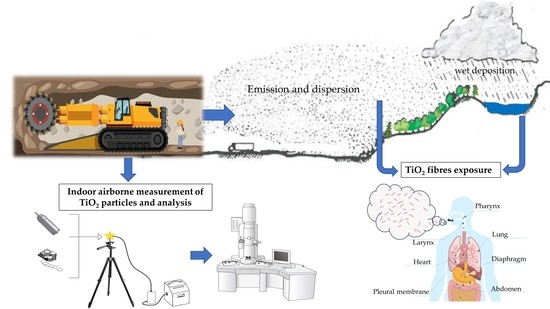
Dispersion of Natural Airborne TiO2 Fibres in Excavation Activity as a Potential Environmental and Human Health Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Rock and Airborne Sample Preparation
2.2. Scanning Electron Microscopy (SEM) analysis
- C = particle concentration
- Nf = total number of fibres counted
- a = field area at 2000× (mm2)
- Nc = total number of fields examined on the filter
- Af = effective collecting area of filter (mm2)
- V = the volume of sampled air (L)
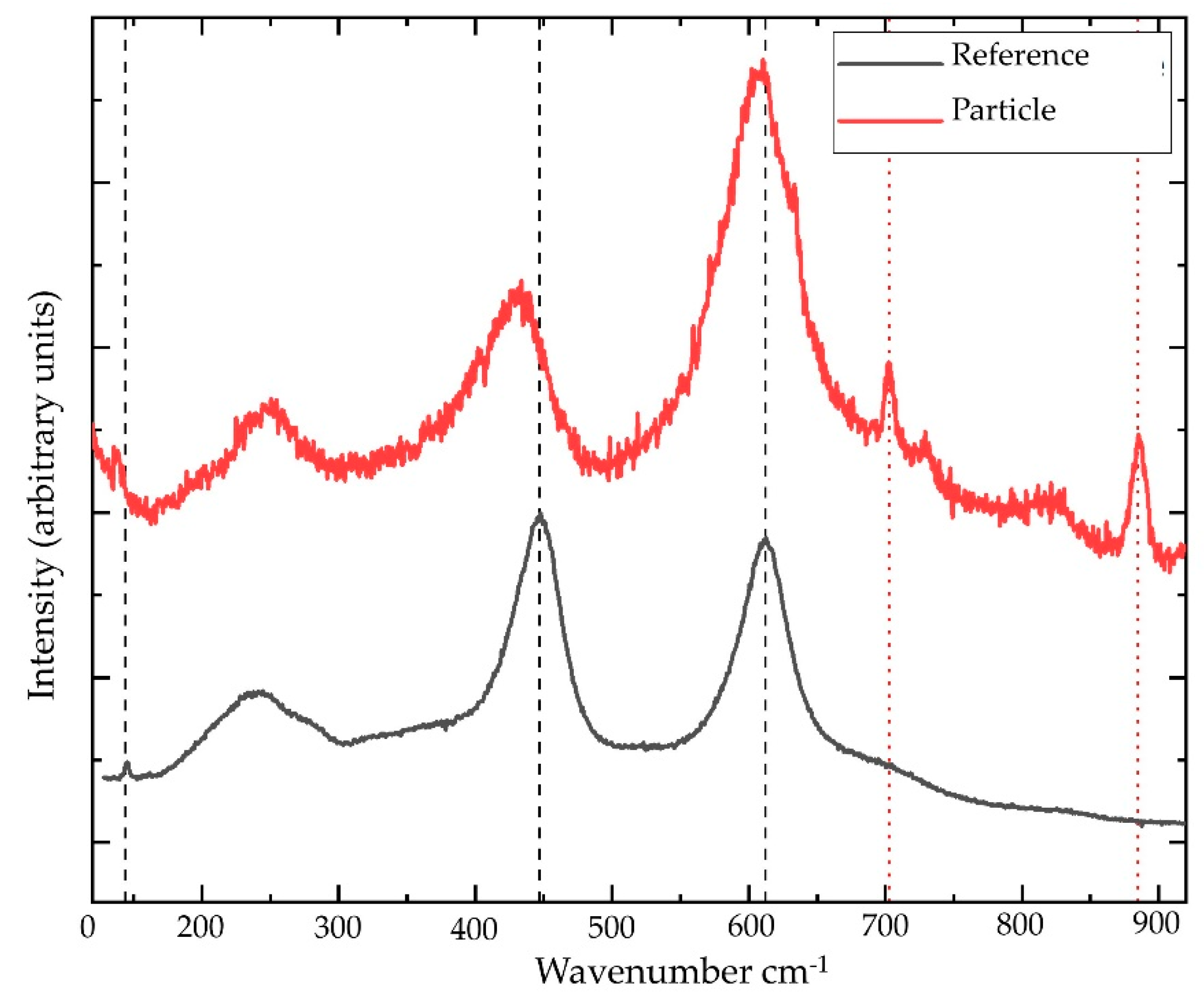
2.3. Micro-Raman Spectroscopy
3. Results
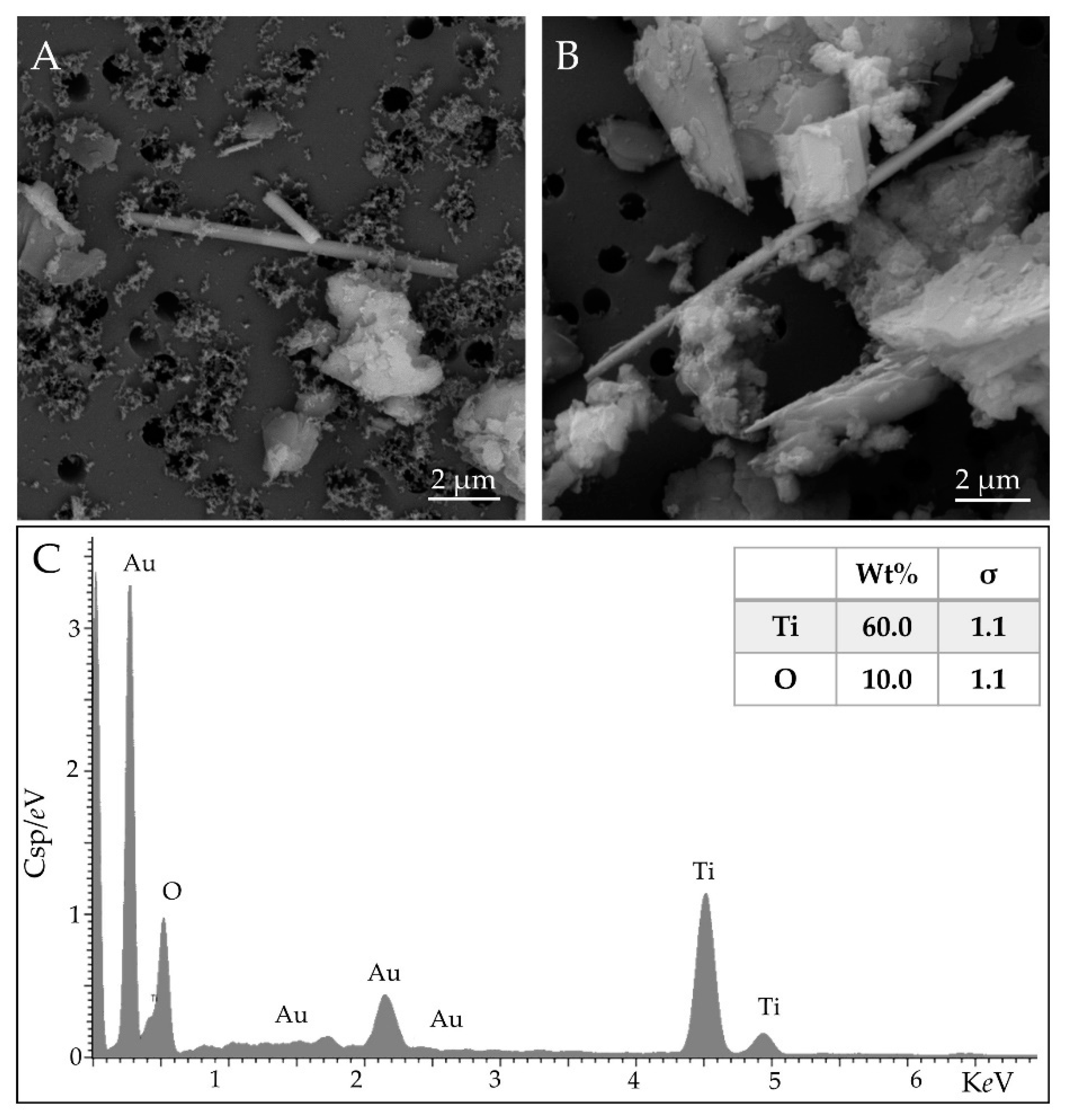
3.1. TiO2-EMPs in Massive Rock Samples
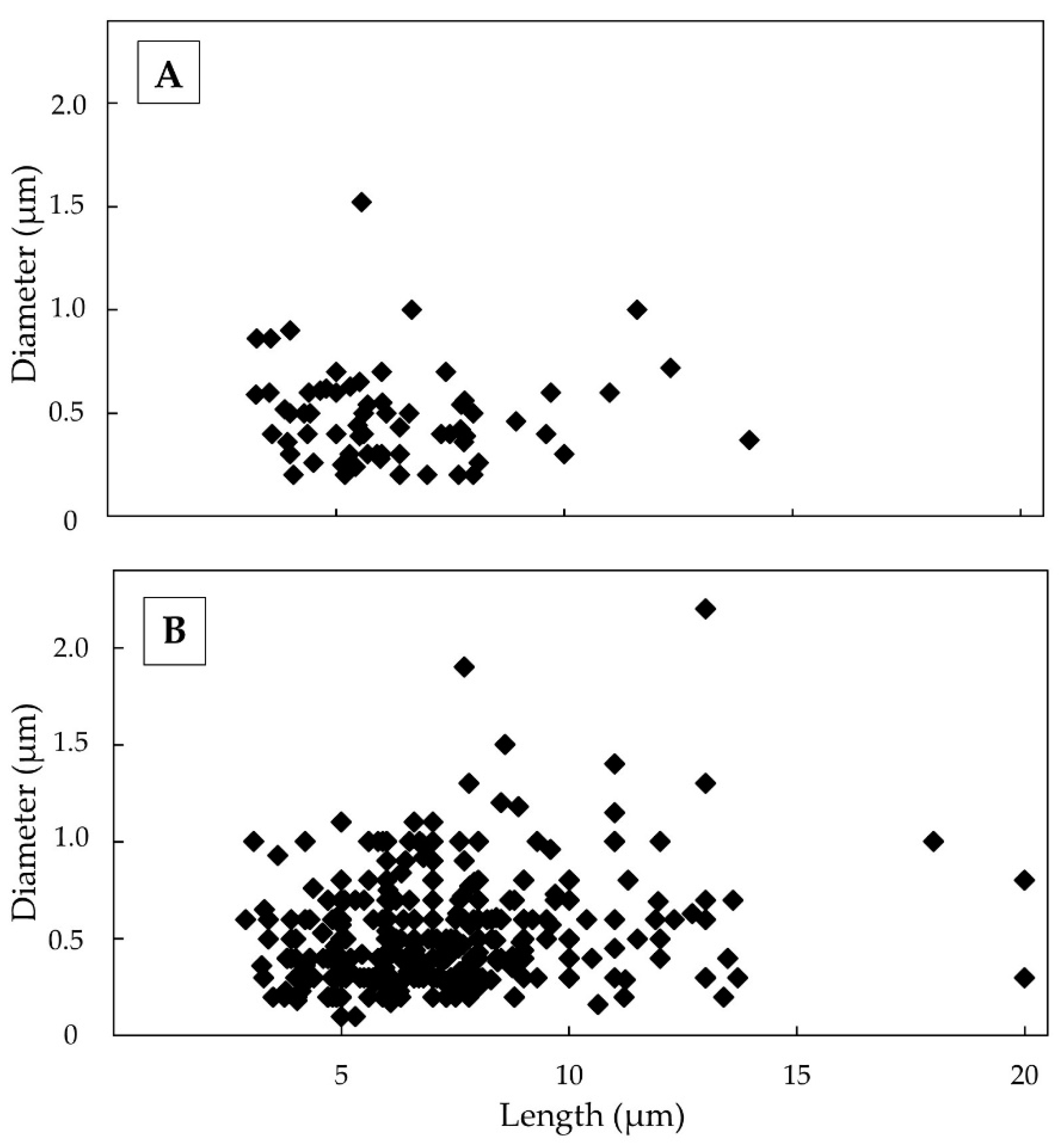
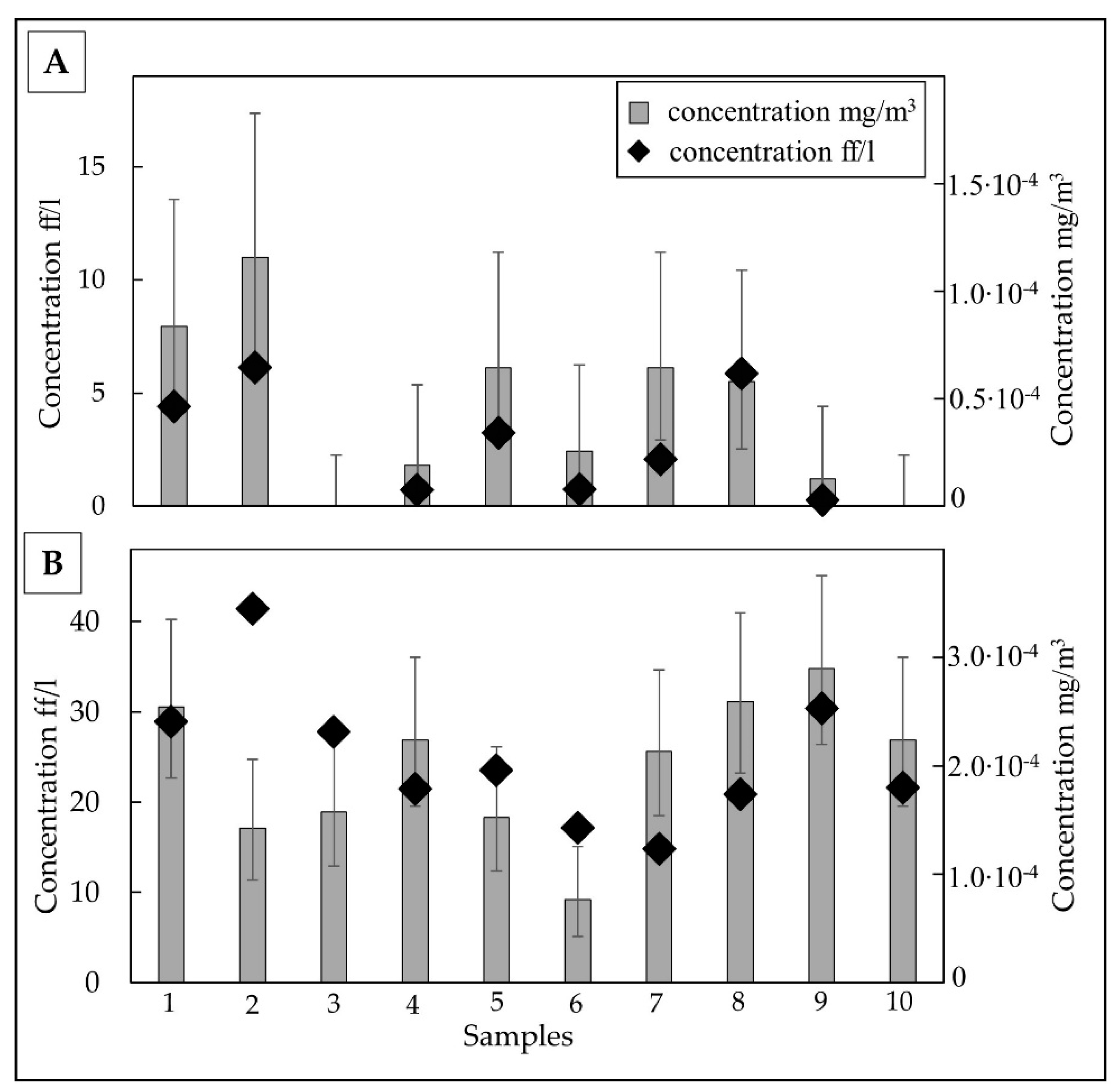
3.2. TiO2-EMPs in Airborne Sample
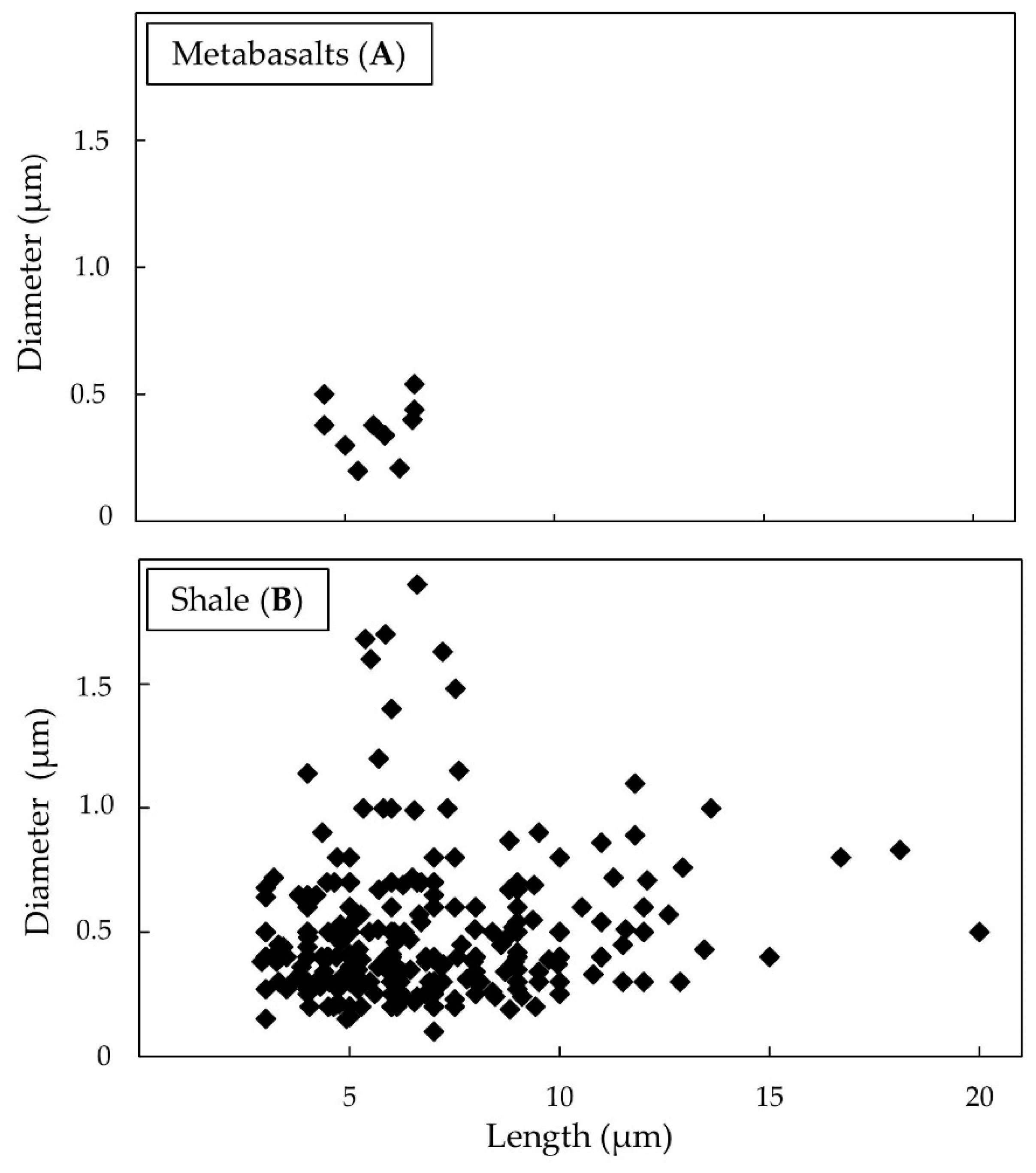
3.2.1. Meta-basalts
3.2.2. Shales
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meinhold, G. Rutile and its applications in earth sciences. Earth Sci. Rev. 2010, 102, 1–28. [Google Scholar] [CrossRef]
- Carocci, E.; Marignac, C.; Cathelineau, M.; Truche, L.; Lecomte, A.; Pinto, F. Rutile from Panasqueira (Central Portugal): An Excellent Pathfinder for Wolframite Deposition. Minerals 2019, 9, 9. [Google Scholar] [CrossRef]
- Lewis, R.J., Sr. Hawley’s Condensed Chemical Dictionary, 12th ed.; Van Nostrand Reinhold Company: New York, NY, USA, 1993; p. 1153. [Google Scholar]
- ACGIH. Industrial Ventilation: A Manual of Recommended Practice, 24th ed.; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2001. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Organic Solvents, Resin Monomers and Related Compounds, Pigments and Occupational Exposures in Paint Manufacture and Painting; World Health Organization, International Agency for Research on Cancer: Lyon, France, 1989; Volume 47. [Google Scholar]
- U.S.G.S. Titanium and titanium dioxide. In Mineral Commodity Summaries; U.S. Department of the Interior, U.S. Geological Survey: Washington, DC, USA, 2008; pp. 180–181. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/titanium/mcs-2008-timet.pdf (accessed on 23 April 2021).
- Research and Markets. Titanium Dioxide (TiO2)—A Global Market IARC Overview; IE: Dublin, Ireland, 2016; p. 322. [Google Scholar]
- Aitken, R.J.; Creely, K.S.; Tran, C.L. Nanoparticles: An Occupational Hygiene Review; HSE Research Report 274; Health & Safety Executive: Edinburgh, UK, 2004. Available online: http://www.hse.gov.uk/research/rrhtm/rr274.htm (accessed on 3 May 2021).
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans: Carbon Black, Titanium Dioxide, and Talc; World Health Organization, International Agency for Research on Cancer: Lyon, France, 2010; Volume 93, Available online: http://monographs.iarc.fr/ENG/Monographs/vol93/index.php (accessed on 3 May 2021).
- National Institute for Occupational Safety and Health (NIOSH). Pocket Guide to Chemical Hazards and Other Databases [CD-ROM]; DHHS Publication No. 2002–140; U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control: Cincinnati, OH, USA, 2002. [Google Scholar]
- NIOSH. Occupational Exposure to Titanium Dioxide; Current Intelligence Bulletin 63; U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety: Cincinnati, OH, USA, 2011. Available online: http://www.cdc.gov/niosh/docs/2011-160/pdfs/2011-160.pdf (accessed on 28 April 2021).
- Lee, K.P.; Trochimowicz, H.J.; Reinhardt, C.F. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol. Appl. Pharmacol. 1985, 79, 179–192. [Google Scholar] [CrossRef]
- Chen, J.L.; Fayerweather, W.E. Epidemiologic study of workers exposed to titanium dioxide. J. Occup. Med. 1988, 30, 937–942. [Google Scholar] [CrossRef]
- Fryzek, J.P.; Chadda, B.; Marano, D.; White, K.; Schweitzer, S.; McLaughlin, J.K.; Blot, W.J. A cohort mortality study among titanium dioxide manufacturing workers in the United States. J. Occup. Environ. Med. 2003, 45, 400–409. [Google Scholar] [CrossRef]
- Boffetta, P.; Gaborieau, V.; Nadon, L.; Parent, M.E.; Weiderpass, E.; Siemiatycki, J. Exposure to titanium dioxide and risk of lung cancer in a population-based study from Montreal. Scand. J. Work Environ. Health. 2001, 27, 227–232. [Google Scholar] [CrossRef]
- Boffetta, P.; Soutar, A.; Cherrie, J.W.; Granath, F.; Andersen, A.; Anttila, A.; Blettner, M.; Gaborieau, V.; Klug, S.J.; Langard, S.; et al. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control. 2004, 15, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Ramanakumar, A.V.; Parent, M.E.; Latreille, B.; Siemiatycki, J. Risk of lung cancer following exposure to carbon black, titanium dioxide and talc: Results from two case-control studies in Montreal. Int. J. Cancer 2008, 122, 183–189. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10–15, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Lettino, A.; Belviso, C.; Cavalcante, F.; Fiore, S. Environmental risk induced by TiO2 dispersions in waters and sediments: A case study. Environ. Geochem. Health 2016, 38, 73–84. [Google Scholar] [CrossRef]
- Gurr, J.R.; Wang, A.S.; Chen, C.H.; Jan, K.Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.; Handy, R.D.; Garrod, J.; Owen, R. Ecotoxicity test methods and environmental hazard assessment for engineered nanoparticles. Ecotoxicology 2008, 17, 421–437. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef]
- Borm, P.J.; Schins, R.P.; Albrecht, C. Inhaled particles and lung cancer, part B: Paradigms and risk assessment. Int. J. Cancer 2004, 110, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Knaapen, A.M.; Borm, P.J.; Albrecht, C.; Schins, R.P. Inhaled particles and lung cancer. Part A: Mechanisms. Int J Cancer. 2004, 109, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Baan, R.A. Carcinogenic hazards from inhaled carbon black, titanium dioxide, and talc not containing asbestos or asbestiform fibers: Recent evaluations by an IARC Monographs Working Group. Inhal. Toxicol. 2007, 19, 213–228. [Google Scholar] [CrossRef]
- Gaggero, L.; Sanguineti, E.; Yus González, A.; Militello, G.M.; Scuderi, A.; Parisi, G. Airborne asbestos fibres monitoring in tunnel excavation. J. Environ. Manag. 2017, 196, 583–593. [Google Scholar] [CrossRef]
- World Health Organisation. Determination of Airborne Fiber Number Concentrations. In A Recommended Method, by Phase Contrast Microscopy (Membrane Filter Method); World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Jani, P.; McCarthy, D.; Florence, A. Titanium dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. Int. J. Pharm. 1994, 105, 157–168. [Google Scholar] [CrossRef]
- ACGIH: Titanium dioxide. In Documentation of the Threshold Limit Values for Chemical Substances, 7th ed.; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2001.
- OSHA. Available online: http://www.osha.gov/dts/chemicalsampling/data/CH_272100.html (accessed on 20 April 2021).
- Militello, G.M.; Gaggero, L.; La Maestra, S. Asbestiform Amphiboles and Cleavage Fragments Analogues: Overview of Critical Dimensions, Aspect Ratios, Exposure and Health Effects. Minerals 2021, 11, 525. [Google Scholar] [CrossRef]
- McClellan, R.O. Use of mechanistic data in assessing human risks from exposure to particles. Environ. Health Perspect. 1997, 105, 1363–1372. [Google Scholar] [PubMed]
- Greim, H.A. Research needs to improve risk assessment of fiber toxicity. Mutat. Res. 2004, 553, 11–22. [Google Scholar] [CrossRef]
- La Maestra, S.; Micale, R.T.; Ferretti, M.; Izzotti, A.; Gaggero, L. Attenuation of oxidative stress and chromosomal aberrations in cultured macrophages and pulmonary cells following self-sustained high temperature synthesis of asbestos. Sci. Rep. 2020, 10, 8581. [Google Scholar] [CrossRef]
- Heinrich, U.; Fuhst, R.; Rittinghausen, S.; Creutzenberg, O.; Bellmann, B.; Koch, W.; Levsen, K. Chronic inhalation exposure of Wistar rats and two different strains of mice to diesel engine exhaust, carbon black, and titanium dioxide. Inhal. Toxicol. 1994, 7, 533–556. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 20, 7–40373. [Google Scholar] [CrossRef] [PubMed]
- Proquin, H.; Jetten, M.J.; Jonkhout, M.C.M.; Garduño-Balderas, L.G.; Briedé, J.J.; de Kok, T.M.; Chirino, Y.I.; van Loveren, H. Gene expression profiling in colon of mice exposed to food additive titanium dioxide (E171). Food Chem. Toxicol. 2018, 111, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, H.M.; Gosens, I.; Heringa, M.B.; Oomen, A.G.; Vandebriel, R.J.; Groenewold, M.; Cassee, F.R. Mechanism of Action of TiO2: Recommendations to Reduce Uncertainties Related to Carcinogenic Potential. Annu. Rev. Pharmacol. Toxicol. 2021, 6, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Marucco, A.; Prono, M.; Beal, D.; Alasonati, E.; Fisicaro, P.; Bergamaschi, E.; Carriere, M.; Fenoglio, I. Biotransformation of Food-Grade and Nanometric TiO2 in the Oral-Gastro-Intestinal Tract: Driving Forces and Effect on the Toxicity toward Intestinal Epithelial Cells. Nanomaterials 2020, 10, 2132. [Google Scholar] [CrossRef] [PubMed]
- Zender, C.S.; Bian, H.S.; Newman, D. Mineral dust entrainment and deposition (DEAD) model: Description and 1990s dust climatology. J. Geophys. Res. 2003, 108, 4416. [Google Scholar] [CrossRef]
- Miller, R.L.; Cakmur, R.V.; Perlwitz, J.; Geogdzhayev, I.V.; Ginoux, P.; Koch, D.; Prigent, C.; Ruedy, R.; Schmidt, G.A.; Tegen, I. Mineral dust aerosols in the NASA Goddard Institute for Space Sciences ModelE atmospheric general circulation model. J. Geophys. Res. 2006, 111, 1–19. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Maestra, S.; D’Agostini, F.; Sanguineti, E.; Yus González, A.; Annis, S.; Militello, G.M.; Parisi, G.; Scuderi, A.; Gaggero, L. Dispersion of Natural Airborne TiO2 Fibres in Excavation Activity as a Potential Environmental and Human Health Risk. Int. J. Environ. Res. Public Health 2021, 18, 6587. https://doi.org/10.3390/ijerph18126587
La Maestra S, D’Agostini F, Sanguineti E, Yus González A, Annis S, Militello GM, Parisi G, Scuderi A, Gaggero L. Dispersion of Natural Airborne TiO2 Fibres in Excavation Activity as a Potential Environmental and Human Health Risk. International Journal of Environmental Research and Public Health. 2021; 18(12):6587. https://doi.org/10.3390/ijerph18126587
Chicago/Turabian StyleLa Maestra, Sebastiano, Francesco D’Agostini, Elisa Sanguineti, Adrián Yus González, Samanta Annis, Gaia M. Militello, Giovanni Parisi, Alberto Scuderi, and Laura Gaggero. 2021. "Dispersion of Natural Airborne TiO2 Fibres in Excavation Activity as a Potential Environmental and Human Health Risk" International Journal of Environmental Research and Public Health 18, no. 12: 6587. https://doi.org/10.3390/ijerph18126587
APA StyleLa Maestra, S., D’Agostini, F., Sanguineti, E., Yus González, A., Annis, S., Militello, G. M., Parisi, G., Scuderi, A., & Gaggero, L. (2021). Dispersion of Natural Airborne TiO2 Fibres in Excavation Activity as a Potential Environmental and Human Health Risk. International Journal of Environmental Research and Public Health, 18(12), 6587. https://doi.org/10.3390/ijerph18126587