Combined Exposure to Metals in Drinking Water Alters the Dopamine System in Mouse Striatum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Motor Coordination
2.3. Measurement of Dopamine
2.4. Real-Time PCR Analysis
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
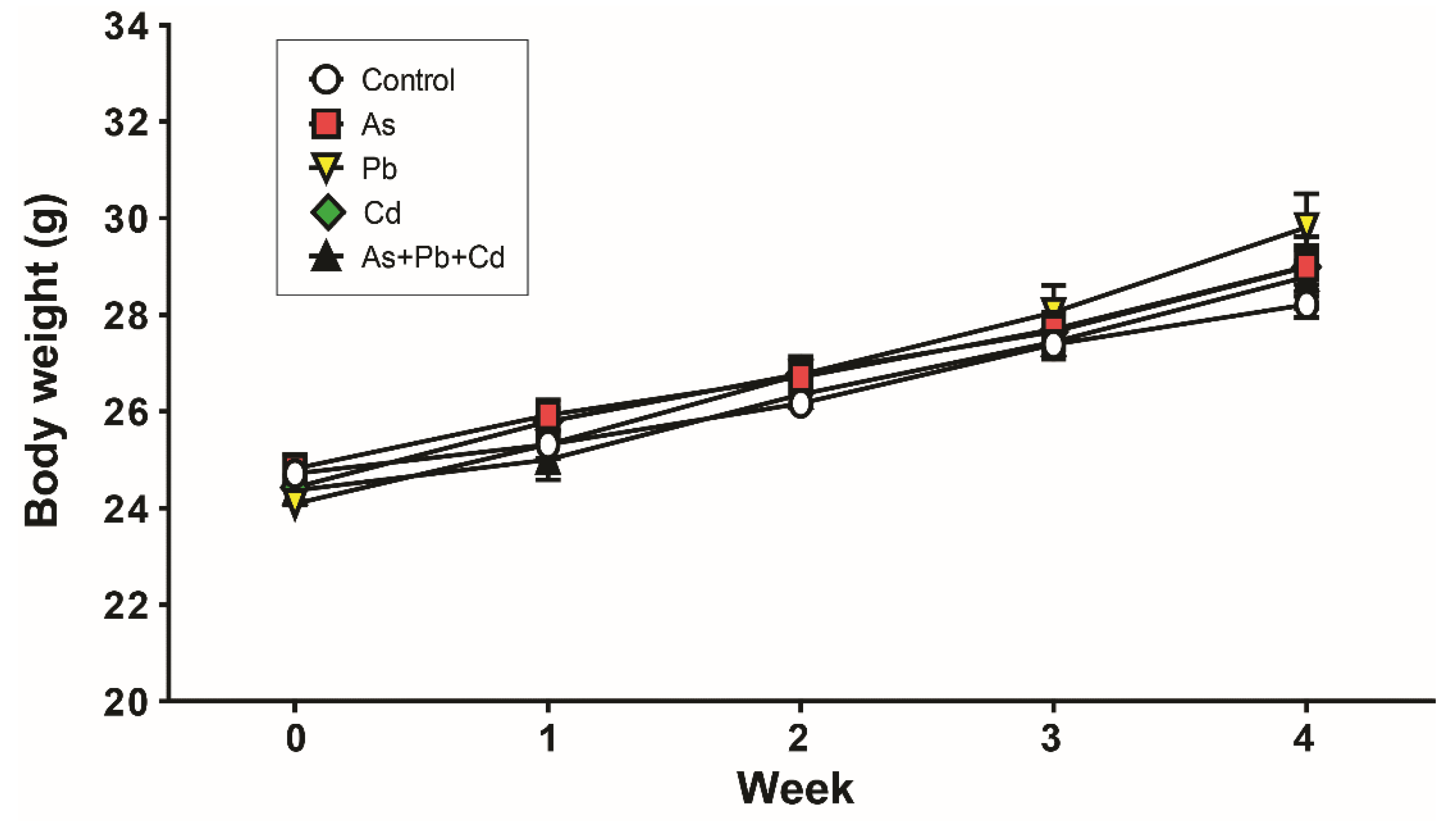
3.1. Body Weight
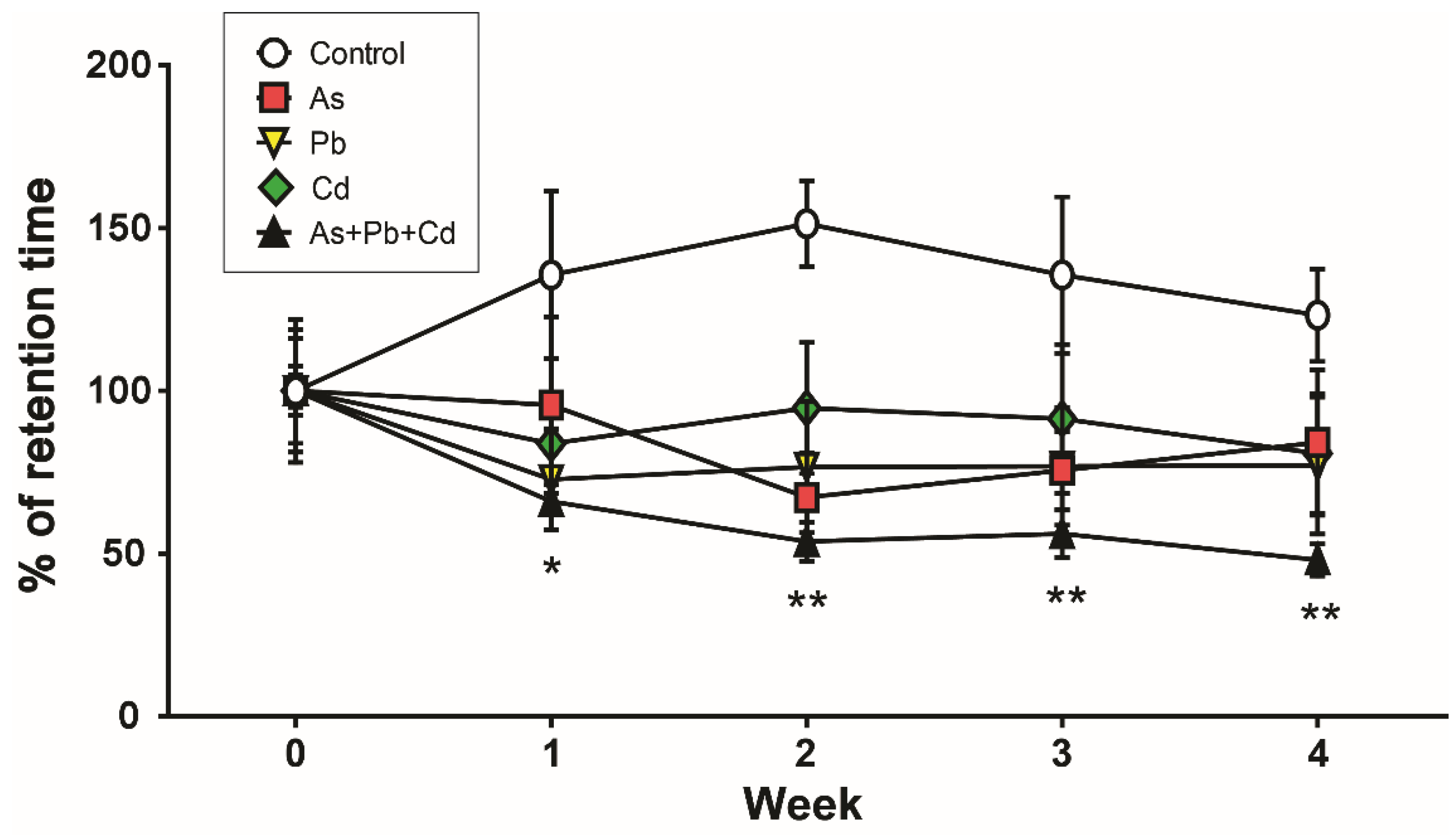
3.2. Motor Coordination
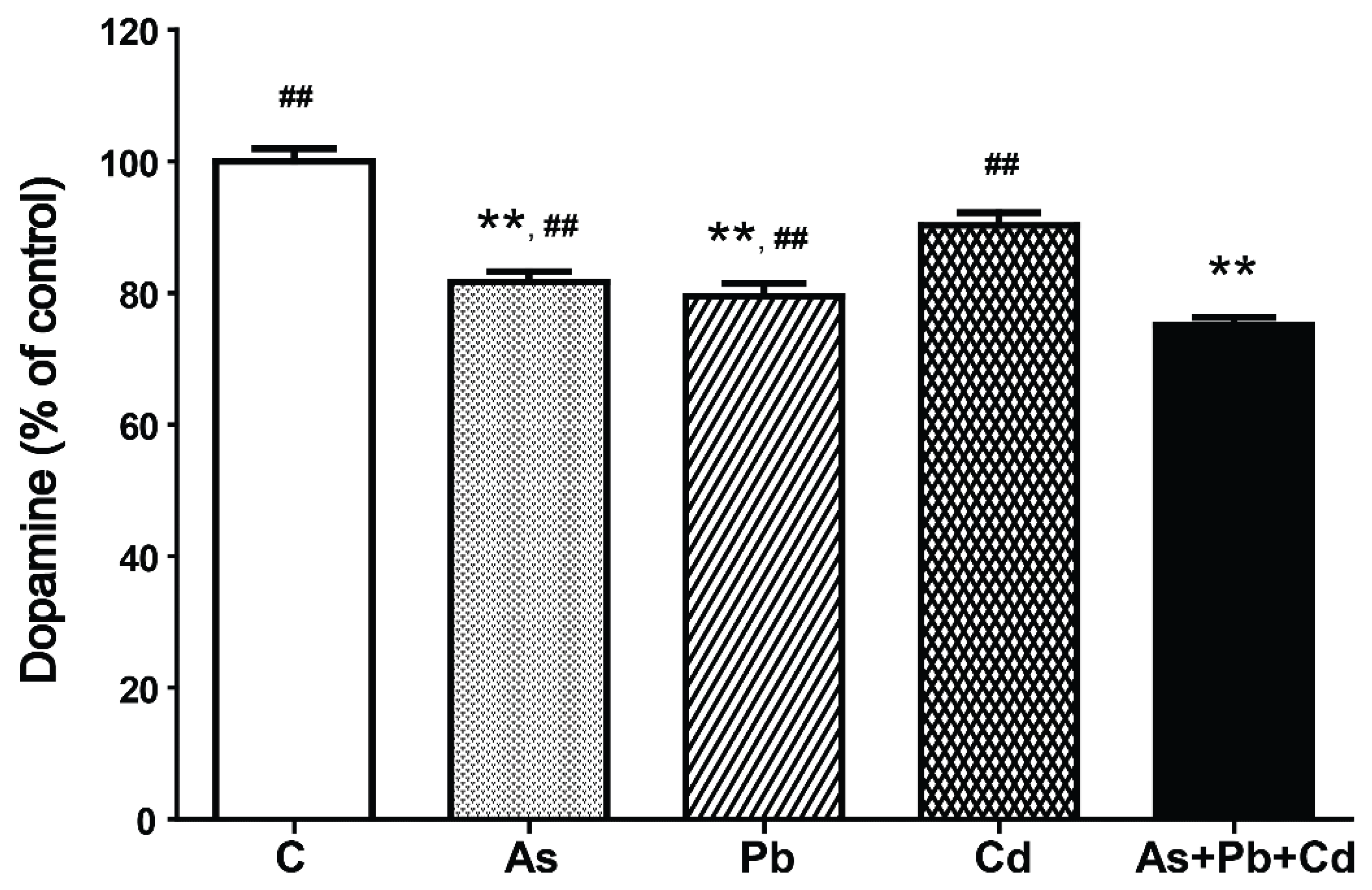
3.3. Dopamine Content
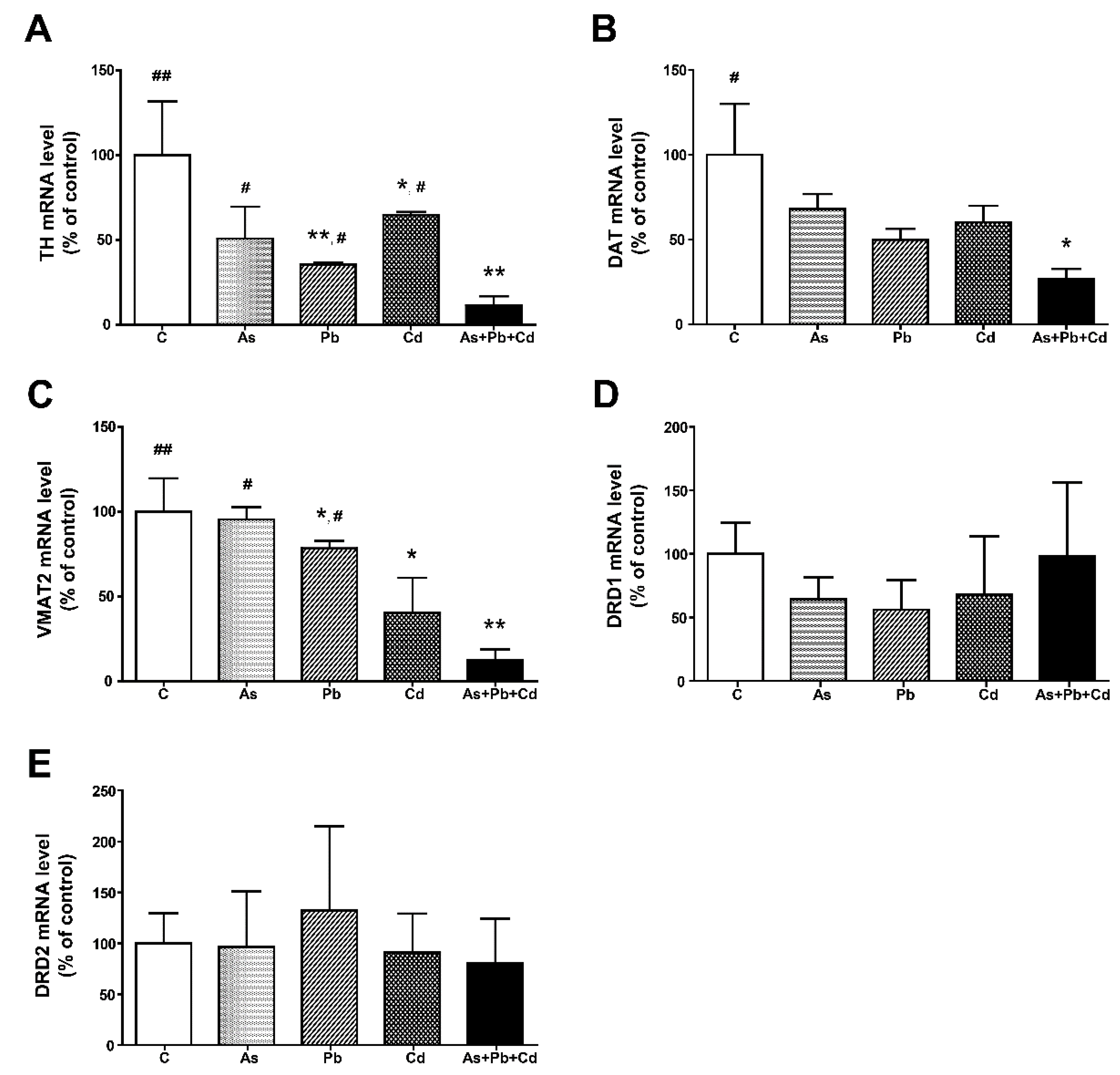
3.4. Gene Expression in the Striatum
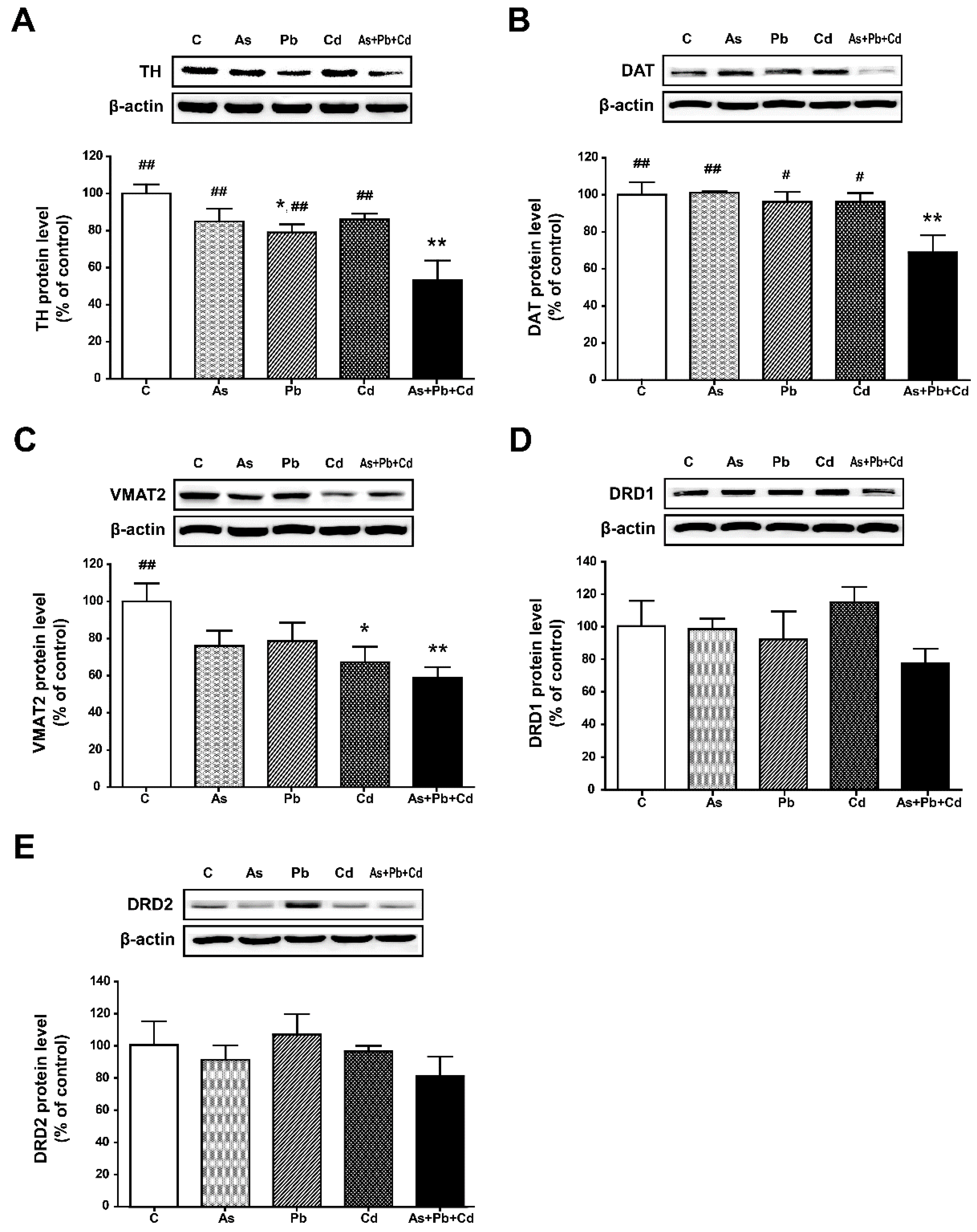
3.5. Protein Expression in the Striatum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Naseri, K.; Esform, A.; Aramjoo, H.; Naghizadeh, A. Drinking water heavy metal toxicity and chronic kidney diseases: A systematic review. Rev. Environ. Health 2020, in press. [Google Scholar] [CrossRef]
- Whittaker, M.H.; Wang, G.; Chen, X.Q.; Lipsky, M.; Smith, D.; Gwiazda, R.; Fowler, B.A. Exposure to Pb, Cd, and As mixtures potentiates the production of oxidative stress precursors: 30-day, 90-day, and 180-day drinking water studies in rats. Toxicol. Appl. Pharmacol. 2011, 254, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.R.; Allan, A.M. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: A review. Curr. Environ. Health Rep. 2014, 1, 132–147. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Scicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin disturbances produced by sub-toxic concentration of heavy metals: The role of oligodendrocyte dysfunction. Int. J. Mol. Sci. 2019, 20, 4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserman, G.A.; Liu, X.; Parvez, F.; Ahsan, H.; Factor-Litvak, P.; van Geen, A.; Slavkovich, V.; LoIacono, N.J.; Cheng, Z.; Hussain, I.; et al. Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2004, 112, 1329–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, V.M.; Jiménez-Capdeville, M.E.; Giordano, M. The effects of arsenic exposure on the nervous system. Toxicol. Lett. 2003, 145, 1–18. [Google Scholar] [CrossRef]
- Ramírez Ortega, D.; González Esquivel, D.F.; Blanco Ayala, T.; Pineda, B.; Gómez Manzo, S.; Marcial Quino, J.; Carrillo Mora, P.; Pérez de la Cruz, V. Cognitive impairment induced by lead exposure during lifespan: Mechanisms of lead neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Fu, Z.; Yan, M.; Wu, N.; Wu, H.; Yin, P. Associations between blood cadmium levels and cognitive function in a cross-sectional study of US adults aged 60 years or older. BMJ Open 2018, 8, e020533. [Google Scholar] [CrossRef]
- Taylor, C.M.; Emond, A.M.; Lingam, R.; Golding, J. Prenatal lead, cadmium and mercury exposure and associations with motor skills at age 7 years in a UK observational birth cohort. Environ. Int. 2018, 117, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Bardullas, U.; Limón-Pacheco, J.H.; Giordano, M.; Carrizales, L.; Mendoza-Trejo, M.S.; Rodríguez, V.M. Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol. Appl. Pharmacol. 2009, 239, 169–177. [Google Scholar] [CrossRef]
- Stansfield, K.H.; Ruby, K.N.; Soares, B.D.; McGlothan, J.L.; Liu, X.; Guilarte, T.R. Early-life lead exposure recapitulates the selective loss of parvalbumin-positive GABAergic interneurons and subcortical dopamine system hyperactivity present in schizophrenia. Transl. Psychiatry 2015, 5, e522. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, V.M.; Limón-Pacheco, J.H.; Carrizales, L.; Mendoza-Trejo, M.S.; Giordano, M. Chronic exposure to low levels of inorganic arsenic causes alterations in locomotor activity and in the expression of dopaminergic and antioxidant systems in the albino rat. Neurotoxicol. Teratol. 2010, 32, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Bouyatas, M.M.; Abbaoui, A.; Gamrani, H. Neurobehavioral effects of acute and chronic lead exposure in a desert rodent Meriones shawi: Involvement of serotonin and dopamine. J. Chem. Neuroanat. 2019, 102, 101689. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moneim, A.E.; Bauomy, A.A.; Diab, M.M.; Shata, M.T.; Al-Olayan, E.M.; El-Khadragy, M.F. The protective effect of Physalis peruviana L. against cadmium-induced neurotoxicity in rats. Biol. Trace Elem. Res. 2014, 160, 392–399. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, J.P.; Scherman, D. Radioligands of the vesicular monoamine transporter and their use as markers of monoamine storage vesicles. Biochem. Pharmacol. 1989, 38, 2395–2404. [Google Scholar] [CrossRef]
- Lachowicz, J.E.; Sibley, D.R. Molecular characteristics of mammalian dopamine receptors. Pharmacol. Toxicol. 1997, 81, 105–113. [Google Scholar] [CrossRef]
- Vallone, D.; Picetti, R.; Borrelli, E. Structure and function of dopamine receptors. Neurosci. Biobehav. Rev. 2000, 24, 125–132. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Naghizadeh, B.; López-Larrubia, P.; Cauli, O. Behavioral deficits induced by lead exposure are accompanied by serotonergic and cholinergic alterations in the prefrontal cortex. Neurochem. Int. 2013, 62, 232–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Guidelines for Drinking-Water Quality. 2017. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 11 June 2021).
- Zhou, F.; Xie, J.; Zhang, S.; Yin, G.; Gao, Y.; Zhang, Y.; Bo, D.; Li, Z.; Liu, S.; Feng, C.; et al. Lead, cadmium, arsenic, and mercury combined exposure disrupted synaptic homeostasis through activating the Snk-SPAR pathway. Ecotoxicol. Environ. Saf. 2018, 163, 674–684. [Google Scholar] [CrossRef]
- Ali, M.M.; Murthy, R.C.; Chandra, S.V. Developmental and longterm neurobehavioral toxicity of low level in-utero cadmium exposure in rats. Neurobehav. Toxicol. Teratol. 1986, 8, 463–468. [Google Scholar] [PubMed]
- Parvez, F.; Wasserman, G.A.; Factor-Litvak, P.; Liu, X.; Slavkovich, V.; Siddique, A.B.; Sultana, R.; Sultana, R.; Islam, T.; Levy, D.; et al. Arsenic exposure and motor function among children in Bangladesh. Environ. Health Perspect. 2011, 119, 1665–1670. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhong, H.Q.; Chu, Z.W.; Zuo, X.; Wang, L.; Ren, X.L.; Ma, H.; Du, R.Y.; Ju, J.J.; Ye, X.L.; et al. Arsenic induces transgenerational behavior disorders in Caenorhabditis elegans and its underlying mechanisms. Chemosphere 2020, 252, 126510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Zhang, B.; Xu, T.; Yin, D.; Gu, W.; Bai, J. Developmental exposure to lead at environmentally relevant concentrations impaired neurobehavior and NMDAR-dependent BDNF signaling in zebrafish larvae. Environ. Pollut. 2020, 257, 113627. [Google Scholar] [CrossRef]
- Tian, J.; Hu, J.; Liu, D.; Yin, J.; Chen, M.; Zhou, L.; Yin, H. Cadmium chloride-induced transgenerational neurotoxicity in zebrafish development. Environ. Toxicol. Pharmacol. 2021, 81, 103545. [Google Scholar] [CrossRef]
- Saritha, S.; Davuljigari, C.B.; Kumar, K.P.; Reddy, G.R. Effects of combined arsenic and lead exposure on the brain monoaminergic system and behavioral functions in rats: Reversal effect of MiADMSA. Toxicol. Ind. Health 2019, 35, 89–108. [Google Scholar] [CrossRef]
- Pradhan, N.; Arunasmitha, S. Correlations of motility, defecatory behavior and striatal dopaminergic activity in rats. Physiol. Behav. 1991, 50, 135–138. [Google Scholar] [CrossRef]
- Foley, P.B. Dopamine in psychiatry: A historical perspective. J. Neural Transm. 2019, 126, 473–479. [Google Scholar] [CrossRef]
- Agrawal, S.; Bhatnagar, P.; Flora, S.J. Changes in tissue oxidative stress, brain biogenic amines and acetylcholinesterase following co-exposure to lead, arsenic and mercury in rats. Food Chem. Toxicol. 2015, 86, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Y.; Palmiter, R.D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 1995, 83, 1197–1209. [Google Scholar] [CrossRef] [Green Version]
- Chandravanshi, L.P.; Gupta, R.; Shukla, R.K. Arsenic-induced neurotoxicity by dysfunctioning cholinergic and dopaminergic system in brain of developing rats. Biol. Trace Elem. Res. 2019, 189, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Dhuriya, Y.K.; Gupta, R.; Shukla, R.K.; Yadav, R.S.; Dwivedi, H.N.; Pant, A.B.; Khanna, V.K. Protective effect of curcumin by modulating BDNF/DARPP32/CREB in arsenic-induced alterations in dopaminergic signaling in rat corpus striatum. Mol. Neurobiol. 2018, 55, 445–461. [Google Scholar] [CrossRef]
- Kim, M.; Seo, S.; Sung, K.; Kim, K. Arsenic exposure in drinking water alters the dopamine system in the brains of C57BL/6 mice. Biol. Trace Elem. Res. 2014, 162, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli-Nezhad, M.; Barron, A.J.; Pitts, D.K. Postnatal inorganic lead exposure decreases the number of spontaneously active midbrain dopamine neurons in the rat. Neurotoxicology 2001, 22, 259–269. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Miah, M.R.; Ijomone, O.M.; Tsatsakis, A.; Soares, F.A.A.; Tinkov, A.A.; Skalny, A.V.; Venkataramani, V.; Aschner, M. Lead (Pb) exposure induces dopaminergic neurotoxicity in Caenorhabditis elegans: Involvement of the dopamine transporter. Toxicol. Rep. 2019, 6, 833–840. [Google Scholar] [CrossRef]
- El-Tarras, A.; Attia, H.F.; Soliman, M.M.; El Awady, M.A.; Amin, A.A. Neuroprotective effect of grape seed extract against cadmium toxicity in male albino rats. Int. J. Immunopathol. Pharmacol. 2016, 29, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Fortune, T.; Lurie, D.I. Chronic low-level lead exposure affects the monoaminergic system in the mouse superior olivary complex. J. Comp. Neurol. 2009, 513, 542–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, S.; Kitayama, S.; Lin, C.L.; Patel, A.; Nanthakumar, E.; Gregor, P.; Kuhar, M.; Uhl, G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science 1991, 254, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Cass, W.A.; Zahniser, N.R.; Flach, K.A.; Gerhardt, G.A. Clearance of exogenous dopamine in rat dorsal striatum and nucleus accumbens: Role of metabolism and effects of locally applied uptake inhibitors. J. Neurochem. 1993, 61, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Kilty, J.E.; Lorang, D.; Amara, S.G. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science 1991, 254, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Eiden, L.E.; Weihe, E. VMAT2: A dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N. Y. Acad. Sci. 2011, 1216, 86–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US EPA. Issue Paper on the Human Health Effects of Metals. 2004. Available online: https://www.epa.gov/sites/production/files/2014-11/documents/human_health_effects.pdf (accessed on 11 June 2021).
- Smith, Y.; Villalba, R. Striatal and extrastriatal dopamine in the basal ganglia: An overview of its anatomical organization in normal and Parkinsonian brains. Mov. Disord. 2008, 23 (Suppl. S3), S534–S547. [Google Scholar] [CrossRef] [PubMed]
| Gene | NCBI Ref Seq Number | Sense | Antisense |
|---|---|---|---|
| TH | NM_009377.1 | CAGCTGGAGGATGTGTCTCA | GGCATGACGGATGTACTGTG |
| DAT | NM_010020.3 | TTGCAGCTGGCACATCTATC | ATGCTGACCACGACCACATA |
| VMAT2 | NM_172523.3 | ATGTGTTCCCGAAAGTGGCA | AAGTTGGGAGCGATGAGTCC |
| DRD1 | NM_010076 | GGAAGATGCCGAGGATGA | TAGATACTGGTGTAAGTGACGAT |
| DRD2 | NM_010077 | CTCAACAACACAGACCAGAAT | GAACGAGACGATGGAGGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Lee, D.; Kim, K. Combined Exposure to Metals in Drinking Water Alters the Dopamine System in Mouse Striatum. Int. J. Environ. Res. Public Health 2021, 18, 6558. https://doi.org/10.3390/ijerph18126558
Kim H, Lee D, Kim K. Combined Exposure to Metals in Drinking Water Alters the Dopamine System in Mouse Striatum. International Journal of Environmental Research and Public Health. 2021; 18(12):6558. https://doi.org/10.3390/ijerph18126558
Chicago/Turabian StyleKim, Haesoo, Daeun Lee, and Kisok Kim. 2021. "Combined Exposure to Metals in Drinking Water Alters the Dopamine System in Mouse Striatum" International Journal of Environmental Research and Public Health 18, no. 12: 6558. https://doi.org/10.3390/ijerph18126558






