Prenatal Exposure to Di-Ethyl Phthalate (DEP) Is Related to Increasing Neonatal IgE Levels and the Altering of the Immune Polarization of Helper-T Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Questionnaire
2.3. Measurement of Total IgE in Maternal Plasma and Cord Blood Plasma
2.4. Measurement of Cytokines of Maternal Plasma and Cord Blood Plasma
2.5. Measurement of Phthalate Metabolites and BPA in Urine
2.6. Induction of Cytokine Release by MNCS
2.7. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Participants
3.2. Urinary Phthalate Metabolites
3.3. Plasma Total IgE Levels
3.4. Correlation of Maternal Characteristics with Maternal Phthalate Metabolites, Maternal IgE, and Cord Blood IgE
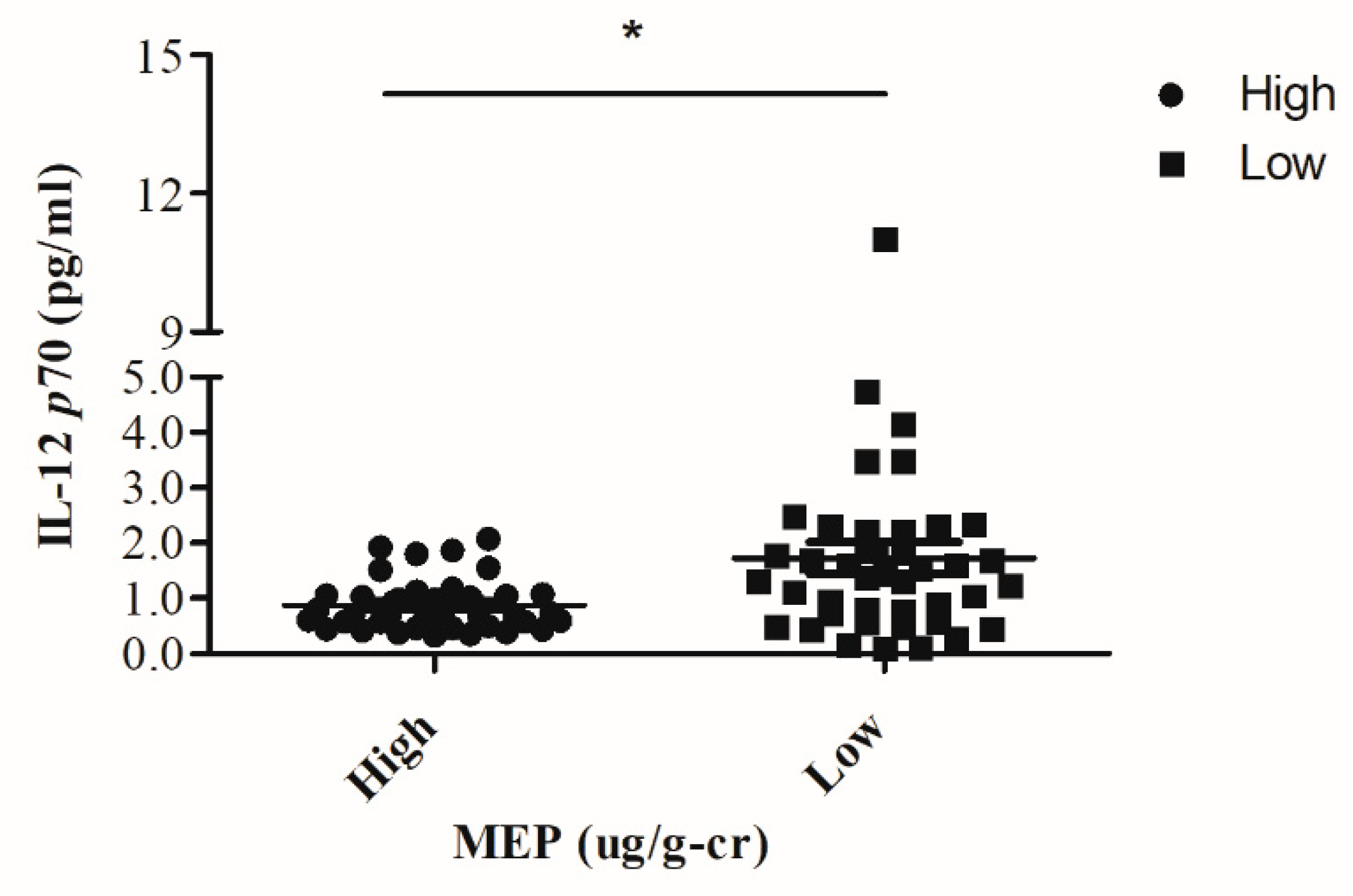
3.5. Correlation of Maternal Phthalate Metabolites, Maternal and Cord Blood Cytokines
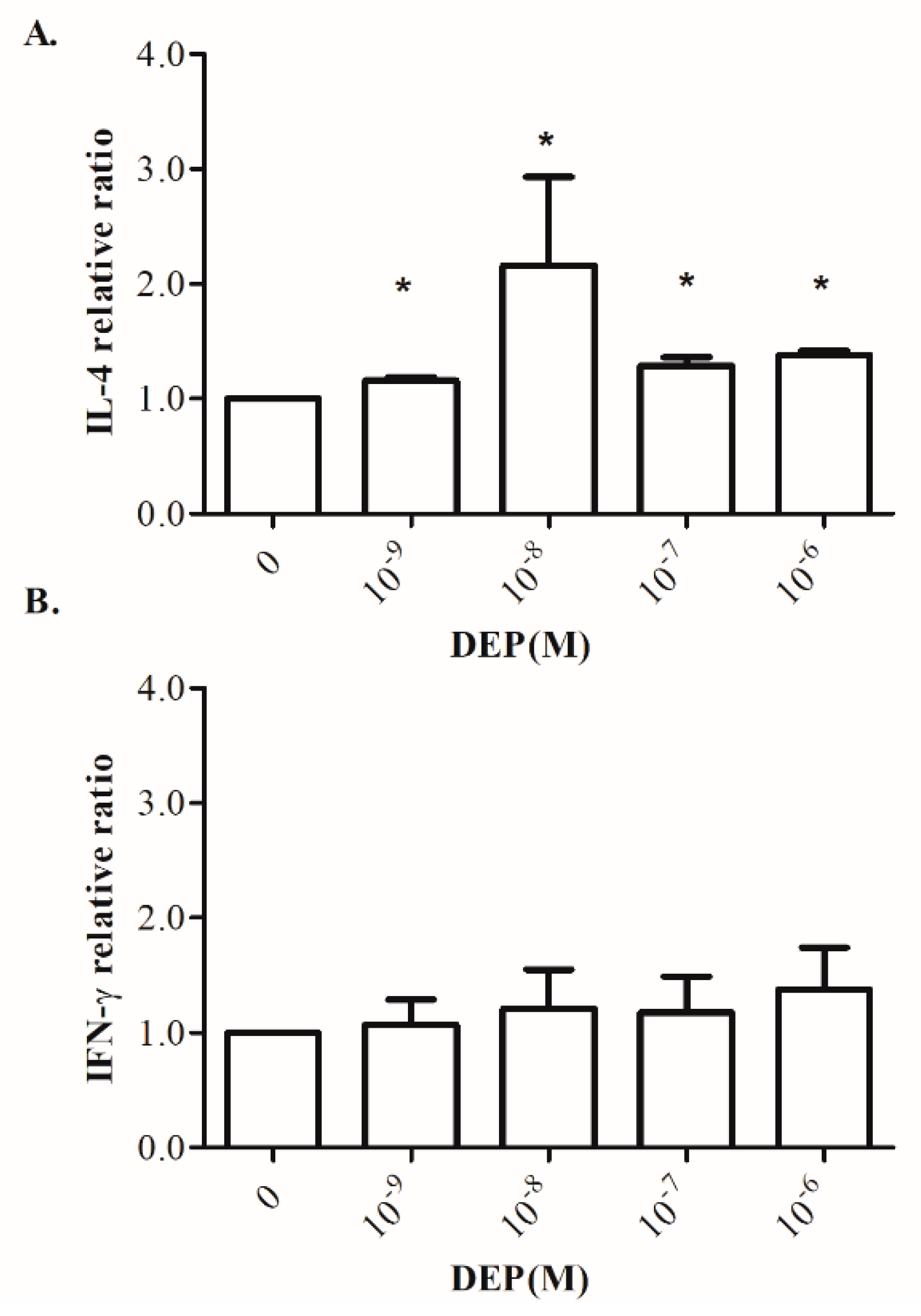
3.6. Effects of DEP on Th1/Th2-Related Cytokine Production by Cord Blood MNCs
4. Discussion
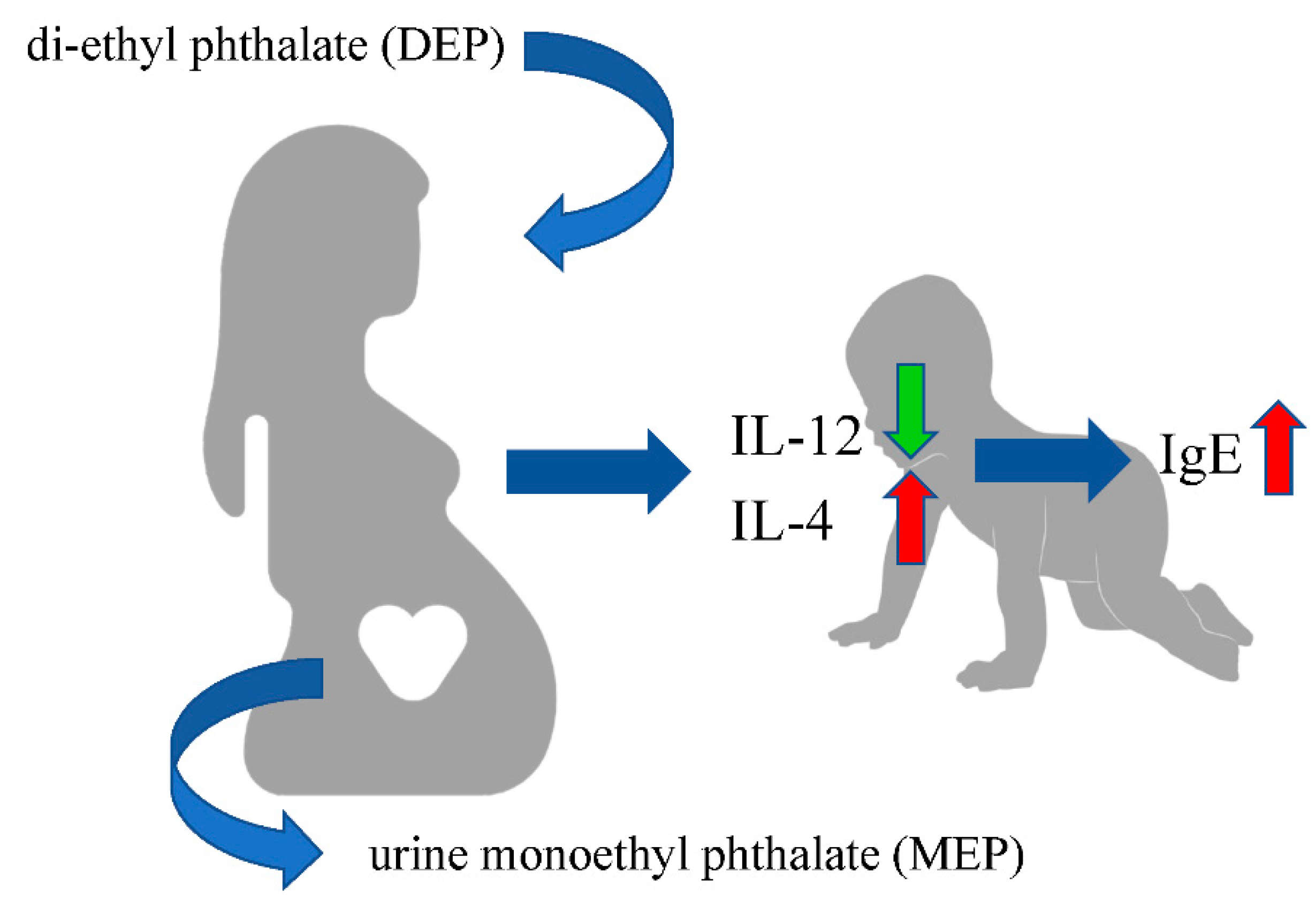
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hsieh, K.H.; Shen, J.J. Prevalence of childhood asthma in Taipei, Taiwan, and other Asian Pacific countries. J. Asthma 1988, 25, 73–82. [Google Scholar] [CrossRef]
- Asher, M.I.; Montefort, S.; Bjorksten, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; Group, I.P.T.S. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Wu, W.F.; Wan, K.S.; Wang, S.J.; Yang, W.; Liu, W.L. Prevalence, severity, and time trends of allergic conditions in 6-to-7-year-old schoolchildren in Taipei. J. Investig. Allergol. Clin. Immunol. 2011, 21, 556–562. [Google Scholar]
- Frederiksen, H.; Jensen, T.K.; Jorgensen, N.; Kyhl, H.B.; Husby, S.; Skakkebaek, N.E.; Main, K.M.; Juul, A.; Andersson, A.M. Human urinary excretion of non-persistent environmental chemicals: An overview of Danish data collected between 2006 and 2012. Reproduction 2014, 147, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Kay, V.R.; Bloom, M.S.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in males. Crit. Rev. Toxicol. 2014, 44, 467–498. [Google Scholar] [CrossRef] [PubMed]
- Erler, C.; Novak, J. Bisphenol a exposure: Human risk and health policy. J. Pediatr. Nurs. 2010, 25, 400–407. [Google Scholar] [CrossRef]
- Wu, M.T.; Wu, C.F.; Wu, J.R.; Chen, B.H.; Chen, E.K.; Chao, M.C.; Liu, C.K.; Ho, C.K. The public health threat of phthalate-tainted foodstuffs in Taiwan: The policies the government implemented and the lessons we learned. Environ. Int. 2012, 44, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Nagel, S.C.; Coe, B.L.; Angle, B.M.; Taylor, J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell Endocrinol. 2012, 354, 74–84. [Google Scholar] [CrossRef]
- Ku, H.Y.; Su, P.H.; Wen, H.J.; Sun, H.L.; Wang, C.J.; Chen, H.Y.; Jaakkola, J.J.; Wang, S.L.; Group, T. Prenatal and postnatal exposure to phthalate esters and asthma: A 9-year follow-up study of a taiwanese birth cohort. PLoS ONE 2015, 10, e0123309. [Google Scholar] [CrossRef]
- Ait Bamai, Y.; Shibata, E.; Saito, I.; Araki, A.; Kanazawa, A.; Morimoto, K.; Nakayama, K.; Tanaka, M.; Takigawa, T.; Yoshimura, T.; et al. Exposure to house dust phthalates in relation to asthma and allergies in both children and adults. Sci. Total Environ. 2014, 485, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Hoppin, J.A.; Jaramillo, R.; London, S.J.; Bertelsen, R.J.; Salo, P.M.; Sandler, D.P.; Zeldin, D.C. Phthalate exposure and allergy in the U.S. population: Results from NHANES 2005–2006. Environ. Health Perspect. 2013, 121, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.R.; Tsai, C.C.; Chang, L.S.; Huang, H.C.; Cheng, H.H.; Wang, J.Y.; Sheen, J.M.; Kuo, H.C.; Hsieh, K.S.; Huang, Y.H.; et al. l-Arginine-Dependent Epigenetic Regulation of Interleukin-10, but Not Transforming Growth Factor-beta, Production by Neonatal Regulatory T Lymphocytes. Front. Immunol. 2017, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Ownby, D.R.; McCullough, J.; Johnson, C.C.; Peterson, E.L. Evaluation of IgA measurements as a method for detecting maternal blood contamination of cord blood samples. Pediatr. Allergy Immunol. 1996, 7, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Milgram, K.E.; Silva, M.J.; Malek, N.A.; Reidy, J.A.; Needham, L.L.; Brock, J.W. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal. Chem. 2000, 72, 4127–4134. [Google Scholar] [CrossRef]
- Yu, H.R.; Chang, J.C.; Chen, R.F.; Chuang, H.; Hong, K.C.; Wang, L.; Yang, K.D. Different antigens trigger different Th1/Th2 reactions in neonatal mononuclear cells (MNCs) relating to T-bet/GATA-3 expression. J. Leukoc. Biol. 2003, 74, 952–958. [Google Scholar] [CrossRef]
- O’Connell, E.J. Pediatric allergy: A brief review of risk factors associated with developing allergic disease in childhood. Ann. Allergy Asthma Immunol. 2003, 90, 53–58. [Google Scholar] [CrossRef]
- Nabavi, M.; Ghorbani, R.; Asadi, A.M.; Faranoush, M. Factors associated with cord blood IgE levels. Asian Pac. J. Allergy Immunol. 2013, 31, 157–162. [Google Scholar] [CrossRef]
- De Amici, M.; Perotti, F.; Marseglia, G.L.; Ierullo, A.M.; Bollani, L.; Decembrino, L.; Licari, A.; Quaglini, S.; Stronati, M.; Spinillo, A. Cord and blood levels of newborn IgE: Correlation, role and influence of maternal IgE. Immunobiology 2017, 222, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T. A conceptual framework for the developmental origins of health and disease. J. Dev. Orig. Health Dis. 2010, 1, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.; Prescott, S. Epigenetics and prenatal influences on asthma and allergic airways disease. Chest 2011, 139, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Kimber, I.; Dearman, R.J. An assessment of the ability of phthalates to influence immune and allergic responses. Toxicology 2010, 271, 73–82. [Google Scholar] [CrossRef]
- Gascon, M.; Casas, M.; Morales, E.; Valvi, D.; Ballesteros-Gomez, A.; Luque, N.; Rubio, S.; Monfort, N.; Ventura, R.; Martinez, D.; et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J. Allergy Clin. Immunol. 2015, 135, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Whyatt, R.M.; Perzanowski, M.S.; Just, A.C.; Rundle, A.G.; Donohue, K.M.; Calafat, A.M.; Hoepner, L.A.; Perera, F.P.; Miller, R.L. Asthma in inner-city children at 5–11 years of age and prenatal exposure to phthalates: The Columbia Center for Children’s Environmental Health Cohort. Environ. Health Perspect. 2014, 122, 1141–1146. [Google Scholar] [CrossRef]
- Wang, I.J.; Lin, C.C.; Lin, Y.J.; Hsieh, W.S.; Chen, P.C. Early life phthalate exposure and atopic disorders in children: A prospective birth cohort study. Environ. Int. 2014, 62, 48–54. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, J.; Zhang, Q.; Yin, X.; Wang, Y.; Fu, J.; Zou, L.; Kong, W. Maternofetal transfer of antibodies and the influence of maternal atopic status on the neonate. Am. J. Rhinol. Allergy 2015, 29, 119–123. [Google Scholar] [CrossRef]
- Duty, S.M.; Ackerman, R.M.; Calafat, A.M.; Hauser, R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ. Health Perspect. 2005, 113, 1530–1535. [Google Scholar] [CrossRef]
- Hoppin, J.A.; Brock, J.W.; Davis, B.J.; Baird, D.D. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ. Health Perspect. 2002, 110, 515–518. [Google Scholar] [CrossRef]
- Hauser, R.; Meeker, J.D.; Park, S.; Silva, M.J.; Calafat, A.M. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ. Health Perspect. 2004, 112, 1734–1740. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Ko, Y.A.; Mukherjee, B.; Meeker, J.D. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ. Int. 2014, 70, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; McElrath, T.F.; Mukherjee, B.; Loch-Caruso, R.; Meeker, J.D. Associations between Maternal Biomarkers of Phthalate Exposure and Inflammation Using Repeated Measurements across Pregnancy. PLoS ONE 2015, 10, e0135601. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.M.; Bennett, D.H.; Barkoski, J.; Ye, X.; Calafat, A.M.; Tancredi, D.; Hertz-Picciotto, I. Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ. Int. 2019, 122, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Sadeghnejad, A.; Karmaus, W.; Davis, S.; Kurukulaaratchy, R.J.; Matthews, S.; Arshad, S.H. Raised cord serum immunoglobulin E increases the risk of allergic sensitisation at ages 4 and 10 and asthma at age 10. Thorax 2004, 59, 936–942. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kjellman, N.I.; Croner, S. Cord blood IgE determination for allergy prediction--a follow-up to seven years of age in 1651 children. Ann. Allergy 1984, 53, 167–171. [Google Scholar]
- Ait Bamai, Y.; Miyashita, C.; Araki, A.; Nakajima, T.; Sasaki, S.; Kishi, R. Effects of prenatal di(2-ethylhexyl) phthalate exposure on childhood allergies and infectious diseases: The Hokkaido Study on Environment and Children’s Health. Sci. Total Environ. 2018, 618, 1408–1415. [Google Scholar] [CrossRef]
- Ashley-Martin, J.; Dodds, L.; Levy, A.R.; Platt, R.W.; Marshall, J.S.; Arbuckle, T.E. Prenatal exposure to phthalates, bisphenol A and perfluoroalkyl substances and cord blood levels of IgE, TSLP and IL-33. Environ. Res. 2015, 140, 360–368. [Google Scholar] [CrossRef]
- Poulsen, L.K.; Hummelshoj, L. Triggers of IgE class switching and allergy development. Ann. Med. 2007, 39, 440–456. [Google Scholar] [CrossRef]
- Ozdemir, C.; Akdis, M.; Akdis, C.A. T-cell response to allergens. Chem. Immunol. Allergy 2010, 95, 22–44. [Google Scholar]
- Bornehag, C.G.; Nanberg, E. Phthalate exposure and asthma in children. Int. J. Androl. 2010, 33, 333–345. [Google Scholar] [CrossRef]
- Hansen, J.F.; Nielsen, C.H.; Brorson, M.M.; Frederiksen, H.; Hartoft-Nielsen, M.L.; Rasmussen, A.K.; Bendtzen, K.; Feldt-Rasmussen, U. Influence of phthalates on in vitro innate and adaptive immune responses. PLoS ONE 2015, 10, e0131168. [Google Scholar] [CrossRef]
- Hsieh, C.S.; Macatonia, S.E.; Tripp, C.S.; Wolf, S.F.; O’Garra, A.; Murphy, K.M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 1993, 260, 547–549. [Google Scholar] [CrossRef]
- Zheng, H.; Ban, Y.; Wei, F.; Ma, X. Regulation of Interleukin-12 Production in Antigen-Presenting Cells. Adv. Exp. Med. Biol. 2016, 941, 117–138. [Google Scholar]
- Kuo, F.C.; Su, S.W.; Wu, C.F.; Huang, M.C.; Shiea, J.; Chen, B.H.; Chen, Y.L.; Wu, M.T. Relationship of urinary phthalate metabolites with serum thyroid hormones in pregnant women and their newborns: A prospective birth cohort in Taiwan. PLoS ONE 2015, 10, e0123884. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Li, S.S. Phthalates: Toxicogenomics and inferred human diseases. Genomics 2011, 97, 148–157. [Google Scholar] [CrossRef]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D.; et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect. 2013, 121, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Wang, Y.H.; Wang, S.L.; Huang, P.C.; Chuang, S.C.; Chen, M.H.; Chen, B.H.; Sun, C.W.; Fu, H.C.; Lee, C.C.; et al. Exposure sources and their relative contributions to urinary phthalate metabolites among children in Taiwan. Int. J. Hyg. Environ. Health 2017, 220, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Shi, H.; Zhang, Y.; Cao, Y. Dietary intake and phthalates body burden in boys and girls. Arch. Public Health 2015, 73, 5. [Google Scholar] [CrossRef]
- Cheng, Z.; Nie, X.P.; Wang, H.S.; Wong, M.H. Risk assessments of human exposure to bioaccessible phthalate esters through market fish consumption. Environ. Int. 2013, 57, 75–80. [Google Scholar] [CrossRef] [PubMed]

| Variable | Subjects (n = 101) |
|---|---|
| Mean ± SD or n (%) | |
| Mother | |
| Age at delivery (years) | 34.12 ± 3.99 |
| BMI at delivery (Kg/m2) | 27.08 ± 4.42 |
| History of atopic disease | |
| Asthma | 7 (6.9) |
| Allergic rhinitis | 35 (34.7) |
| Atopic dermatitis | 16 (15.8) |
| Newborn | |
| Gestation age (weeks) | 38.04 ± 2.42 |
| Birth height (cm) | 48.34 ± 3.04 |
| Birth weight (g) | 2932.66 ± 555.48 |
| Birth head circumference (cm) | 33.16 ± 1.78 |
| Variables | Percentiles | |||||
|---|---|---|---|---|---|---|
| n | Min | 25th | 50th | 75th | Max | |
| MEP (μg/g-creatinine) | 101 | 0.69 | 3.38 | 9.80 | 22.00 | 39.40 |
| MnBP (μg/g-creatinine) | 101 | 0.01 | 3.59 | 6.20 | 13.75 | 67.00 |
| MBzP (μg/g-creatinine) | 101 | 0.02 | 0.14 | 0.25 | 0.46 | 1.36 |
| MEHP (μg/g-creatinine) | 101 | 0.01 | 0.65 | 1.70 | 3.04 | 19.10 |
| BPA (μg/g-creatinine) | 101 | 0.001 | 0.86 | 1.58 | 3.07 | 51.90 |
| Maternal IgE (KU/L) | 101 | 1.00 | 16.65 | 38.20 | 97.30 | 616.00 |
| Cord blood IgE (KU/L) | 101 | 0.0001 | 0.09 | 0.20 | 0.41 | 9.00 |
| Characteristics | % | MEP | MnBP | MBzP | MEHP | BPA | Maternal IgE | Cord Blood IgE |
|---|---|---|---|---|---|---|---|---|
| Age at delivery (years) | ||||||||
| ≤35 | 59.4 | 12.24 ± 1.27 | 9.57 ± 1.34 | 0.32 ± 0.03 | 2.34 ± 0.34 | 1.94 ± 0.28 * | 80.24 ± 13.75 | 0.52 ± 0.16 |
| >35 | 40.6 | 14.22 ± 1.89 | 9.77 ± 1.41 | 0.31 ± 0.04 | 2.96 ± 0.64 | 4.22 ± 1.35 | 65.66 ± 14.19 | 0.25 ± 0.05 |
| Maternal education | ||||||||
| <high school | 9.9 | 12.9 ± 2.95 | 15.92 ± 6.09 | 0.37 ± 0.06 | 2.43 ± 0.85 | 2.18 ± 0.37 | 117.94 ± 44.48 | 0.46 ± 0.14 |
| ≥high school | 90.1 | 13.06 ± 1.15 | 8.96 ± 0.85 | 0.31 ± 0.03 | 2.61 ± 0.35 | 2.94 ± 0.64 | 69.53 ± 9.92 | 0.40 ± 0.10 |
| Maternal history of allergies | ||||||||
| Asthma | ||||||||
| Yes | 6.9 | 13.45 ± 4.49 | 14.27 ± 8.98 | 0.36 ± 0.18 | 2.29 ± 1.23 | 1.92 ± 0.40 | 178.23 ± 52.42 † | 0.50 ± 0.12 |
| No | 93.1 | 13.01 ± 1.11 | 9.31 ± 0.83 | 0.31 ± 0.02 | 2.61 ± 0.34 | 2.94 ± 0.62 | 66.58 ± 9.62 | 0.40 ± 0.10 |
| Allergic rhinitis | ||||||||
| Yes | 34.7 | 12.84 ± 2.02 | 6.09 ± 0.81 † | 0.27 ± 0.05 * | 2.57 ± 0.65 | 1.85 ± 0.24 | 113.06 ± 22.95 † | 0.57 ± 0.26 |
| No | 65.3 | 13.15 ± 1.26 | 11.54 ± 1.38 | 0.34 ± 0.03 | 2.60 ± 0.37 | 3.41 ± 0.87 | 53.78 ± 8.33 | 0.33 ± 0.05 |
| Atopic dermatitis | ||||||||
| Yes | 15.8 | 14.50 ± 2.77 | 8.36 ± 1.75 | 0.45 ± 0.09 | 3.00 ± 0.86 | 1.84 ± 0.35 | 97.92 ± 36.46 | 0.48 ± 0.15 |
| No | 84.2 | 12.77 ± 1.17 | 9.89 ± 1.11 | 0.29 ± 0.02 | 2.52 ± 0.36 | 3.06 ± 0.69 | 69.88 ± 9.72 | 0.40 ± 0.11 |
| Maternal dietary intake | ||||||||
| Milk | ||||||||
| Seldom | 24.8 | 10.03 ± 1.73 | 8.87 ± 1.67 | 0.30 ± 0.04 | 2.05 ± 0.37 | 3.72 ± 2.03 | 70.64 ± 18.03 | 0.34 ± 0.08 |
| Often | 75.2 | 14.03 ± 1.30 | 9.91 ± 1.18 | 0.32 ± 0.03 | 2.77 ± 0.42 | 2.59 ± 0.40 | 75.53 ± 11.92 | 0.43 ± 0.12 |
| Egg | ||||||||
| Seldom | 12.9 | 8.79 ± 1.87 | 9.47 ± 1.55 | 0.30 ± 0.04 | 1.93 ± 0.42 | 1.62 ± 0.37 | 87.77 ± 33.23 | 0.29 ± 0.11 |
| Often | 87.1 | 13.67 ± 1.19 | 9.68 ± 1.10 | 0.32 ± 0.03 | 2.69 ± 0.37 | 3.05 ± 0.66 | 72.33 ± 10.41 | 0.43 ± 0.11 |
| Nut | ||||||||
| Seldom | 77.2 | 12.28 ± 1.24 | 8.85 ± 0.86 | 0.30 ± 0.03 | 2.56 ± 0.39 | 2.34 ± 0.36 | 79.12 ± 12.15 | 0.41 ± 0.12 |
| Often | 22.8 | 15.60 ± 2.13 | 12.39 ± 3.13 | 0.36 ± 0.06 | 2.69 ± 0.59 | 4.64 ± 2.23 | 58.05 ± 14.81 | 0.42 ± 0.09 |
| Shell seafood | ||||||||
| Seldom | 91.1 | 12.46 ± 1.10 | 8.80 ± 0.85 * | 0.32 ± 0.26 | 2.56 ± 0.35 | 2.89 ± 0.64 | 75.03 ± 10.59 | 0.41 ± 0.10 |
| Often | 8.9 | 18.96 ± 4.03 | 18.39 ± 6.28 | 0.30 ± 0.06 | 2.95 ± 0.92 | 2.64 ± 0.48 | 67.03 ± 30.16 | 0.37 ± 0.10 |
| Fish | ||||||||
| Seldom | 40.6 | 12.49 ± 1.67 | 8.22 ± 1.34 | 0.39 ± 0.05 | 2.43 ± 0.43 | 2.89 ± 0.71 | 58.40 ± 16.03 * | 0.56 ± 0.23 |
| Often | 59.4 | 13.41 ± 1.42 | 10.63 + 1.36 | 0.27 ± 0.02 | 2.70 ± 0.47 | 2.85 ± 0.85 | 85.20 ± 12.65 | 0.31 ± 0.04 |
| Furry pets at home during pregnancy | ||||||||
| Yes | 34.7 | 12.35 ± 1.78 | 9.11 ± 1.24 | 0.30 ± 0.03 | 2.23 ± 0.41 | 3.09 ± 0.74 | 55.77 ± 11.28 | 0.32 ± 0.07 |
| No | 65.3 | 13.41 ± 1.36 | 9.94 ± 1.35 | 0.33 ± 0.03 | 2.78 ± 0.45 | 2.74 ± 0.80 | 84.16 ± 13.95 | 0.46 ± 0.14 |
| Family smoking exposure | ||||||||
| Yes | 18.8 | 8.95 ± 1.83 | 7.53 ± 1.22 | 0.35 ± 0.08 | 1.62 ± 0.43 | 2.82 ± 0.77 | 99.72 ± 32.60 | 0.32 ± 0.11 |
| No | 81.2 | 13.99 ± 1.24 | 10.14 ± 1.17 | 0.31 ± 0.02 | 2.82 ± 0.39 | 2.88 ± 0.69 | 68.44 ± 9.71 | 0.43 ± 0.11 |
| MEP | MnBP | MBzP | MEHP | BPA | Maternal IgE | Cord Blood IgE | |
|---|---|---|---|---|---|---|---|
| MEP | 1.0 | ||||||
| MnBP | 0.237 † | 1.0 | |||||
| MBzP | 0.381 † | 0.364 † | 1.0 | ||||
| MEHP | 0.318 † | 0.204 * | 0.379 † | 1.0 | |||
| BPA | 0.230 * | 0.225 * | 0.229 * | 0.251 † | 1.0 | ||
| Maternal IgE | 0.097 | 0.004 | 0.064 | 0.027 | −0.064 | 1.0 | |
| Cord blood IgE | 0.226 * | −0.054 | 0.028 | 0.088 | −0.086 | 0.393 † | 1.0 |
| Maternal Plasma | |||||||
| MEP | MnBP | MBzP | MEHP | BPA | Maternal IgE | Cord blood IgE | |
| IL-10 | 0.142 | 0.221 | −0.031 | 0.01 | −0.122 | 0.091 | 0.089 |
| IL-12p70 | −0.171 | 0.072 | −0.134 | −0.04 | 0.073 | 0.122 | −0.101 |
| Cord Blood | |||||||
| MEP | MnBP | MBzP | MEHP | BPA | Maternal IgE | Cord blood IgE | |
| IL-10 | 0.221 * | 0.040 | 0.032 | −0.118 | −0.130 | 0.044 | 0.264 * |
| IL-12p70 | −0.309 * | −0.036 | −0.050 | −0.033 | −0.019 | −0.083 | −0.186 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-K.; Cheng, H.-H.; Hsu, T.-Y.; Wang, J.-Y.; Hung, C.-H.; Tsai, C.-C.; Lai, Y.-J.; Lin, Y.-J.; Huang, H.-C.; Chan, J.Y.H.; et al. Prenatal Exposure to Di-Ethyl Phthalate (DEP) Is Related to Increasing Neonatal IgE Levels and the Altering of the Immune Polarization of Helper-T Cells. Int. J. Environ. Res. Public Health 2021, 18, 6364. https://doi.org/10.3390/ijerph18126364
Tsai C-K, Cheng H-H, Hsu T-Y, Wang J-Y, Hung C-H, Tsai C-C, Lai Y-J, Lin Y-J, Huang H-C, Chan JYH, et al. Prenatal Exposure to Di-Ethyl Phthalate (DEP) Is Related to Increasing Neonatal IgE Levels and the Altering of the Immune Polarization of Helper-T Cells. International Journal of Environmental Research and Public Health. 2021; 18(12):6364. https://doi.org/10.3390/ijerph18126364
Chicago/Turabian StyleTsai, Chang-Ku, Hsin-Hsin Cheng, Te-Yao Hsu, Jiu-Yao Wang, Chih-Hsing Hung, Ching-Chang Tsai, Yun-Ju Lai, Yu-Ju Lin, Hsin-Chun Huang, Julie Y. H. Chan, and et al. 2021. "Prenatal Exposure to Di-Ethyl Phthalate (DEP) Is Related to Increasing Neonatal IgE Levels and the Altering of the Immune Polarization of Helper-T Cells" International Journal of Environmental Research and Public Health 18, no. 12: 6364. https://doi.org/10.3390/ijerph18126364
APA StyleTsai, C.-K., Cheng, H.-H., Hsu, T.-Y., Wang, J.-Y., Hung, C.-H., Tsai, C.-C., Lai, Y.-J., Lin, Y.-J., Huang, H.-C., Chan, J. Y. H., Tain, Y.-L., Chen, C.-C., Tsai, T.-A., & Yu, H.-R. (2021). Prenatal Exposure to Di-Ethyl Phthalate (DEP) Is Related to Increasing Neonatal IgE Levels and the Altering of the Immune Polarization of Helper-T Cells. International Journal of Environmental Research and Public Health, 18(12), 6364. https://doi.org/10.3390/ijerph18126364









