Muscle Recovery after a Single Bout of Functional Fitness Training
Abstract
:1. Introduction
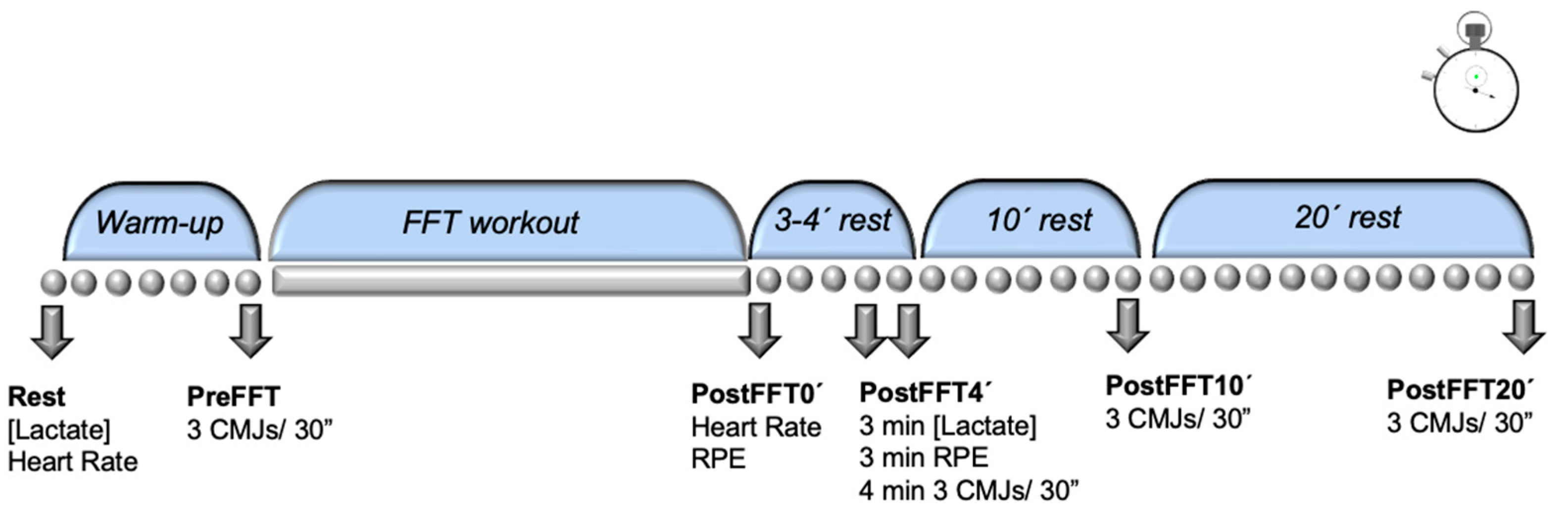
2. Materials and Methods
2.1. Experimental Design
2.2. Subjects
2.3. Functional Fitness Training (FFT) Workout
2.4. Heart Rate
2.5. Blood Lactate
2.6. Rating of Perceived Exertion
2.7. Muscular Fatigue
2.8. Statistical Analysis
3. Results
4. Discussion
4.1. Metabolic Factors
4.2. Mechanical Factors
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Heinrich, K.M.; Becker, C.; Carlisle, T.; Gilmore, K.; Hauser, J.; Frye, J.; Harms, C.A. High-intensity functional training improves functional movement and body composition among cancer survivors: A pilot study. Eur. J. Cancer Care 2015, 24, 812–817. [Google Scholar] [CrossRef]
- Feito, Y.; Heinrich, K.M.; Butcher, S.J.; Poston, W.S.C. High-Intensity Functional Training (FFT): Definition and Research Implications for Improved Fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, K.M.; Spencer, V.; Fehl, N.; Poston, W.S. Mission essential fitness: Comparison of functional circuit training to traditional army physical training for active duty military. Mil. Med. 2012, 177, 1125–1130. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, K.M.; Patel, P.M.; O’Neal, J.L.; Heinrich, B.S. High-intensity compared to moderate-intensity training for exercise initiation, enjoyment, adherence, and intentions: An intervention study. BMC Public Health 2014, 14, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poston, W.S.; Haddock, C.K.; Heinrich, K.M.; Jahnke, S.A.; Jitnarin, N.; Batchelor, D.B. Is high-intensity functional training (FFT)/CrossFit safe for military fitness training? Mil. Med. 2016, 181, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcaraz, P.E.; Sanchez-Lorente, J.; Blazevich, A.J. Physical performance and cardiovascular responses to an acute bout of heavy resistance circuit training versus traditional strength training. J. Strength Cond. Res. 2008, 22, 667–671. [Google Scholar] [CrossRef] [Green Version]
- Gosker, H.R.; Schols, A.M. Fatigued muscles in COPD but no finishing line in sight. Eur. Respir. J. 2008, 31, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Rainoldi, A.; Gazzoni, M.; Merletti, R.; Minetto, M.A. Mechanical and EMG responses of the vastus lateralis and changes in biochemical variables to isokinetic exercise in endurance and power athletes. J. Sports Sci. 2008, 26, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Dis. 2012, 13, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef]
- Smilios, I.; Hakkinen, K.; Tokmakidis, S.P. Power Output and Electromyographic Activity during and after a Moderate Load Muscular Endurance Session. J. Strength Cond. Res. 2010, 24, 2122–2131. [Google Scholar] [CrossRef]
- Bobbert, M.F.; van Soest, A.J. Why do people jump the way they do? Exerc. Sport Sci. Rev. 2001, 29, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Medina, L.; González-Badillo, J.J. Velocity Loss as an indicator of neuromuscular fatigue during resistance training. Med. Sci. Sports Exerc. 2011, 43, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Castaño, M.V.; Domínguez, R.; Ruiz-Solano, P.; Maté-Muñoz, J.L. Acute physiological and mechanical responses during resistance exercise executed at the lactate threshold workload. J. Strength Cond. Res. 2015, 29, 2867–2873. [Google Scholar] [CrossRef]
- Horita, T.; Komi, P.V.; Hamalainen, I.; Avela, J. Exhausting stretch-shortening cycle (SSC) exercise causes greater impairment in SSC performance than in pure concentric performance. Eur. J. Appl. Physiol. 2003, 88, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Dousset, E.; Avela, J.; Kyrolainen, H.; Kallio, J.; Linnamo, V.; Komi, P.V. Changes in the soleus muscle architecture after exhausting stretch-shortening cycle exercise in humans. Eur. J. Appl. Physiol. 2006, 97, 298–306. [Google Scholar] [CrossRef]
- Ishikawa, M.; Komi, P.V.; Finni, T.; Kuitunen, S. Contribution of the tendinous tissue to force enhancement during stretch-shortening cycle exercise depends on the prestretch and concentric phase intensities. J. Electromyogr. Kinesiol. 2006, 16, 423–431. [Google Scholar] [CrossRef]
- Maté-Muñoz, J.L.; Lougedo, J.H.; Barba, M.; García-Fernández, P.; Garnacho-Castaño, M.V.; Domínguez, R. Muscular fatigue in response to different modalities of CrossFit sessions. PLoS ONE 2017, 12, e0181855. [Google Scholar] [CrossRef] [Green Version]
- Maté-Muñoz, J.L.; Lougedo, J.H.; Barba, M.; Cañuelo-Márquez, A.M.; Guodemar-Pérez, J.; García-Fernández, P.; Lozano-Estevan, M.C.; Alonso-Melero, R.; Sánchez-Calabuig, M.A.; Ruiz-López, M.; et al. Cardiometabolic and Muscular Fatigue Responses to Different Crossfit® Workouts. J. Sports Sci. Med. 2018, 20, 668–679. [Google Scholar]
- Tomlin, D.L.; Wenger, H.A. The Relationship between Aerobic Fitness and Recovery from High Intensity Intermittent Exercise. Sports Med. 2001, 31, 1–11. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- McNaughton, L.R.; Thompson, D.; Philips, G.; Backx, K.; Crickmore, L. A comparison of the lactate Pro, Accusport, Analox GM7 and Kodak Ektachem lactate analysers in normal, hot and humid conditions. Int. J. Sports Med. 2002, 23, 130–135. [Google Scholar] [CrossRef]
- McLean, S.R.; Norris, S.R.; Smith, D.J. Comparison of the Lactate Pro and the YSI 1500 Sport Blood Lactate Analyzers. Int. J. Appl. Sports Sci. 2004, 16, 22–30. [Google Scholar]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [PubMed]
- Domínguez, R.; Garnacho-Castaño, M.V.; Cuenca, E.; García-Fernández, P.; Muñoz-González, A.; De Jesús, F.; Lozano-Estevan, M.C.; Fernandes da Silva, S.; Veiga-Herreros, P.; Maté-Muñoz, J.L. Effects of Beetroot Juice Supplementation on a 30-s High-Intensity Inertial Cycle Ergometer Test. Nutrients 2017, 9, 1360. [Google Scholar] [CrossRef] [Green Version]
- Gorostiaga, E.M.; Asiain, X.; Izquierdo, M.; Postigo, A.; Aguado, R.; Alonso, J.M.; Ibánez, J. Vertical jump performance and blood ammonia and lactate levels during typical training sessions in elite 400-m runners. J. Strength Cond. Res. 2010, 24, 1138–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markovic, G.; Dizdar, D.; Jukic, I.; Cardinale, M. Reliability and factorial validity of squat and countermovement jump tests. J. Strength Cond. Res. 2004, 18, 551–555. [Google Scholar]
- Bobbert, M.F.; Gerritsen, K.G.M.; Litjens, M.C.A.; Van Soest, A.J. Why is countermovement jump height greater than squat jump height? Med. Sci. Sports Exerc. 1996, 28, 1402–1412. [Google Scholar] [CrossRef]
- Linthorne, N.P. Analysis of standing vertical jumps using a force platform. Am. J. Phys. 2001, 69, 1198–1204. [Google Scholar] [CrossRef] [Green Version]
- Street, G.M.; McMillan, S.; Board, W.; Rasmussen, M.; Heneghan, J.M. Sources of error in determining countermovement jump height with the impulse method. J. Appl. Biomech. 2001, 17, 43–54. [Google Scholar] [CrossRef]
- Flora, P.; Gómez-Landero, L.A.; Suárez-Arrones, L.; Harrison, A.J. Kinetic and kinematic analysis for assessing the differences in countermovement jump performance in rugby players. J. Strength Cond. Res. 2016, 30, 2533–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, N.J.; Watkins, J.; Kilduff, L.P.; Bevan, H.R.; Bennett, M.A. Development of a criterion method to determine peak mechanical power output in a countermovement jump. J. Strength Cond. Res. 2014, 28, 1552–1558. [Google Scholar] [CrossRef] [Green Version]
- Rodacki, A.L.F.; Fowler, N.E.; Bennet, S.J. Vertical jump coordination: Fatigue effects. Med. Sci. Sports Exerc. 2002, 34, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Freitas, P.B.; Barela, J.A.; Ugrinowitsch, C.; Rodacki, A.L.F.; Kokubun, E.; Fowler, N.E. Vertical jump fatigue does not affect intersegmental coordination and segmental contribution. Motriz 2014, 20, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; Sage: London, UK, 2013; pp. 473–474. [Google Scholar]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Alberton, C.L.; Antunes, A.H.; Beilke, D.D.; Pinto, S.S.; Kanitz, A.C.; Tartaruga, M.P.; Kruel, L.F.M. Maximal and Ventilatory Thresholds of Oxygen Uptake and Rating of Perceived Exertion Responses to Water Aerobic Exercises. J. Strength Cond. Res. 2013, 27, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Rodacki, A.L.F.; Fowler, N.E.; Bennett, S.J. Multi-segment coordination: Fatigue effects. Med. Sci. Sports Exerc. 2001, 33, 1157–1167. [Google Scholar] [CrossRef]
- Skare, O.C.; Skadberg, O.; Wisnes, A.R. Creatine supplementation improves sprint performance in male sprinters. Scand. J. Med. Sci. Sports 2001, 11, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, J.; Sabio-Solana, R.; Moya, D.; Sarabia, J.M.; Moya, M. Acute physiological responses during Crossfit® workouts. Eur. J. Hum. Mov. 2015, 35, 114–124. [Google Scholar]
- Lucía, A.; Sánchez, O.; Carvajal, A.; Chicharro, J.L. Analysis of the aerobic- anaerobic transition in elite cyclists during incremental exercise with the use of electromyography. Br. J. Sports Med. 1999, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Burnley, B.; Jones, A.M. Oxygen uptake kinetics as a determinant of sports performance. Eur. J. Sport Sci. 2007, 7, 63–79. [Google Scholar] [CrossRef]
- Korhonen, M.T.; Cristea, A.; Markku, A.; Hakkinen, K.; Sipila, S.; Mero, A.; Viitasalo, J.T.; Larsson, L.; Suominen, H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J. Appl. Physiol. 2006, 101, 906–917. [Google Scholar] [CrossRef]
- Lucertini, F.; Gervasi, M.; D’Amen, G.; Sisti, D.; Rocchi, M.B.L.; Stocchi, V.; Benelli, P. Effect of water-based recovery on blood lactate removal after high-intensity exercise. PLoS ONE 2017, 12, e0184240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchini, E.; Bertuzzi, R.C.M.; Takito, M.Y.; Kiss, M.A.P.D.M. Effects of recovery type after a judo match on blood lactate and performance in specific and non-specific judo tasks. Eur. J. Appl. Physiol. 2009, 107, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Ikebukuro, T. Changes in joint, muscle, and tendon stiffness following repeated hopping exercise. Physiol. Rep. 2019, 7, e14237. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, J.; Cronin, N.J.; Avela, J.; Finni, T. In vivo mechanical response of human Achilles tendon to a single bout of hopping exercise. J. Exp. Biol. 2010, 213, 1259–1265. [Google Scholar] [CrossRef] [Green Version]
- Peltonen, J.; Cronin, N.J.; Stenroth, L.; Finni, T.; Avela, J. Achilles tendon stiffness is unchanged one hour after a marathon. J. Exp. Biol. 2012, 215, 3665–3671. [Google Scholar] [CrossRef] [Green Version]
- Klich, S.; Ficek, K.; Krymski, I.; Klimek, A.; Kawczynski, A.; Madeleine, P.; Fernández de las Peñas, C. Quadriceps and Patellar Tendon Thickness and Stiffness in Elite Track Cyclists: An Ultrasonographic and Myotonometric Evaluation. Front. Physiol. 2020, 11, 607208. [Google Scholar] [CrossRef] [PubMed]
- Ditroilo, M.; Watsford, M.; Fernández-Peña, E.; D´Amen, G.; Lucertini, F.; De Vito, G. Effects of fatigue on muscle stiffness and intermittent sprinting during cycling. Med. Sci. Sports Exerc. 2010, 43, 837–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farley, C.T.; Morgenroth, D.C. Leg stiffness primarily depends on ankle stiffness during human hopping. J. Biomech. 1999, 32, 267–273. [Google Scholar] [CrossRef]
- Tibana, R.A.; De Sousa, N.M.F.; Prestes, J.; Voltarelli, F.A. Lactate, Heart Rate and Rating of Perceived Exertion Responses to Shorter and Longer Duration CrossFit® Training Sessions. J. Funct. Morphol. Kinesiol. 2018, 3, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk Neto, J.H.; Tibana, R.A.; de Sousa, N.M.F.; Prestes, J.; Voltarelli, F.A.; Kennedy, M.D. Session Rating of Perceived Exertion Is a Superior Method to Monitor Internal Training Loads of Functional Fitness Training Sessions Performed at Different Intensities When Compared to Training Impulse. Front. Physiol. 2020, 11, 919. [Google Scholar] [CrossRef] [PubMed]

| Functional Fitness Training Workout | |||
|---|---|---|---|
| 2 rounds (r) × (6 power clean) | 2 r × (6 pull-up) | ||
| A power clean requires the athlete to catch the bar in the front rack position without reaching the bottom of a squat. |  | To complete the pull-up, the subject grips a suspended horizontal bar with both hands and fully extends both arms at the repetition bottom. At the repetition top, the chin should break the uppermost horizontal plane of the bar. A kip of any style may be used. The repetition is counted when the athlete’s chin breaks the top-most horizontal plane of the bar. |  |
| Men: 50 kg | Women: 35 kg | ||
| 2 r × (10 object over shoulder) | 2 r × (10 bodyweight squat) | ||
| For the object over shoulder, the athlete must pick up the designated object and toss or push it over the shoulder while the athlete reaches full extension of the hips. The athlete may lift the object using any technique. |  | The bodyweight squat is a bodyweight movement where, the athlete begins in a standing position with an open hip angle, descends to a full squat, with the creases of both hips below the plane of the top of the knees, and returns to the standing position with hips returning to an open angle. |  |
| Men: 20 kg | Women: 15 kg | ||
| 2 r × (14 wall ball shot) | 2 r × (14 power snatch) | ||
| The wallball shot is performed with a medicine ball and an elevated target. With the medicine ball in the frontal plane, the athlete must descend to a bottom-of-squat position and then, while ascending, throw the ball so that it makes contact at or above a designated height. Jumping during the repetition is permissible but not required. |  | The power snatch requires the athlete to catch the dumbbell overhead with elbows fully extended but without achieving the bottom of a squat during the task. |  |
| Men: 9 kg | Women: 7 kg | Men: 20 kg | Women: 10 kg |
| 2 r × (18 shoulder to overhead) | 2 r × (18 box jump) | ||
| Shoulder to overhead movements involve elevating dumbells from a static position at the shoulder to a static position overhead. The athlete may use a single, simultaneous bending of the hips and/or knees to assist in elevating the dumbbells to the top of the repetition. |  | The box jump requires the athlete to initiate a jump from the ground with both feet simultaneously, land on top of a designated object (e.g., box) with both feet and demonstrate static control. |  |
| Men: 20 kg | Women: 10 kg | Men: 60 cm (23.6 inch) | Women: 50 cm (19.7 inch) |
| 2 r × (200 m run) | 2 r × (100 m run) | ||
| Variable | Group | M ± SD | t | p |
|---|---|---|---|---|
| Final RPE Muscular | PostFFT0′ | 16.50 ± 2.43 | 6.02 | 0.000 * |
| PostFFT3′ | 13.18 ± 3.30 | |||
| Final RPE Cardio | PostFFT0′ | 14.64 ± 2.75 | 6.32 | 0.000 * |
| PostFFT3′ | 11.80 ± 2.40 | |||
| Final RPE General | PostFFT0′ | 15.67 ± 2.01 | 6.82 | 0.000 * |
| PostFFT3′ | 12.83 ± 2.58 |
| Variable | Pre (M ± SD, 95% CI) | Post 4′ (M ± SD, 95% CI) | Post 10′ (M ± SD, 95% CI) | Post 20′ (M ± SD, 95% CI) | % Loss Pre–Post 4′ | % Loss Pre–Post 10′ | % Loss Pre–Post 20′ | p for the Time Effect | ηp2 | SP |
|---|---|---|---|---|---|---|---|---|---|---|
| Jump height (cm) | 28.13 ± 8.35 ‡, * (24.89–31.36) | 26.45 ± 7.45 (23.57–29.34) | 26.22 ± 7.90 (23.16–29.28) | 26.90 ± 7.64 (23.93–29.86) | −5.97 | −6.79 | −4.37 | 0.005 * | 0.144 | 0.871 |
| Vmax (m·s−1) | 2.52 ± 0.30 ‡, * (2.40–2.64) | 2.46 ± 0.31 (2.34–2.58) | 2.44 ± 0.33 (2.32–2.57) | 2.47 ± 0.31 (2.35–2.59) | −2.38 | −3.17 | −1.98 | 0.001 * | 0.178 | 0.926 |
| Fmax (Newtons) | 1849.92 ± 295.19 (1735.46–1964.39) | 1864.41 ± 323.59 (1738.93–1989.88) | 1883.60 ± 317.92 (1760.33–2006.88) | 1825.58 ± 358.28 (1686.65–1964.50) | 0.78 | 1.82 | −1.31 | 0.213 | 0.055 | 0.342 |
| APR (watts·kg−1) | 26.50 ± 4.58 ‡, * (24.73–28.28) | 25.50 ± 4.70 (23.68–27.32) | 25.73 ± 4.82 (23.86–27.60) | 26.20 ± 4.69 (24.39–28.02) | −3.77 | −2.91 | −1.13 | 0.151 | 0.069 | 0.370 |
| APT (watts) | 2005.10 ± 458.11 ‡, * (1827.45–2182.73) | 1930.96 ± 465.85 (1750.32–2111.60) | 1947.61 ± 464.40 (1767.54–2127.68) | 1977.61 ± 466.68 (1796.65–2158.57) | −3.70 | −2.87 | −1.37 | 0.021 * | 0.113 | 0.754 |
| PPR (watts·kg−1) | 48.54 ± 8.25 (45.34–51.74) | 47.90 ± 8.45 (44.62–51.18) | 47.62 ± 8.59 (44.29–50.95) | 47.83 ± 8.04 (44.71–50.95) | −1.32 | −1.90 | −1.46 | 0.322 | 0.042 | 0.307 |
| PPT (watts) | 3672.85 ± 819.42 (3355.11–3990.58) | 3629.43 ± 849.57 (3300.00–3958.86) | 3607.57 ± 842.39 (3280.93–3934.22) | 3624.46 ± 819.34 (3306.75–3942.17) | −1.18 | −1.78 | −1.32 | 0.384 | 0.037 | 0.270 |
| Total jump duration (s) | 0.760 ± 0.13 *,† (0.708–0.812) | 0.738 ± 0.13 (0.689–0.787) | 0.719 ± 0.16 (0.656–0.782) | 0.708 ± 0.13 (0.658–0.759) | −2.89 | −5.39 | −6.84 | 0.005 * | 0.144 | 0.871 |
| ECC duration (s) | 0.577 ± 0.15 (0.519–0.634) | 0.580 ± 0.17 (0.525–0.636) | 0.567 ± 0.17 (0.501–0.636) | 0.553 ± 0.14 (0.498–0.608) | 0.52 | −1.73 | −4.16 | 0.323 | 0.042 | 0.306 |
| ISO duration (s) | 0.0126 ± 0.005 (0.011–0.015) | 0.0113 ± 0.004 (0.010–0.013) | 0.0111 ± 0.005 (0.009–0.013) | 0.0139 ± 0.011 (0.010–0.018) | −10.32 | −11.90 | 10.32 | 0.325 | 0.040 | 0.225 |
| CON duration (s) | 0.171 ± 0.06 ‡, *, † (0.146–0.195) | 0.146 ± 0.06 (0.122–0.170) | 0.139 ± 0.05 (0.118–0.160) | 0.142 ± 0.06 (0.120–0.163) | −14.62 | −18.71 | −16.96 | 0.000 * | 0.205 | 0.974 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Fernández, P.; Cimadevilla, E.; Guodemar-Pérez, J.; Cañuelo-Márquez, A.M.; Heredia-Elvar, J.R.; Fernández-Rodríguez, T.; Lozano-Estevan, M.d.C.; Hervás-Pérez, J.P.; Sánchez-Calabuig, M.A.; Garnacho-Castaño, M.V.; et al. Muscle Recovery after a Single Bout of Functional Fitness Training. Int. J. Environ. Res. Public Health 2021, 18, 6634. https://doi.org/10.3390/ijerph18126634
García-Fernández P, Cimadevilla E, Guodemar-Pérez J, Cañuelo-Márquez AM, Heredia-Elvar JR, Fernández-Rodríguez T, Lozano-Estevan MdC, Hervás-Pérez JP, Sánchez-Calabuig MA, Garnacho-Castaño MV, et al. Muscle Recovery after a Single Bout of Functional Fitness Training. International Journal of Environmental Research and Public Health. 2021; 18(12):6634. https://doi.org/10.3390/ijerph18126634
Chicago/Turabian StyleGarcía-Fernández, Pablo, Eduardo Cimadevilla, Jesús Guodemar-Pérez, Ana María Cañuelo-Márquez, Juan Ramón Heredia-Elvar, Tomás Fernández-Rodríguez, María del Carmen Lozano-Estevan, Juan Pablo Hervás-Pérez, María Aránzazu Sánchez-Calabuig, Manuel Vicente Garnacho-Castaño, and et al. 2021. "Muscle Recovery after a Single Bout of Functional Fitness Training" International Journal of Environmental Research and Public Health 18, no. 12: 6634. https://doi.org/10.3390/ijerph18126634






