013, 19, 506–508. [Google Scholar] [CrossRef]
- Amer, O.S.; Waly, M.I.; Burhan, I.W.; Al-Malki, E.S.; Smida, A.; Al-Benasy, K.S. Epidemiological trends of malaria in the Western regions of Saudi Arabia: A cross sectional study. J. Infect. Dev. Ctries. 2020, 14, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Wills, W.M.; Jakob, W.L.; Francy, D.B.; Oertley, R.E.; Anani, E.; Calisher, C.H.; Monath, T.P. Sindbis virus isolations from Saudi Arabian mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 63–66. [Google Scholar] [CrossRef]
- Al Ali, K.; Elbadry, A.A.; Eassa, A.H.; Aljuhan, A.M.; AlZubiany, S.F.; Ibrahim, E.D. A Study on Culex Species and Culex Transmitted Diseases in AI-Madinah AI-Munawarah, Saudi Arabia. Parasitol. United J. 2008, 1, 101–108. [Google Scholar]
- Altassan, K.K.; Morin, C.; Shocket, M.S.; Ebi, K.; Hess, J. Dengue fever in Saudi Arabia: A review of environmental and population factors impacting emergence and spread. Travel Med. Infect. Dis. 2019, 30, 46–53. [Google Scholar] [CrossRef]
- Ashshi, A.M. The prevalence of dengue virus serotypes in asymptomatic blood donors reveals the emergence of serotype 4 in Saudi Arabia. Virol. J. 2017, 14, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, M.A.; Merdan, A.I. Distribution and ecology of the mosquito fauna in the southwestern Saudi Arabia. J. Egypt. Soc. Parasitol. 1995, 25, 815–837. [Google Scholar]
- Al-Sheik, A.A. Larval habitat, ecology, seasonal abundance and vectorial role in malaria transmission of Anopheles arabiensis in Jazan Region of Saudi Arabia. J. Egypt. Soc. Parasitol. 2011, 41, 615–634. [Google Scholar] [PubMed]
- Alahmed, A.M.; Al Kuriji, M.A.; Kheir, S.M.; Alahmedi, S.A.; Al Hatabbi, M.J.; Al Gashmari, M.A. Mosquito fauna (Diptera: Culicidae) and seasonal activity in Makka Al Mukarramah Region, Saudi Arabia. J. Egypt. Soc. Parasitol. 2009, 39, 991–1013. [Google Scholar] [PubMed]
- Aziz, A.T.; Dieng, H.; Ahmad, A.H.; Mahyoub, J.A.; Turkistani, A.M.; Mesed, H.; Koshike, S.; Satho, T.; Salmah, M.C.; Ahmad, H.; et al. Household survey of container-breeding mosquitoes and climatic factors influencing the prevalence of Aedes aegypti (Diptera: Culicidae) in Makkah City, Saudi Arabia. Asian Pac. J. Trop. Biomed. 2012, 2, 849–857. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Countries and Territories with Current or Previous Zika Virus Transmission; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Aranda, C.; Sanchez-Seco, M.P.; Caceres, F.; Escosa, R.; Galvez, J.C.; Masia, M.; Marques, E.; Ruiz, S.; Alba, A.; Busquets, N.; et al. Detection and monitoring of mosquito flaviviruses in Spain between 2001 and 2005. Vector-Borne Zoonotic Dis. 2009, 9, 171–178. [Google Scholar] [CrossRef]
- Shimoda, H.; Hayasaka, D.; Yoshii, K.; Yokoyama, M.; Suzuki, K.; Kodera, Y.; Takeda, T.; Mizuno, J.; Noguchi, K.; Yonemitsu, K.; et al. Detection of a novel tick-borne flavivirus and its serological surveillance. Ticks Tick borne Dis. 2019, 10, 742–748. [Google Scholar] [CrossRef]
- Hobson-Peters, J.; Yam, A.W.; Lu, J.W.; Setoh, Y.X.; May, F.J.; Kurucz, N.; Walsh, S.; Prow, N.A.; Davis, S.S.; Weir, R.; et al. A new insect-specific flavivirus from northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co-infected mosquito cells. PLoS ONE 2013, 8, e56534. [Google Scholar] [CrossRef]
- Guzman, H.; Contreras-Gutierrez, M.A.; Travassos da Rosa, A.P.A.; Nunes, M.R.T.; Cardoso, J.F.; Popov, V.L.; Young, K.I.; Savit, C.; Wood, T.G.; Widen, S.G.; et al. Characterization of Three New Insect-Specific Flaviviruses: Their Relationship to the Mosquito-Borne Flavivirus Pathogens. Am. J. Trop. Med. Hyg. 2018, 98, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Zumla, A.; Alhakeem, R.F.; Assiri, A.; Turkestani, A.; Al Harby, K.D.; Alyemni, M.; Dhafar, K.; Gautret, P.; Barbeschi, M.; et al. Hajj: Infectious disease surveillance and control. Lancet 2014, 383, 2073–2082. [Google Scholar] [CrossRef]
- Saudi Ministry of Health. Health Requirements and Recommendations for Travellers to Saudi Arabia for Hajj and Umrah; Saudi Ministry of Health: Riyadh, Saudi Arabia, 2019. [Google Scholar]
- Tambo, E.; El Dessouky, A.G.; Khater, E.I.M. Innovative Preventive and Resilience Approaches Against Aedes-linked Vector-borne Arboviral Diseases Threat and Epidemics Burden in Gulf Council Countries. Oman Med. J. 2019, 34, 391–396. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yezli, S.; Yasir, M.; Yassin, Y.; Almazrua, A.; Al-Subhi, T.; Othman, N.; Omar, A.; Abdoon, A.; Elamin, Y.; Abuzaid, A.; et al. Lack of Zika Virus and Other Recognized Flaviviruses among the Mosquito Vectors during and Post the Hajj Mass Gathering. Int. J. Environ. Res. Public Health 2021, 18, 6275. https://doi.org/10.3390/ijerph18126275
Yezli S, Yasir M, Yassin Y, Almazrua A, Al-Subhi T, Othman N, Omar A, Abdoon A, Elamin Y, Abuzaid A, et al. Lack of Zika Virus and Other Recognized Flaviviruses among the Mosquito Vectors during and Post the Hajj Mass Gathering. International Journal of Environmental Research and Public Health. 2021; 18(12):6275. https://doi.org/10.3390/ijerph18126275
Chicago/Turabian StyleYezli, Saber, Muhammad Yasir, Yara Yassin, Afnan Almazrua, Tagreed Al-Subhi, Norah Othman, Abdiasiis Omar, Abdelmohsin Abdoon, Yousif Elamin, Abuzaid Abuzaid, and et al. 2021. "Lack of Zika Virus and Other Recognized Flaviviruses among the Mosquito Vectors during and Post the Hajj Mass Gathering" International Journal of Environmental Research and Public Health 18, no. 12: 6275. https://doi.org/10.3390/ijerph18126275
APA StyleYezli, S., Yasir, M., Yassin, Y., Almazrua, A., Al-Subhi, T., Othman, N., Omar, A., Abdoon, A., Elamin, Y., Abuzaid, A., Bafaraj, T., Alzahrani, H., Almahmoodi, S., Alzahrani, H., Bieh, K., Alotaibi, B., Khan, A., Alzahrani, M., & Azhar, E. I. (2021). Lack of Zika Virus and Other Recognized Flaviviruses among the Mosquito Vectors during and Post the Hajj Mass Gathering. International Journal of Environmental Research and Public Health, 18(12), 6275. https://doi.org/10.3390/ijerph18126275







