Effect of PM2.5 Levels on Respiratory Pediatric ED Visits in a Semi-Urban Greek Peninsula
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Patient Data
2.3. Network Implementation and PM2.5 Levels Measurements
2.4. Statistical Analysis
3. Results
3.1. Data Collection and Patient Characteristics
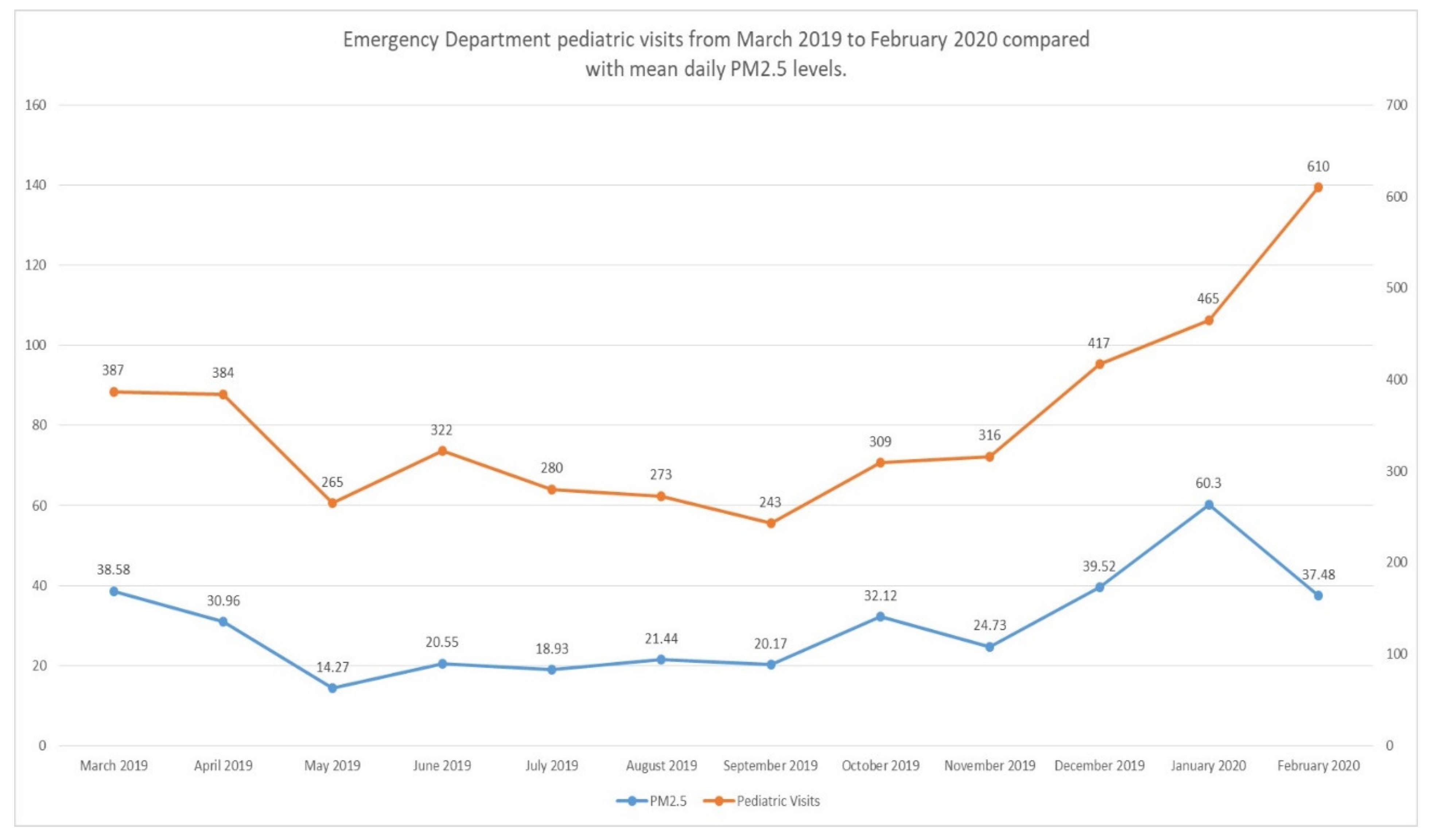
3.2. ED Visits and PM2.5 Levels
3.3. Association of Upper Respiratory Tract Infection, Asthma and Pneumonia with PM2.5 Levels
4. Discussion
4.1. PM2.5 Concentrations
4.2. Health Impacts of PM2.5
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Air Pollution. Available online: https://www.who.int/westernpacific/health-topics/air-pollution (accessed on 1 November 2020).
- Park, M.; Joo, H.S.; Lee, K.; Jang, M.; Kim, S.D.; Kim, I.; Borlaza, L.J.S.; Lim, H.; Shin, H.; Chung, K.H.; et al. Differential Toxicities of Fine Particulate Matters from Various Sources. Sci. Rep. 2018, 8, 17007. [Google Scholar] [CrossRef]
- GBD 2015 Risk Factors Collaborators. Global, Regional, and National Comparative Risk Assessment of 79 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; Occupational and Environmental Health Team. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment. Lignes Directrices OMS Relatives à la Qualité de L’air: Particules, Ozone, Dioxyde D’azote et Dioxyde de Soufre: Mise à Jour Mondiale 2005: Synthèse de L’évaluation des Risques; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Concentrations of Particulate Matter (PM10 and PM2.5). Available online: https://www.gov.uk/government/publications/air-quality-statistics/concentrations-of-particulate-matter-pm10-and-pm25 (accessed on 1 November 2020).
- Xie, Y.; Wang, Y.; Zhang, K.; Dong, W.; Lv, B.; Bai, Y. Daily Estimation of Ground-Level PM2.5 Concentrations over Beijing Using 3 Km Resolution MODIS AOD. Environ. Sci. Technol. 2015, 49, 12280–12288. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Zhou, L.; Tang, M.; Wang, B. Association of Fine Particles with Respiratory Disease Mortality: A Meta-Analysis. Arch. Environ. Occup. Health 2015, 70, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ruan, Z.; Wang, X.; Yang, Y.; Mason, T.G.; Lin, H.; Tian, L. Short-Term and Long-Term Exposures to Fine Particulate Matter Constituents and Health: A Systematic Review and Meta-Analysis. Environ. Pollut. 2019, 247, 874–882. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Kang, S.; Anderson, H.R.; Mills, I.C.; Walton, H.A. Epidemiological Time Series Studies of PM2.5 and Daily Mortality and Hospital Admissions: A Systematic Review and Meta-Analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Cao, X.; Guo, M.; Xie, M.; Liu, X. Trends and Risk Factors of Mortality and Disability Adjusted Life Years for Chronic Respiratory Diseases from 1990 to 2017: Systematic Analysis for the Global Burden of Disease Study 2017. BMJ 2020, 368, m234. [Google Scholar] [CrossRef] [Green Version]
- Krall, J.R.; Mulholland, J.A.; Russell, A.G.; Balachandran, S.; Winquist, A.; Tolbert, P.E.; Waller, L.A.; Sarnat, S.E. Associations between Source-Specific Fine Particulate Matter and Emergency Department Visits for Respiratory Disease in Four U.S. Cities. Environ. Health Perspect. 2017, 125, 97–103. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, Y.; Mulholland, J.A.; Russell, A.G.; Darrow, L.A.; Tolbert, P.E.; Strickland, M.J. Pediatric Emergency Department Visits and Ambient Air Pollution in the U.S. State of Georgia: A Case-Crossover Study. Environ. Health 2016, 15, 115. [Google Scholar] [CrossRef] [Green Version]
- Norris, G.; YoungPong, S.N.; Koenig, J.Q.; Larson, T.V.; Sheppard, L.; Stout, J.W. An Association between Fine Particles and Asthma Emergency Department Visits for Children in Seattle. Environ. Health Perspect. 1999, 107, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Moustris, K.P.; Proias, G.T.; Larissi, I.K.; Nastos, P.T.; Koukouletsos, K.V.; Paliatsos, A.G. Health Impacts Due to Particulate Air Pollution in Volos City, Greece. J. Environ. Sci. Health Part A 2016, 51, 15–20. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Insurance Portability and Accountability Act of 1996 (HIPAA)|CDC. Available online: https://www.cdc.gov/phlp/publications/topic/hipaa.html (accessed on 19 August 2020).
- Rogulski, M. Low-Cost PM Monitors as an Opportunity to Increase the Spatiotemporal Resolution of Measurements of Air Quality. Energy Procedia 2017, 128, 437–444. [Google Scholar] [CrossRef]
- Tagle, M.; Rojas, F.; Reyes, F.; Vásquez, Y.; Hallgren, F.; Lindén, J.; Kolev, D.; Watne, Å.K.; Oyola, P. Field Performance of a Low-Cost Sensor in the Monitoring of Particulate Matter in Santiago, Chile. Environ. Monit. Assess. 2020, 192, 171. [Google Scholar] [CrossRef] [Green Version]
- Flu News Europe—Joint ECDC and WHO/Europe Weekly Influenza Update. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/surveillance-and-disease-data/flu-news-europe (accessed on 5 June 2021).
- Das, M.; Maiti, S.K.; Mukhopadhyay, U. Distribution of PM2.5 and PM10-2.5 in PM10 Fraction in Ambient Air Due to Vehicular Pollution in Kolkata Megacity. Environ. Monit. Assess. 2006, 122, 111–123. [Google Scholar] [CrossRef] [PubMed]
- World’s Most Polluted Cities in 2019—PM2.5 Ranking|AirVisual. Available online: https://www.airvisual.com/world-most-polluted-cities (accessed on 6 January 2021).
- Sicard, P.; Khaniabadi, Y.O.; Perez, S.; Gualtieri, M.; De Marco, A. Effect of O3, PM10 and PM2.5 on Cardiovascular and Respiratory Diseases in Cities of France, Iran and Italy. Environ. Sci. Pollut. Res. Int. 2019, 26, 32645–32665. [Google Scholar] [CrossRef]
- Grivas, G.; Chaloulakou, A.; Samara, C.; Spyrellis, N. Spatial and Temporal Variation of PM10 Mass Concentrations within the Greater Area of Athens, Greece. Water Air Soil Pollut. 2004, 158, 357–371. [Google Scholar] [CrossRef]
- Lekkas, P.T. Atmospheric PM10 Particle Concentration Measurements at Central and Peripheral Urban Sites in Athens and Thessaloniki, Greece. Available online: https://journal.gnest.org/publication/691 (accessed on 6 January 2021).
- Zhang, R.; Wang, G.; Guo, S.; Zamora, M.L.; Ying, Q.; Lin, Y.; Wang, W.; Hu, M.; Wang, Y. Formation of Urban Fine Particulate Matter. Chem. Rev. 2015, 115, 3803–3855. [Google Scholar] [CrossRef]
- Pacca, L.; Antonarakis, A.; Schröder, P.; Antoniades, A. The Effect of Financial Crises on Air Pollutant Emissions: An Assessment of the Short vs. Medium-Term Effects. Sci. Total Environ. 2020, 698, 133614. [Google Scholar] [CrossRef]
- Molnar, P.; Gustafson, P.; Johannesson, S.; Boman, J.; Barregård, L.; Sällsten, G. Domestic wood burning and PM2.5 trace elements: Personal exposures, indoor and outdoor levels. Atmos. Environ. 2005, 39, 2643–2653. [Google Scholar] [CrossRef]
- In Crisis, Greeks Turn to Wood-Burning—and Choke, By Nicholas Paphitis|Kathimerini. Available online: https://www.ekathimerini.com/147932/article/ekathimerini/community/in-crisis-greeks-turn-to-wood-burning--and-choke (accessed on 6 January 2021).
- Lo, C.W.-H.; Fryxell, G.E.; Wong, W.W.-H. Effective Regulations with Little Effect? The Antecedents of the Perceptions of Environmental Officials on Enforcement Effectiveness in China. Environ. Manag. 2006, 38, 388–410. [Google Scholar] [CrossRef]
- Sierra-Vargas, M.P.; Teran, L.M. Air pollution: Impact and prevention. Respirology 2012, 17, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Beijing’s Air Quality Improvements Are a Model for Other Cities. Available online: https://www.ccacoalition.org/en/news/beijing%E2%80%99s-air-quality-improvements-are-model-other-cities (accessed on 11 January 2021).
- Zhang, L.; Morisaki, H.; Wei, Y.; Li, Z.; Yang, L.; Zhou, Q.; Zhang, X.; Xing, W.; Hu, M.; Shima, M.; et al. Characteristics of Air Pollutants inside and Outside a Primary School Classroom in Beijing and Respiratory Health Impact on Children. Environ. Pollut. 2019, 255, 113147. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.-F.; Xu, Y.-H.; Shi, M.-H.; Lian, Y.-X. The Impact of PM2.5 on the Human Respiratory System. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef]
- Chi, R.; Li, H.; Wang, Q.; Zhai, Q.; Wang, D.; Wu, M.; Liu, Q.; Wu, S.; Ma, Q.; Deng, F.; et al. Association of Emergency Room Visits for Respiratory Diseases with Sources of Ambient PM2.5. J. Environ. Sci. 2019, 86, 154–163. [Google Scholar] [CrossRef]
- Pennington, A.F.; Strickland, M.J.; Gass, K.; Klein, M.; Sarnat, S.E.; Tolbert, P.E.; Balachandran, S.; Chang, H.H.; Russell, A.G.; Mulholland, J.A.; et al. Source-Apportioned PM2.5 and Cardiorespiratory Emergency Department Visits: Accounting for Source Contribution Uncertainty. Epidemiology 2019, 30, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhong, T.; Zhu, Y.; Ge, D.; Lin, X.; Li, Q. Effects of Particulate Matter (PM) on Childhood Asthma Exacerbation and Control in Xiamen, China. BMC Pediatr. 2019, 19, 194. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Xu, C.; Ji, G.; Liu, H.; Shao, W.; Zhang, C.; Gu, A.; Zhao, P. Effect of Exposure to Ambient PM2.5 Pollution on the Risk of Respiratory Tract Diseases: A Meta-Analysis of Cohort Studies. J. Biomed. Res. 2017, 31, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.L.; Mangum, J.B.; De Lorme, M.P.; Everitt, J.I. Ultrafine Carbon Black Particles Enhance Respiratory Syncytial Virus-Induced Airway Reactivity, Pulmonary Inflammation, and Chemokine Expression. Toxicol. Sci. 2003, 72, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Huo, X.; Zhang, Y.; Huang, Y.; Zheng, X.; Xu, X. Ambient Fine Particulate Matter Inhibits Innate Airway Antimicrobial Activity in Preschool Children in E-Waste Areas. Environ. Int. 2019, 123, 535–542. [Google Scholar] [CrossRef]
- Ostro, B.; Roth, L.; Malig, B.; Marty, M. The Effects of Fine Particle Components on Respiratory Hospital Admissions in Children. Environ. Health Perspect. 2009, 117, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Horne, B.D.; Joy, E.A.; Hofmann, M.G.; Gesteland, P.H.; Cannon, J.B.; Lefler, J.S.; Blagev, D.P.; Korgenski, E.K.; Torosyan, N.; Hansen, G.I.; et al. Short-Term Elevation of Fine Particulate Matter Air Pollution and Acute Lower Respiratory Infection. Am. J. Respir. Crit. Care Med. 2018, 198, 759–766. [Google Scholar] [CrossRef]
- Kim, K.-N.; Kim, S.; Lim, Y.-H.; Song, I.G.; Hong, Y.-C. Effects of Short-Term Fine Particulate Matter Exposure on Acute Respiratory Infection in Children. Int. J. Hyg. Environ. Health 2020, 229, 113571. [Google Scholar] [CrossRef]
- Tolbert, P.E.; Mulholland, J.A.; MacIntosh, D.L.; Xu, F.; Daniels, D.; Devine, O.J.; Carlin, B.P.; Klein, M.; Dorley, J.; Butler, A.J.; et al. Air Quality and Pediatric Emergency Room Visits for Asthma in Atlanta, Georgia, USA. Am. J. Epidemiol. 2000, 151, 798–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.-Y.; Rull, R.P.; Wilhelm, M.; Lombardi, C.; Balmes, J.; Ritz, B. Outdoor Air Pollution and Uncontrolled Asthma in the San Joaquin Valley, California. J. Epidemiol. Community Health 2010, 64, 142–147. [Google Scholar] [CrossRef]
- Baldacci, S.; Maio, S.; Cerrai, S.; Sarno, G.; Baïz, N.; Simoni, M.; Annesi-Maesano, I.; Viegi, G.; HEALS Study. Allergy and Asthma: Effects of the Exposure to Particulate Matter and Biological Allergens. Respir. Med. 2015, 109, 1089–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babin, S.M.; Burkom, H.S.; Holtry, R.S.; Tabernero, N.R.; Stokes, L.D.; Davies-Cole, J.O.; DeHaan, K.; Lee, D.H. Pediatric Patient Asthma-Related Emergency Department Visits and Admissions in Washington, DC, from 2001–2004, and Associations with Air Quality, Socio-Economic Status and Age Group. Environ. Health 2007, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, P.; Kelley, J.K. Physicians’ Knowledge, Attitudes, and Practice Regarding Environmental Health Hazards. Ind. Environ. Crisis Q. 1996, 9, 512–542. [Google Scholar] [CrossRef]
- WHO|Air Pollution and Child Health: Prescribing Clean Air. Available online: http://www.who.int/ceh/publications/air-pollution-child-health/en/ (accessed on 16 January 2021).
- Laumbach, R.; Meng, Q.; Kipen, H. What Can Individuals Do to Reduce Personal Health Risks from Air Pollution? J. Thorac. Dis. 2015, 7, 96–107. [Google Scholar] [CrossRef]

| Population | PM2.5 Level | Total Days | Number of ED Visits | Mean ED Visits/Day (SD) | % Increase in Mean ED Visits | p-Value |
|---|---|---|---|---|---|---|
| All patients | - | 366 | 4271 | 11.67 (5.665) | - | - |
| All patients | <25 μg/m3 | 188 | 1895 | 10.08 (4.087) | 32.44 | <0.001 |
| ≥25 μg/m3 | 178 | 2376 | 13.35 (6.564) | |||
| URI | <25 μg/m3 | 188 | 1744 | 9.28 (3.88) | 32 | <0.001 |
| ≥25 μg/m3 | 178 | 2180 | 12.25 (6.216) | |||
| Asthma | <25 μg/m3 | 188 | 97 | 0.52 (0.817) | 19.23 | 0.23 |
| ≥25 μg/m3 | 178 | 111 | 0.62 (0.876) | |||
| Pneumonia | <25 μg/m3 | 188 | 39 | 0.21 (0.468) | 42.86 | 0.08 |
| ≥25 μg/m3 | 178 | 54 | 0.30 (0.561) |
| Season | PM2.5 Level | Total Days | Number of ED Visits | Mean ED Visits/Day (SD) | % Increase in Mean ED Visits | p-Value |
|---|---|---|---|---|---|---|
| Winter | <25 μg/m3 | 18 | 265 | 14.72 (4.663) | 14.20 | 0.271 |
| ≥25 μg/m3 | 73 | 1227 | 16.81 (7.626) | |||
| Spring | <25 μg/m3 | 42 | 424 | 10.10 (3.58) | 21.19 | 0.018 |
| ≥25 μg/m3 | 50 | 612 | 12.24 (4.728) | |||
| Summer | <25 μg/m3 | 74 | 695 | 9.39 (3.572) | 6.50 | 0.520 |
| ≥25 μg/m3 | 18 | 180 | 10.00 (3.614) | |||
| Autumn | <25 μg/m3 | 54 | 511 | 9.46 (3.98) | 2 | 0.822 |
| ≥25 μg/m3 | 37 | 357 | 9.65 (3.646) |
| Season | PM2.5 Level | Total Days | Number of ED Visits | Mean ED Visits/Day (SD) | % Increase in Mean ED Visits | p-Value |
|---|---|---|---|---|---|---|
| Winter | <25 μg/m3 | 18 | 242 | 13.44 (4.592) | 13.84 | 0.302 |
| ≥25 μg/m3 | 73 | 1117 | 15.30 (7.222) | |||
| Spring | <25 μg/m3 | 42 | 385 | 9.17 (3.435) | 23.66 | 0.016 |
| ≥25 μg/m3 | 50 | 567 | 11.34 (4.792) | |||
| Summer | <25 μg/m3 | 74 | 656 | 8.86 (3.410) | 7.22 | 0.479 |
| ≥25 μg/m3 | 18 | 171 | 9.50 (3.365) | |||
| Autumn | <25 μg/m3 | 54 | 461 | 8.54 (3.81) | 2.81 | 0.757 |
| ≥25 μg/m3 | 37 | 325 | 8.78 (3.591) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanellopoulos, N.; Pantazopoulos, I.; Mermiri, M.; Mavrovounis, G.; Kalantzis, G.; Saharidis, G.; Gourgoulianis, K. Effect of PM2.5 Levels on Respiratory Pediatric ED Visits in a Semi-Urban Greek Peninsula. Int. J. Environ. Res. Public Health 2021, 18, 6384. https://doi.org/10.3390/ijerph18126384
Kanellopoulos N, Pantazopoulos I, Mermiri M, Mavrovounis G, Kalantzis G, Saharidis G, Gourgoulianis K. Effect of PM2.5 Levels on Respiratory Pediatric ED Visits in a Semi-Urban Greek Peninsula. International Journal of Environmental Research and Public Health. 2021; 18(12):6384. https://doi.org/10.3390/ijerph18126384
Chicago/Turabian StyleKanellopoulos, Nikolaos, Ioannis Pantazopoulos, Maria Mermiri, Georgios Mavrovounis, Georgios Kalantzis, Georgios Saharidis, and Konstantinos Gourgoulianis. 2021. "Effect of PM2.5 Levels on Respiratory Pediatric ED Visits in a Semi-Urban Greek Peninsula" International Journal of Environmental Research and Public Health 18, no. 12: 6384. https://doi.org/10.3390/ijerph18126384








