High Performance of Biohydrogen Production in Packed-Filter Bioreactor via Optimizing Packed-Filter Position
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Sludge and Substrate
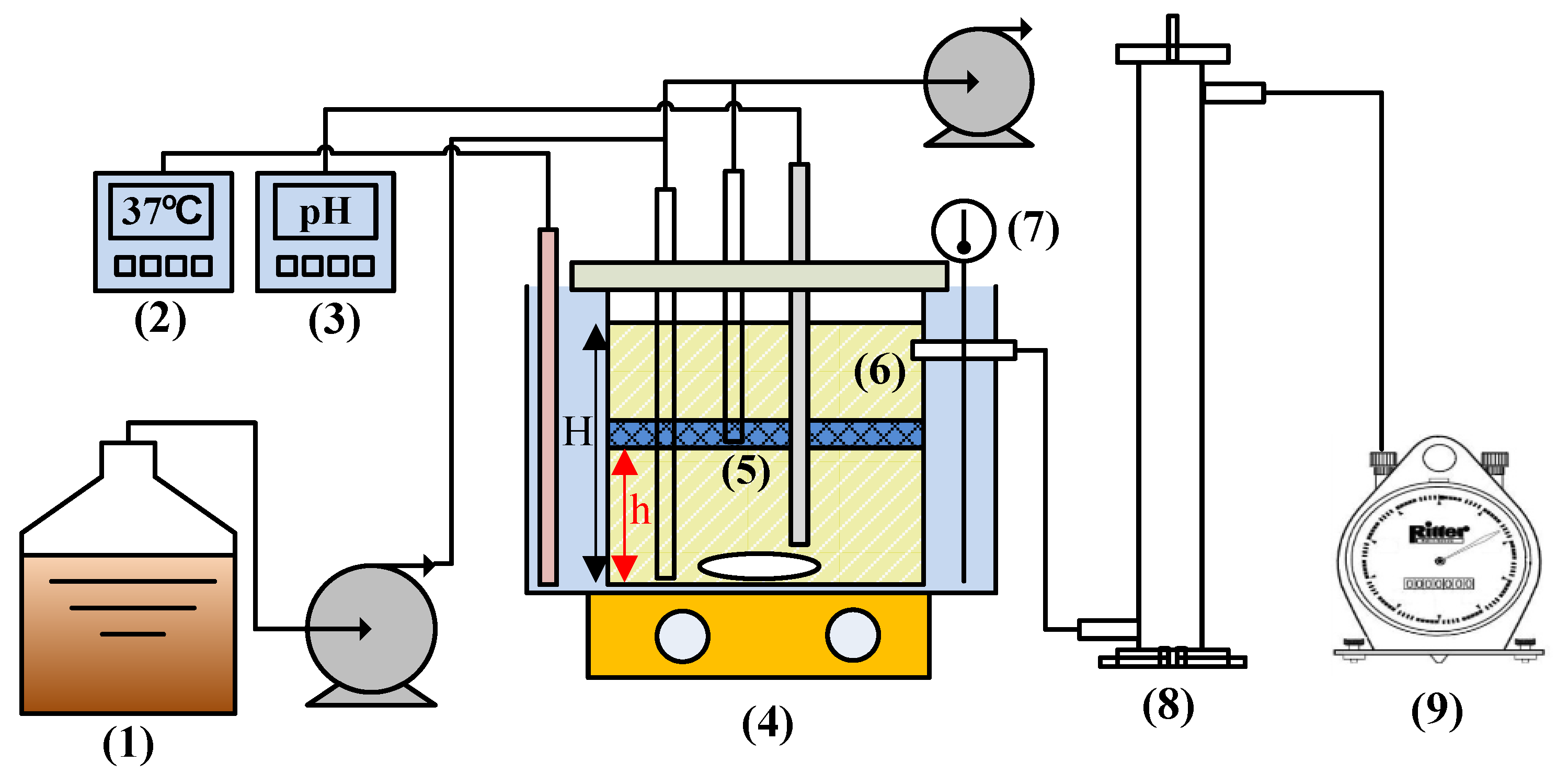
2.2. Bioreactor Design
2.3. Experimental Procedures
2.4. GC and DGGE Analysis
3. Results
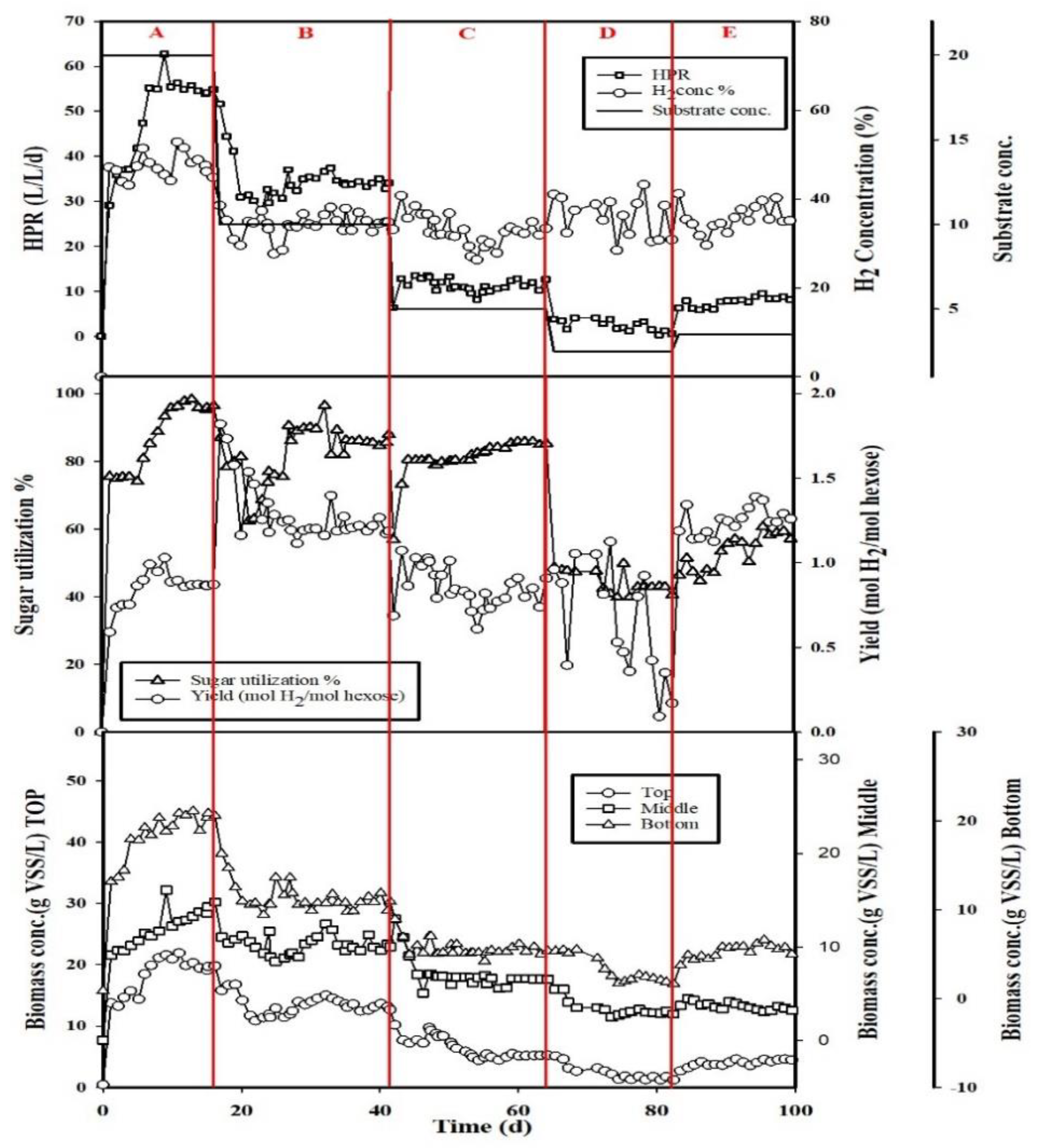
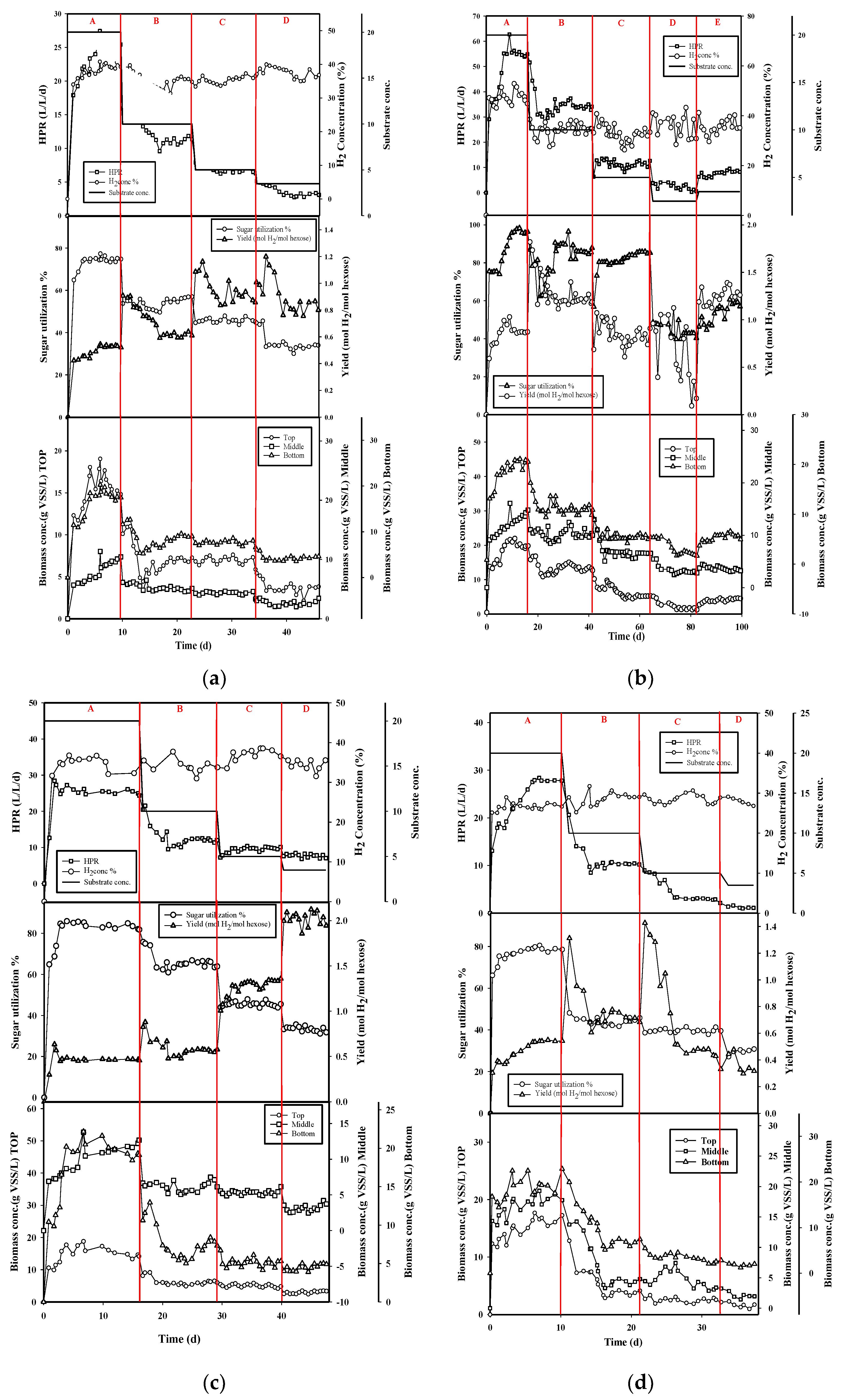
3.1. Effect of Substrate Concentration
3.2. Effect of Packed-Filter Position
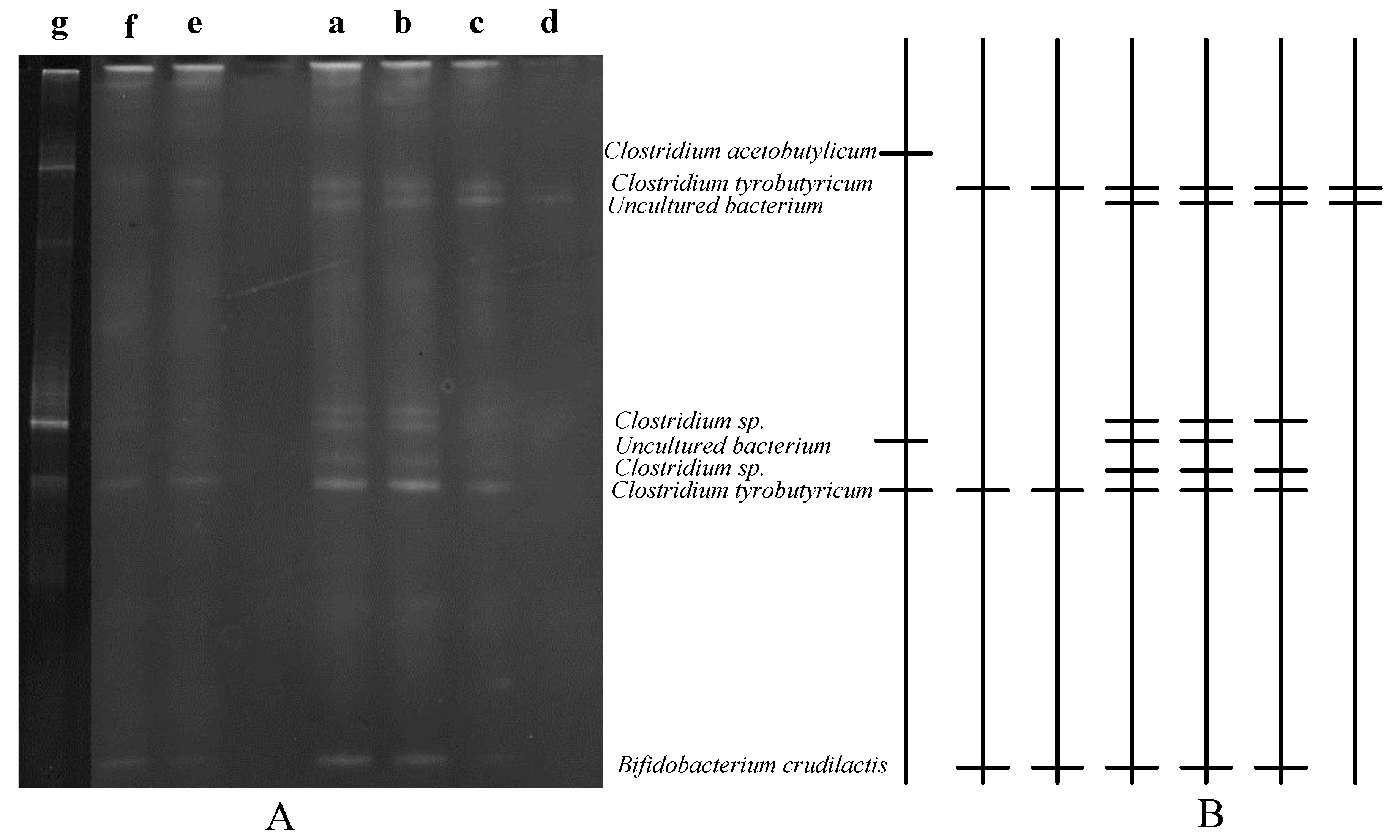
3.3. Microbial Community Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamza, R.A.; Iorhemen, O.T.; Tay, J.H. Advances in biological systems for the treatment of high-strength wastewater. J. Water Process. Eng. 2016, 10, 128–142. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D. A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Hamza, R.A.; Iorhemen, O.T.; Zaghloul, M.S.; Tay, J.H. Rapid formation and characterization of aerobic granules in pilot-scale sequential batch reactor for high-strength organic wastewater treatment. J. Water Process. Eng. 2018, 22, 27–33. [Google Scholar] [CrossRef]
- Ren, N.; Chen, Z.; Wang, X.; Hu, D.; Wang, A. Optimized operational parameters of a pilot scale membrane bioreactor for high-strength organic wastewater treatment. Int. Biodeterior. Biodegrad. 2005, 56, 216–223. [Google Scholar] [CrossRef]
- Sowmeyan, R.; Swaminathan, G. Evaluation of inverse anaerobic fluidized bed reactor for treating high strength organ-ic wastewater. Bioresour. Technol. 2008, 99, 3877–3880. [Google Scholar] [CrossRef]
- Hamza, R.A.; Zaghloul, M.; Iorhemen, O.T.; Sheng, Z.; Tay, J.H. Optimization of organics to nutrients (COD:N:P) ratio for aerobic granular sludge treating high-strength organic wastewater. Sci. Total Environ. 2019, 650, 3168–3179. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wu, S.; Chang, J. Fermentative hydrogen production with a draft tube fluidized bed reactor containing silicone-gel-immobilized anaerobic sludge. Int. J. Hydrogen Energy 2006, 31, 2200–2210. [Google Scholar] [CrossRef]
- Liu, C.-M.; Chu, C.-Y.; Lee, W.-Y.; Li, Y.-C.; Wu, S.-Y.; Chou, Y.-P. Biohydrogen production evaluation from rice straw hydrolysate by concentrated acid pre-treatment in both batch and continuous systems. Int. J. Hydrogen Energy 2013, 38, 15823–15829. [Google Scholar] [CrossRef]
- Chu, C.-F.; Xu, K.; Li, Y.-Y.; Inamori, Y. Hydrogen and methane potential based on the nature of food waste materials in a two-stage thermophilic fermentation process. Int. J. Hydrogen Energy 2012, 37, 10611–10618. [Google Scholar] [CrossRef]
- Park, M.J.; Jo, J.H.; Park, D.; Lee, D.S.; Park, J.M. Comprehensive study on a two-stage anaerobic digestion process for the sequential production of hydro-gen and methane from cost-effective molasses. Int. J. Hydrogen Energy 2010, 35, 6194–6202. [Google Scholar] [CrossRef]
- Ruggeri, B.; Tommasi, T.; Sassi, G. Energy balance of dark anaerobic fermentation as a tool for sustainability analysis. Int. J. Hydrogen Energy 2010, 35, 10202–10211. [Google Scholar] [CrossRef]
- Ventura, J.-R.S.; Lee, J.; Jahng, D. A comparative study on the alternating mesophilic and thermophilic two-stage anaer-obic digestion of food waste. J. Environ. Sci. 2014, 26, 1274–1283. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, R.; Xu, X.; Fan, X.; Luo, S. Hydrogen and methane production from lipid-extracted microalgal biomass residues. Int. J. Hydrogen Energy 2011, 36, 3465–3470. [Google Scholar] [CrossRef]
- Herrmann, A.P.; Janke, H.D. Cofermentation of rutin and hesperidin during two-stage anaerobic pre-treatment of high-loaded brewery wastewater. Water Res. 2001, 35, 2583–2588. [Google Scholar] [CrossRef]
- Cooney, M.; Maynard, N.; Cannizzaro, C.; Benemann, J. Two-phase anaerobic digestion for production of hydrogen–methane mixtures. Bioresour. Technol. 2007, 98, 2641–2651. [Google Scholar] [CrossRef]
- Mari, A.G.; Andreani, C.L.; Tonello, T.U.; Leite, L.C.; Fernandes, J.R.; Lopes, D.D.; Rodrigues, J.; Gomes, S.D. Biohydrogen and biomethane production from cassava wastewater in a two-stage anaerobic sequencing batch biofilm reactor. Int. J. Hydrogen Energy 2020, 45, 5165–5174. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Chu, C.-Y.; Ou, C.-M. Pre-treatment study on two-stage biohydrogen and biomethane productions in a continuous co-digestion process from a mixture of swine manure and pineapple waste. Int. J. Hydrogen Energy 2021, 46, 11325–11336. [Google Scholar] [CrossRef]
- Kisielewska, M.; Wysocka, I.; Rynkiewicz, M.R. Continuous biohydrogen and biomethane production from whey per-meate in a two-stage fermentation process. Environ. Prog. Sustain. Energy 2014, 33, 1411–1418. [Google Scholar]
- Wu, S.-Y.; Chu, C.-Y.; Yeh, W.-Z. Aspect ratio effect of bioreactor on fermentative hydrogen production with immobi-lized sludge. Int. J. Hydrogen Energy 2013, 38, 6154–6160. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Tung, L.; Lin, C.-Y. Effect of substrate concentration and pH on biohydrogen production kinetics from food industry wastewater by mixed culture. Int. J. Hydrogen Energy 2013, 38, 15849–15855. [Google Scholar] [CrossRef]
- Tran, V.G.; Chu, C.-Y.; Unpaprom, Y.; Ramaraj, R.; Chen, T.-H. Effects of substrate concentration and hydraulic retention time on hydrogen production from common reed by enriched mixed culture in continuous anaerobic bioreactor. Int. J. Hydrogen Energy 2021, 46, 14036–14044. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.; Yang, F.; Ma, Y. Current Progress on Butyric Acid Production by Fermentation. Curr. Microbiol. 2009, 59, 656–663. [Google Scholar] [CrossRef]
- Van Ginkel, S.; Logan, B.E. Inhibition of biohydrogen production by undissociated acetic and butyric acids. Environ. Sci. Technol. 2005, 39, 9351–9356. [Google Scholar] [CrossRef]
- Hastuti, Z.D.; Chu, C.-Y.; Rachman, M.A.; Purwanto, W.W.; Dewi, E.L.; Lin, C.-Y. Effect of concentration on biohydrogen production in a continuous stirred bioreactor using biofilm induced packed-carrier. Int. J. Hydrogen Energy 2016, 41, 21649–21656. [Google Scholar] [CrossRef]
- Fang, H.H.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Morimoto, M. Biological production of hydrogen from glucose by natural anaerobic microflora. Int. J. Hydrogen Energy 2004, 29, 709–713. [Google Scholar] [CrossRef]
- Ueno, Y.; Haruta, S.; Ishii, M.; Igarashi, Y. Characterization of a microorganism isolated from the effluent of hydrogen fermentation by microflora. J. Biosci. Bioeng. 2001, 92, 397–400. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chang, R.-C. Fermentative hydrogen production at ambient temperature. Int. J. Hydrogen Energy 2004, 29, 715–720. [Google Scholar] [CrossRef]
- Gao, C.; Ma, C.; Xu, P. Biotechnological routes based on lactic acid production from biomass. Biotechnol. Adv. 2011, 29, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cai, J.; Wang, J.; Zhu, X.; Huang, L.; Yang, S.-T.; Xu, Z. Efficient production of butyric acid from Jerusalem artichoke by immobilized Clostridium tyrobutyricum in a fibrous-bed bioreactor. Bioresour. Technol. 2011, 102, 3923–3926. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-S.; Im, J.A.; Choi, S.Y.; Lee, J.I.; Lee, S.Y. Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity. Metab. Eng. 2014, 23, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-S.; Malaviya, A.; Cho, C.; Lee, J.; Lee, S.Y. Butanol production from renewable biomass by clostridia. Bioresour. Technol. 2012, 123, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, A.; Jang, Y.-S.; Lee, S.Y. Continuous butanol production with reduced byproducts formation from glycerol by a hyper producing mutant of Clostridium pasteurianum. Appl. Microbiol. Biotechnol. 2012, 93, 1485–1494. [Google Scholar] [CrossRef]
- Jones, S.W.; Paredes, C.J.; Tracy, B.; Cheng, N.; Sillers, R.; Senger, R.S.; Papoutsakis, E.T. The transcriptional program underlying the physiology of clostridial sporulation. Genome Biol. 2008, 9, R114. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, D.; Lütke-Eversloh, T. Switching Clostridium acetobutylicum to an ethanol producer by disruption of the butyrate/butanol fermentative pathway. Metab. Eng. 2011, 13, 464–473. [Google Scholar] [CrossRef]
- Liu, G.; Shen, J. Effects of culture and medium conditions on hydrogen production from starch using anaerobic bacteria. J. Biosci. Bioeng. 2004, 98, 251–256. [Google Scholar] [CrossRef]
- Mullai, P.; Vishali, S.; Yogeswari, M.; López, M.E.; Rene, E.R. Methane production and recovery from wastewater. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–36. [Google Scholar]
| Substrate Conc. (g Total Sugar/L) | Hydrogen Conc. (%) | HPR (L/L/d) | Yield (mol H2/mol hexose) | Total Sugar Utilization (%) | Biomass Conc. Top (g VSS/L) | Biomass Conc. Filter (g VSS/L) | Biomass Conc. Bottom (g VSS/L) |
|---|---|---|---|---|---|---|---|
| 19.51 ± 1.06 | 48.00 ± 3.03 | 54.98 ± 0.81 | 0.90 ± 0.06 | 96.43 ± 1.05 | 20.14 ± 0.91 | 13.45 ± 0.85 | 20.33 ± 0.78 |
| 10.19 ± 1.68 | 34.77 ± 1.69 | 34.42 ± 1.27 | 1.21 ± 0.03 | 85.87 ± 1.91 | 13.52 ± 0.76 | 10.33 ± 0.91 | 10.77 ± 0.68 |
| 5.34 ± 1.05 | 31.86 ± 2.17 | 11.03 ± 1.22 | 0.82 ± 0.07 | 84.81 ± 0.99 | 5.83 ± 1.32 | 6.53 ± 0.40 | 5.32 ± 0.45 |
| 2.51 ± 0.76 | 34.40 ± 5.03 | 1.60 ± 0.96 | 0.46 ± 0.27 | 42.72 ± 3.02 | 1.49 ± 0.27 | 3.00 ± 0.18 | 2.08 ± 0.34 |
| 3.48 ± 1.33 | 35.02 ± 3.73 | 8.35 ± 0.55 | 1.31 ± 0.07 | 56.57 ± 2.92 | 4.27 ± 0.33 | 3.53 ± 0.35 | 5.76 ± 0.76 |
| Substrate Conc. (g Total Sugar/L) | HLa (%) | HFo (%) | HAc (%) | HPr (%) | EtOH (%) | HBu (%) | (HAc + HBu) /SMP (%) | TVFA (mg COD/L) | SMP (mg COD/L) | TVFA/SMP |
|---|---|---|---|---|---|---|---|---|---|---|
| 19.51 ± 1.06 | 1.09 | 0.11 | 12.97 | 1.43 | 10.38 | 74.02 | 86.99 | 8707.11 | 9835.21 | 88.53 |
| 10.19 ± 1.68 | 2.13 | 0.09 | 9.71 | 0.84 | 11.34 | 75.89 | 85.60 | 4903.98 | 5667.38 | 86.53 |
| 5.34 ± 1.05 | 1.14 | 0.38 | 9.77 | 6.09 | 15.89 | 66.73 | 76.50 | 2981.72 | 3593.73 | 82.97 |
| 2.51 ± 0.76 | 1.01 | 0.33 | 9.66 | 6.05 | 17.22 | 65.73 | 75.39 | 797.18 | 974.91 | 81.77 |
| 3.48 ± 1.33 | 1.49 | 0.56 | 7.31 | 1.2 | 19.97 | 69.47 | 76.78 | 1062.49 | 1352.81 | 78.54 |
| Height (h/H) | Substrate Conc. (g Total Sugar/L) | Hydrogen Conc. (%) | Production Rate (L/L/d) | Hydrogen Yield (mol H2/mol hexose) | Total Sugar Utilization (%) | Biomass Conc. (g VSS/L) Top | Biomass Conc. (g VSS/L) Filter | Biomass Conc. (g VSS/L) Bottom |
|---|---|---|---|---|---|---|---|---|
| 1/4 | 20.0 | 39.22 ± 1.14 | 25.76 ± 0.81 | 0.53 ± 0.01 | 74.83 ± 1.15 | 16.21 ± 1.48 | 9.23 ± 1.28 | 18.22 ± 1.01 |
| 10.0 | 37.55 ± 0.79 | 11.11 ± 0.64 | 0.61 ± 0.01 | 43.70 ± 1.53 | 6.93 ± 0.62 | 5.00 ± 0.24 | 8.28 ± 1.02 | |
| 5.0 | 34.55 ± 0.89 | 6.78 ± 0.43 | 0.90 ± 0.05 | 39.76 ± 1.16 | 6.70 ± 0.49 | 4.29 ± 0.34 | 7.59 ± 0.55 | |
| 3.5 | 32.18 ± 0.98 | 3.10 ± 0.24 | 0.81 ± 0.04 | 29.56 ± 1.31 | 3.48 ± 0.57 | 2.59 ± 0.40 | 4.16 ± 0.39 | |
| 2/4 | 20.0 | 47.67 ± 2.66 | 55.70 ± 2.42 | 0.90 ± 0.06 | 94.42 ± 3.99 | 20.34 ± 0.92 | 13.32 ± 1.41 | 20.01 ± 0.96 |
| 10.0 | 34.77 ± 1.69 | 34.42 ± 1.27 | 1.21 ± 0.03 | 85.87 ± 1.91 | 13.52 ± 0.76 | 10.33 ± 0.91 | 10.77 ± 0.68 | |
| 5.0 | 31.86 ± 2.17 | 11.03 ± 1.22 | 0.82 ± 0.07 | 84.81 ± 0.99 | 5.27 ± 0.61 | 6.50 ± 0.40 | 5.76 ± 0.46 | |
| 3.5 | 35.02 ± 3.73 | 8.35 ± 0.55 | 1.31 ± 0.07 | 56.57 ± 2.92 | 4.21 ± 0.37 | 3.5 ± 0.33 | 5.32 ± 0.44 | |
| 3/4 | 20.0 | 34.59 ± 1.80 | 25.34 ± 0.60 | 0.46 ± 0.01 | 83.72 ± 1.35 | 15.68 ± 1.63 | 11.20 ± 1.55 | 19.96 ± 1.10 |
| 10.0 | 30.94 ± 5.25 | 11.67 ± 0.81 | 0.56 ± 0.01 | 65.33 ± 1.24 | 5.83 ± 0.53 | 6.19 ± 0.75 | 6.89 ± 1.12 | |
| 5.0 | 45.08 ± 1.49 | 9.69 ± 0.40 | 1.31 ± 0.03 | 45.59 ± 1.15 | 5.06 ± 0.47 | 5.35 ± 0.31 | 5.09 ± 0.48 | |
| 3.5 | 49.14 ± 2.00 | 7.59 ± 0.51 | 2.03 ± 0.08 | 33.00 ± 1.24 | 3.13 ± 0.33 | 3.24 ± 0.51 | 4.68 ± 0.37 | |
| 4/4 | 20.0 | 26.64 ± 0.62 | 27.87 ± 0.26 | 0.54 ± 001 | 79.00 ± 0.98 | 16.59 ± 0.75 | 18.06 ± 0.84 | 19.09 ± 1.91 |
| 10.0 | 28.98 ± 1.40 | 10.26 ± 0.37 | 0.72 ± 0.04 | 54.29 ± 2.65 | 3.96 ± 0.94 | 4.61 ± 1.09 | 7.15 ± 1.71 | |
| 5.0 | 28.78 ± 1.31 | 2.96 ± 0.33 | 0.46 ± 0.05 | 45.75 ± 1.16 | 2.41 ± 0.38 | 4.27 ± 1.50 | 3.06 ± 0.73 | |
| 3.5 | 27.94 ± 0.86 | 1.24 ± 0.22 | 0.37 ± 0.07 | 33.28 ± 1.50 | 1.56 ± 0.44 | 2.05 ± 0.45 | 1.79 ± 0.30 |
| h/H | Substrate Conc. (g Total Sugar/L) | HLa (%) | HFo (%) | HAc (%) | HPr (%) | EtOH (%) | HBu (%) | (HAc + HBu) /SMP (%) | TVFA (mg COD/L) | SMP (mg COD/L) | TVFA/SMP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/4 | 10.0 | 3.30 | 1.54 | 9.43 | 1.57 | 19.78 | 64.38 | 73.81 | 2404.95 | 3126.56 | 76.92 |
| 5.0 | 1.42 | 1.27 | 7.46 | 2.61 | 23.53 | 63.71 | 71.17 | 1006.60 | 1341.24 | 75.05 | |
| 3.5 | 4.51 | 0.26 | 8.57 | 1.37 | 21.82 | 63.47 | 72.04 | 612.47 | 831.37 | 73.67 | |
| 2/4 | 10.0 | 2.13 | 0.09 | 9.71 | 0.84 | 11.34 | 75.89 | 81.31 | 4903.98 | 5667.38 | 82.20 |
| 5.0 | 1.14 | 0.38 | 9.77 | 6.09 | 15.89 | 66.73 | 66.42 | 2981.72 | 3593.73 | 72.04 | |
| 3.5 | 1.49 | 0.56 | 7.31 | 1.2 | 19.97 | 69.47 | 69.98 | 1062.50 | 1352.81 | 71.58 | |
| 3/4 | 10.0 | 2.41 | 2.16 | 6.37 | 2.49 | 18.76 | 67.81 | 74.18 | 3130.21 | 3970.83 | 78.83 |
| 5.0 | 4.62 | 0.92 | 6.45 | 2.79 | 17.09 | 68.13 | 74.58 | 1496.45 | 1911.42 | 78.29 | |
| 3.5 | 1.03 | 1.56 | 6.72 | 2.99 | 23.76 | 63.94 | 70.66 | 702.09 | 933.51 | 75.21 | |
| 4/4 | 10.0 | 1.93 | 2.15 | 7.37 | 4.92 | 20.69 | 62.94 | 70.31 | 2523.52 | 3261.21 | 77.38 |
| 5.0 | 0.39 | 1.21 | 7.83 | 3.79 | 23.67 | 63.11 | 70.94 | 1470.37 | 1936.23 | 75.94 | |
| 3.5 | 2.66 | 1.18 | 8.34 | 1.49 | 22.58 | 63.75 | 72.09 | 765.36 | 1023.75 | 74.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.-Y.; Zheng, J.-L.; Chen, T.-H.; Bhuyar, P. High Performance of Biohydrogen Production in Packed-Filter Bioreactor via Optimizing Packed-Filter Position. Int. J. Environ. Res. Public Health 2021, 18, 7462. https://doi.org/10.3390/ijerph18147462
Chu C-Y, Zheng J-L, Chen T-H, Bhuyar P. High Performance of Biohydrogen Production in Packed-Filter Bioreactor via Optimizing Packed-Filter Position. International Journal of Environmental Research and Public Health. 2021; 18(14):7462. https://doi.org/10.3390/ijerph18147462
Chicago/Turabian StyleChu, Chen-Yeon, Jin-Long Zheng, Tsung-Hsien Chen, and Prakash Bhuyar. 2021. "High Performance of Biohydrogen Production in Packed-Filter Bioreactor via Optimizing Packed-Filter Position" International Journal of Environmental Research and Public Health 18, no. 14: 7462. https://doi.org/10.3390/ijerph18147462







