Metal Ion Release from Engineered Stone Dust in Artificial Lysosomal Fluid—Variation with Time and Stone Type
Abstract
1. Introduction
2. Materials and Methods
2.1. ES Dust Generation
2.2. Preparation of Artificial Lysosomal Fluids (ALF) and Its Extraction
2.3. Determination of Metals and ES Reflected Brightness
2.4. Data Analysis
3. Results
3.1. Reflected Brightness of the Slab and Selected Chemical Characteristics in Unreacted ES Dust Samples
3.2. Release of Metal Ions in Artificial Lysosomal Fluid
3.2.1. Iron
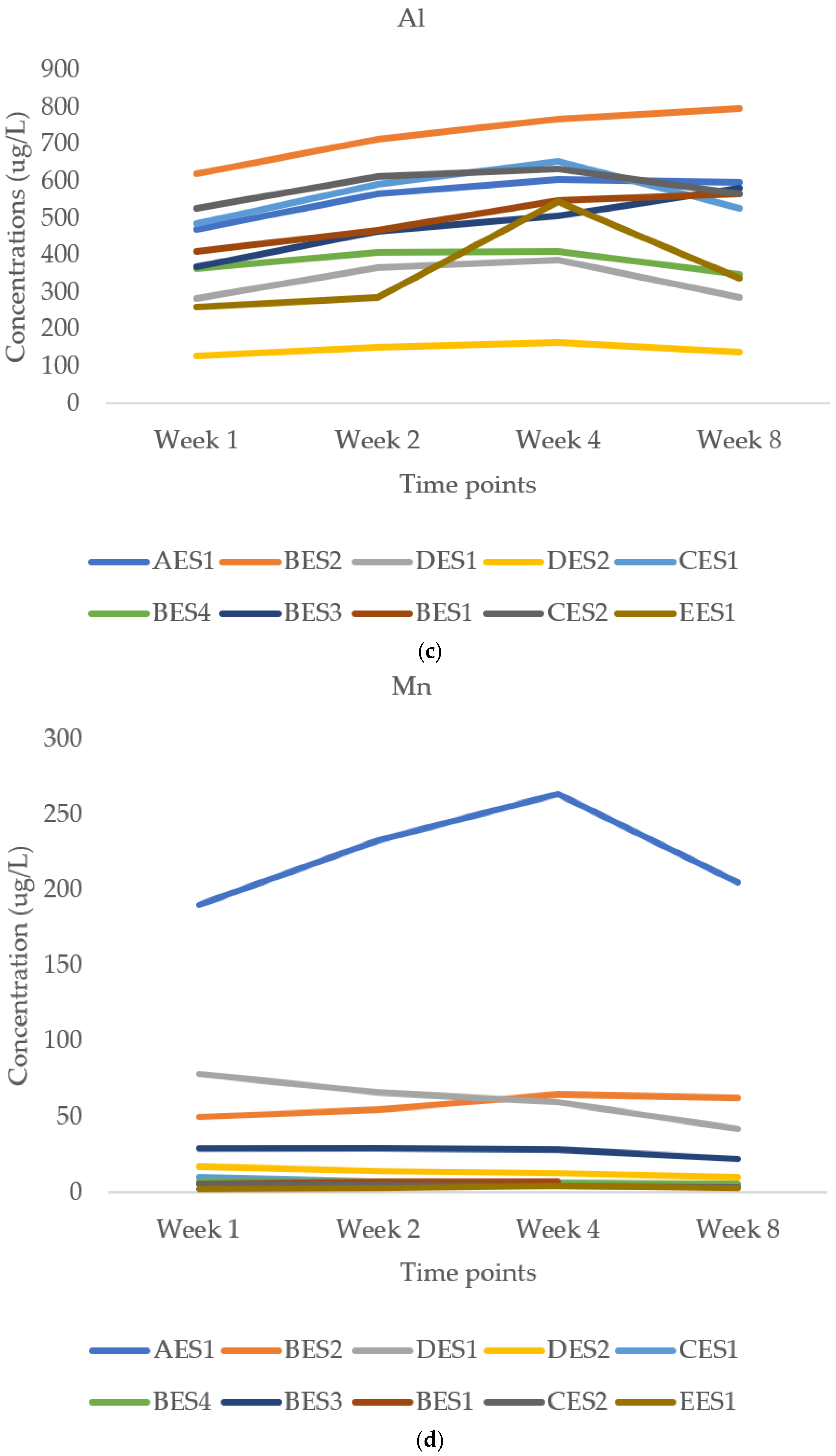
3.2.2. Aluminium
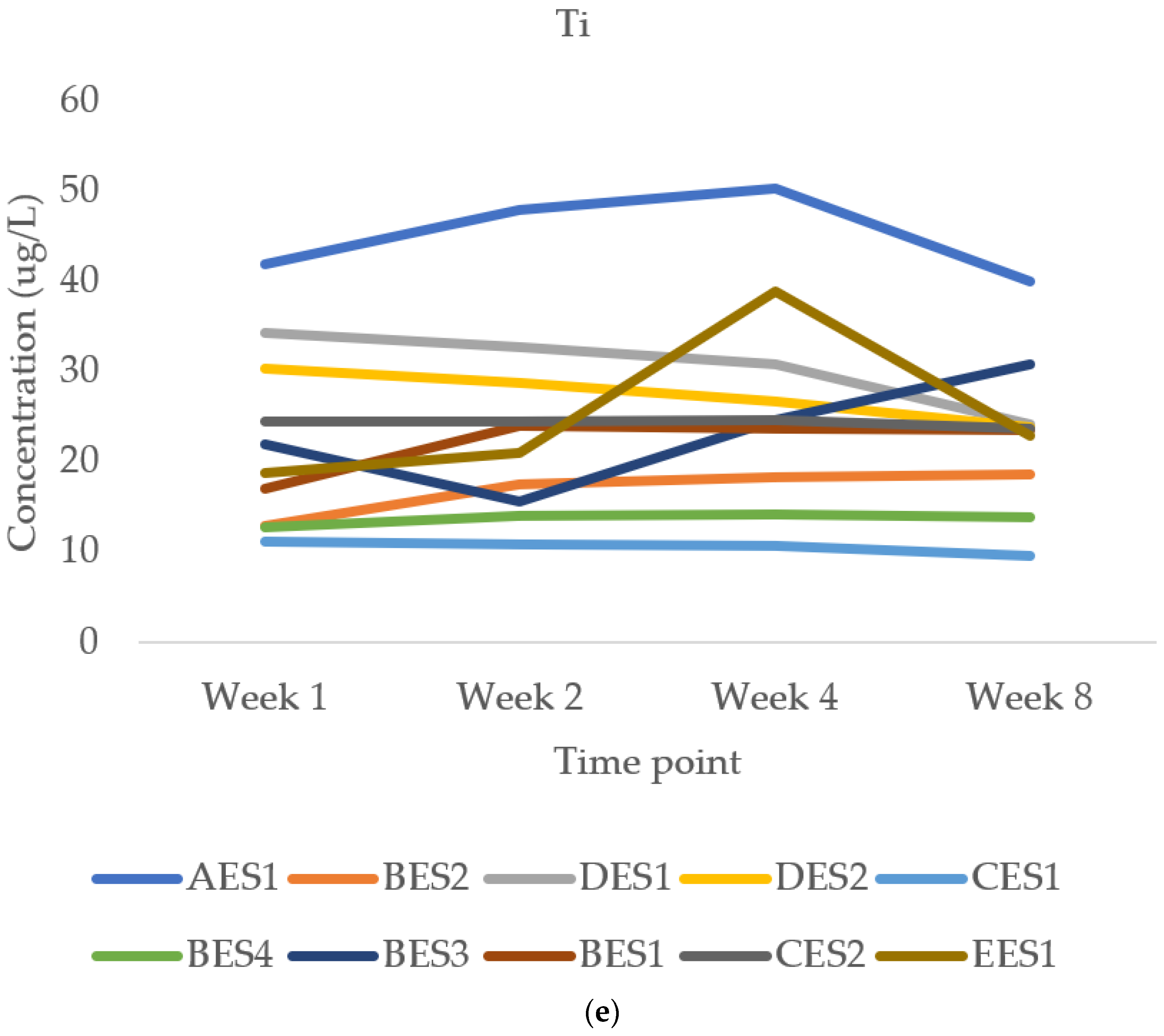
3.2.3. Manganese and Titanium
3.2.4. Correlation of Reflected Brightness with Metal Ion Release
3.3. Degree of Solubilisation of Metals in Artificial Lysosomal Fluid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, J.H.; Johnson, D.L.; Phillips, M.L. Respirable Silica Dust Suppression during Artificial Stone Countertop Cutting. Ann. Occup. Hyg. 2015, 59, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Fazen, L.E.; Linde, B.; Redlich, C.A. Occupational Lung Diseases in the 21st Century: The Changing Landscape and Future Challenges. Curr. Opin. Pulmon. Med. 2020, 26, 142–148. [Google Scholar] [CrossRef]
- Leso, V.; Fontana, L.; Romano, R.; Gervetti, P.; Iavicoli, I. Artificial Stone Associated Silicosis: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 568. [Google Scholar] [CrossRef] [PubMed]
- Ophir, N.; Amir Bar, S.; Korenstein, R.; Kramer, M.R.; Fireman, E. Functional, Inflammatory and Interstitial Impairment due to Artificial Stone Dust Ultrafine Particles Exposure. Occup. Environ. Med. 2019, 76, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Hoy, R.F.; Baird, T.; Hammerschlag, G.; Hart, D.; Johnson, A.R.; King, P.; Putt, M.; Yates, D.H. Artificial Stone-Associated Silicosis: A Rapidly Emerging Occupational Lung Disease. Occup. Environ. Med. 2018, 75, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.; Heinzerling, A.; Patel, K.; Sack, C.; Wolff, J.; Zell-Baran, L.; Weissman, D.; Hall, E.; Sooriash, R.; McCarthy, R.B.; et al. Severe Silicosis in Engineered Stone Fabrication Workers-California, Colorado, Texas, and Washington, 2017–2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alonso, A.; Cordoba-Dona, J.A.; Millares-Lorenzo, J.L.; Figueroa-Murillo, E.; Garcia-Vadillo, C.; Romero-Morillos, J. Outbreak of Silicosis in Spanish Quartz Conglomerate Workers. Int. J. Occup. Environ. Health 2014, 20, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, V.; Romeo, R.; Sisinni, A.G.; Bartoli, D.; Mazzei, M.A.; Sartorelli, P. Silicosis in Workers Exposed to Artificial Quartz Conglomerates: Does it Differ from Chronic Simple Silicosis? Arch. Bronconeumol. 2015, 51, e57–e60. [Google Scholar] [CrossRef]
- Kirby, T. Australia Reports on Audit of Silicosis for Stonecutters. Lancet 2019, 393, 861. [Google Scholar] [CrossRef]
- Australia Government Department of Health. National Dust Disease Taskforce. Available online: https://www1.health.gov.au/internet/main/publishing.nsf/Content/ohp-nat-dust-disease-taskforce.htm (accessed on 25 October 2020).
- Reed, S.; Madden, C.; Davidson, M.; O’Donnell, G. Characterisation of Respiratory Hazards during the Manufacture and Installation of Engineered and Natural Stone Products. In Proceedings of the 37th Annual Conference and Exhibition, Perth, Australia, 20 November–4 December 2019; pp. 55–64, ISBN 978-0-9577703-6-2. Available online: https://www.aioh.org.au/static/uploads/files/aioh-2019-conference-proceedings-combined-file-v2-wfxxyrwknmls.pdf (accessed on 25 October 2020).
- Cohen, M. Pulmonary Immunotoxicology of Select Metals: Aluminum, Arsenic, Cadmium, Chromium, Copper, Manganese, Nickel, Vanadium, and Zinc. J. Immunotoxicol. 2004, 1, 39–69. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Castranova, V.; Vallyathan, V.; Ramsey, D.M.; McLaurin, J.L.; Pack, D.; Leonard, S.; Barger, M.W.; Ma, J.Y.; Dalal, N.S.; Teass, A. Augmentation of Pulmonary Reactions to Quartz Inhalation by Trace Amounts of Iron-Containing Particles. Environ. Health Perspect. 1997, 105 (Suppl. 5), 1319–1324. [Google Scholar] [CrossRef]
- Pavan, C.; Polimeni, M.; Tomatis, M.; Corazzari, I.; Turci, F.; Ghigo, D.; Funini, B. Abrasion of Artificial Stones as a New Cause of an Ancient Disease. Physicochemical Features and Cellular Responses. Toxicol. Sci. 2016, 153, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, F.; Giaccherini, A.; Montegrossi, G.; Pardi, L.A.; Zoleo, A.; Capolupo, F.; Innocenti, M.; Lepore, G.O.; d’Acapito, F.; Capacci, F.; et al. Chemical Variability of Artificial Stone Powders in Relation to their Health Effects. Sci. Rep. 2019, 9, 6531. [Google Scholar] [CrossRef]
- Weggeberg, H.; Benden, T.F.; Steinnes, E.; Flaten, T.P. Element Analysis and Bioaccessibility Assessment of Ultrafine Airborne Particulate Matter (PM0. 1) Using Simulated Lung Fluid Extraction (Artificial Lysosomal Fluid and Gamble’s Solution). Environ. Chem. Ecotoxicol. 2019, 1, 26–35. [Google Scholar] [CrossRef]
- Cannizzaro, A.; Angelosanto, F.; Barrese, E.; Campopiano, A. Biosolubility of High Temperature Insulation Wools in Simulated Lung Fluids. J. Occup. Med. Toxicol. 2019, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Monhemius, A.J.; Plant, J.A. Platinum, Palladium and Rhodium Release from Vehicle Exhaust Catalysts and Road Dust Exposed to Simulated Lung Fluids. Ecotoxicol. Environ. Saf. 2008, 71, 722–730. [Google Scholar] [CrossRef]
- Pelfrêne, A.; Cave, M.R.; Wragg, J.; Douay, F. In Vitro Investigations of Human Bioaccessibility from Reference Materials using Simulated Lung Fluids. Int. J. Environ. Res Public Health 2017, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T. Contamination from Mortars and Mills during Laboratory Crushing and Pulverizing. Bull. Geol. Surv. Jpn. 2018, 69, 201–210. [Google Scholar] [CrossRef]
- Hetland, R.B.; Refsnes, M.; Myran, T.; Johansen, B.V.; Uthus, N.; Schwarze, P.E. Mineral and/or Metal Content as Critical Determinants of Particle-Induced release of IL-6 and IL-8 from A549 cells. J. Toxicol Environ. Health A 2000, 60, 47–65. [Google Scholar] [CrossRef]
- Becher, R.; Hetland, R.B.; Refsnes, M.; Dahl, J.E.; Dahlman, H.J.; Schwarze, P.E. Rat Lung Inflammatory Responses after In Vivo and In Vitro Exposure to Various Stone Particles. Inhal. Toxicol. 2001, 13, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Haider, T.; Gassmann, M.; Muckenthaler, M.U. Iron Homeostasis in the Lungs-A Balance between Health and Disease. Pharmaceuticals 2019, 12, 5. [Google Scholar] [CrossRef]
- Martinez-Finley, E.J.; Gavin, C.E.; Aschner, M.; Gunter, T.E. Manganese Neurotoxicity and the Role of Reactive Oxygen Species. Free Rad. Biol. Med. 2013, 62, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Fubini, F.; Fenoglio, I. Toxic Potential of Mineral Dusts. Elements 2007, 3, 407–414. [Google Scholar] [CrossRef]
- Bischof, H.; Burgstaller, S.; Waldeck-Weiermair, M.; Rauter, T.; Schinagl, M.; Ramadani-Muja, J.; Graier, W.F.; Malli, R. Live-Cell Imaging of Physiologically Relevant Metal Ions Using Genetically Encoded FRET-Based Probes. Cells 2019, 8, 492. [Google Scholar] [CrossRef]
- Jansen, J.F.G.A.; Hilker, I.; Kleuskens, E.; Hensen, G.; Kraeger, I.; Posthumus, W. Cobalt Replacement in Unsaturated Polyester Resins-Going for Sustainable Composites. Macromol. Symp. 2013, 329, 142–149. [Google Scholar] [CrossRef]
- Murashov, V.; Harper, M.; Demchuk, E. Impact of Silanol Surface Density on the Toxicity of Silica Aerosols Measured by Erythrocyte Haemolysis. J. Occup. Environ. Hyg. 2006, 3, 718–723. [Google Scholar] [CrossRef]
- Pavan, C.; Santalucia, R.; Leinardi, R.; Fabbiani, M.; Yakoub, Y.; Uwambayinema, F.; Ugliengo, P.; Tomatis, M.; Martra, G.; Turci, F.; et al. Nearly Free Surface Silanols are the Critical Molecular Moieties that Initiate the Toxicity of Silica Particles. Proc. Natl. Acad. Sci. USA 2020, 117, 27836–27846. [Google Scholar] [CrossRef]


| Stone Type | Brightness (cd/m2) | Fe (mg/kg) | Mn (mg/kg) | Al (mg/kg) | Ti (mg/kg) | Main Crystalline Mineral Species * |
|---|---|---|---|---|---|---|
| AES1 | 3.1 | 3800 | 63 | 150 | 9 | 89% quartz; 3% albite; 0.8% magnetite |
| BES1 | 62 | 40 | 1 | 230 | 7 | 56% quartz, 24% cristobalite, 2% albite |
| BES2 | 5.2 | 710 | 23 | 250 | 4 | 92% quartz |
| BES3 | 60 | 90 | 7 | 160 | 7 | 19% quartz, 47% cristobalite, 6% albite |
| BES4 | 58 | 50 | 3 | 190 | 6 | 23% quartz, 43% cristobalite, 7% albite |
| CES1 | 56 | 50 | 3 | 330 | 6 | 98% quartz, 2% rutile # |
| CES2 | 39 | 40 | 2 | 400 | 10 | 96% quartz, 4% rutile # |
| DES1 | 9.0 | 565 | 21 | 140 | 8 | 90% quartz |
| DES2 | 45 | 80 | 3 | 60 | 7 | 76% quartz, 23% cristobalite # |
| EES1 | 63.2 | 30 | 1 | 240 | 9 | 99% quartz # |
| Metal | AES1 | BES1 | BES2 | BES3 | BES4 | CES1 | CES2 | DES1 | DES2 | EES1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe (mg/kg or μg/g)) | Available metal in solid dust | 3800 | 40 | 710 | 90 | 50 | 50 | 40 | 565 | 80 | 30 |
| Fe (μg/L) | Maximum metal release | 13,300 | 95 | 1257 | 344 | 116 | 87 | 73 | 1940 | 139 | 47 |
| % of metal solubilised in ALF | 87 | 59 | 44 | 96 | 58 | 44 | 46 | 86 | 43 | 39 | |
| Mn (mg/kg) | Available metal in solid dust | 63 | 1 | 23 | 7 | 3 | 3 | 2 | 21 | 3 | 1 |
| Mn (μg/L) | Maximum metal release | 264 | 7 | 65 | 29 | 7 | 10 | 5 | 79 | 17 | 4 |
| % of metal solubilised in ALF | - * | - * | 71 | - * | 58 | 83 | 63 | 94 | - * | - * | |
| Al (mg/kg) | Available metal in solid dust | 150 | 230 | 250 | 160 | 190 | 330 | 400 | 140 | 60 | 240 |
| Al (μg/L) | Maximum metal release | 604 | 568 | 796 | 583 | 410 | 654 | 635 | 389 | 164 | 545 |
| % of metal solubilised in ALF | 100 | 62 | 80 | 91 | 54 | 50 | 40 | 69 | 68 | 57 | |
| Ti (mg/kg) | Available metal in solid dust | 9 | 7 | 4 | 7 | 6 | 6 | 10 | 8 | 7 | 9 |
| Ti (μg/L) | Maximum metal release | 50 | 24 | 19 | 31 | 14 | 11 | 25 | 35 | 30 | 39 |
| % of metal solubilised in ALF | - * | 86 | - * | - * | 58 | 46 | 63 | - * | - * | - * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharjan, P.; Crea, J.; Tkaczuk, M.; Gaskin, S.; Pisaniello, D. Metal Ion Release from Engineered Stone Dust in Artificial Lysosomal Fluid—Variation with Time and Stone Type. Int. J. Environ. Res. Public Health 2021, 18, 6391. https://doi.org/10.3390/ijerph18126391
Maharjan P, Crea J, Tkaczuk M, Gaskin S, Pisaniello D. Metal Ion Release from Engineered Stone Dust in Artificial Lysosomal Fluid—Variation with Time and Stone Type. International Journal of Environmental Research and Public Health. 2021; 18(12):6391. https://doi.org/10.3390/ijerph18126391
Chicago/Turabian StyleMaharjan, Preeti, Joseph Crea, Michael Tkaczuk, Sharyn Gaskin, and Dino Pisaniello. 2021. "Metal Ion Release from Engineered Stone Dust in Artificial Lysosomal Fluid—Variation with Time and Stone Type" International Journal of Environmental Research and Public Health 18, no. 12: 6391. https://doi.org/10.3390/ijerph18126391
APA StyleMaharjan, P., Crea, J., Tkaczuk, M., Gaskin, S., & Pisaniello, D. (2021). Metal Ion Release from Engineered Stone Dust in Artificial Lysosomal Fluid—Variation with Time and Stone Type. International Journal of Environmental Research and Public Health, 18(12), 6391. https://doi.org/10.3390/ijerph18126391






