Therapeutic Management of Rare Primary Ovarian Neoplasms: Carcinosarcoma, Leiomyosarcoma, Melanoma and Carcinoid
Abstract
:1. Introduction
2. Primary Carcinosarcoma of the Ovary (PCSO)
2.1. Characteristics
2.2. Treatment
3. Primary Leiomyosarcoma of the Ovary (PLSO)
3.1. Characteristics
3.2. Treatment
4. Primary Ovarian Melanoma (POM)
4.1. Characteristics
4.2. Treatment
5. Primary Carcinoid of the Ovary (PCO)
5.1. Characteristics
5.2. Treatment
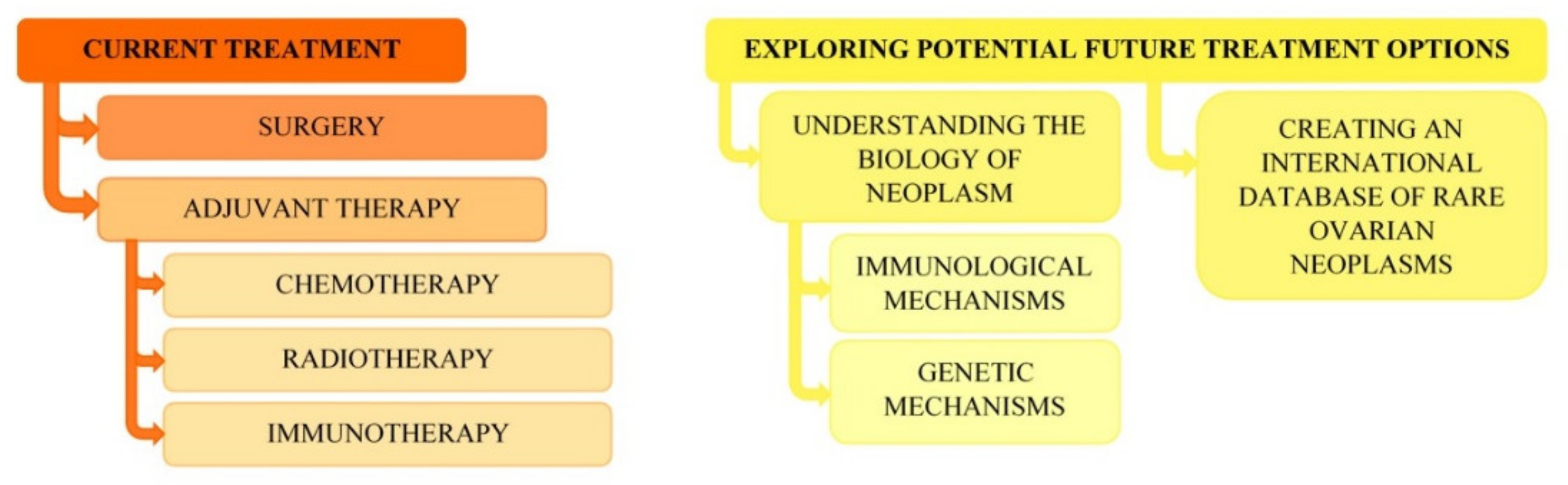
6. Conclusions, Perspectives and Clinical Observations of the Authors
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rauh-Hain, J.A.; Growdon, W.B.; Rodriguez, N.; Goodman, A.; Boruta, D.; Schorge, J.O.; Horowitz, N.S.; Del Carmen, M.G. Carcinosarcoma of the ovary: A case–control study. Gynecol. Oncol. 2011, 121, 477–481. [Google Scholar] [CrossRef]
- George, E.M.; Herzog, T.J.; Neugut, A.I.; Lu, Y.-S.; Burke, W.M.; Lewin, S.N.; Hershman, D.L.; Wright, J.D. Carcinosarcoma of the ovary: Natural history, patterns of treatment, and outcome. Gynecol. Oncol. 2013, 131, 42–45. [Google Scholar] [CrossRef] [Green Version]
- Patnayak, R.; Jena, A.; Prakash, J.; Sundaram, R.; Vijaylaxmi, B.; Lakhmi, A. Primary carcinosarcoma of ovary an unusual tumor case report with review of literature. J. Basic Clin. Reprod. Sci. 2015, 4, 39. [Google Scholar] [CrossRef]
- Dasgupta, S.; Bose, D.; Bhattacharyya, N.; Biswas, P. Carcinosarcoma of ovary with its various immunohistochemical expression: Report of a rare case. J. Cancer Res. Ther. 2015, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Terada, K.Y.; Miki, N.; Miki, K. Non-Islet Cell Tumor Hypoglycemia Associated With Recurrent Carcinosarcoma of the Ovary. Endocr. Pract. 2013, 19, 83–87. [Google Scholar] [CrossRef]
- Chandra, S.; Gaur, D.S.; Saini, S.; Pathak, V.P. Malignant mixed Müllerian tumour of the ovary with probable prognostic factors. J. Obstet. Gynaecol. 2011, 31, 457–458. [Google Scholar] [CrossRef]
- Akhtar, K. Primary Carcinosarcoma of Ovary with Lung Metastasis in a Young Female: A Rare Case Report. MOJ Clin. Med Case Rep. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Nishida, M.; Takebayashi, K.; Sato, H.; Ishii, T.; Nasu, K.; Narahara, H. Carcinosarcoma of the ovary successfully treated with paclitaxel and carboplatin therapy: Two cases. Int. Cancer Conf. J. 2014, 4, 88–91. [Google Scholar] [CrossRef]
- Rocha, J.; Mota, R.; Portugal, R.; Paiva, V.; Costa, A. Carcinosarcoma of ovary: Report of a rare case. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, e96. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshini, P.; Das Majumdar, S.K.; Parida, D.K. Carcinosarcoma of ovary with long-term overall survival. Oncol. J. India 2019, 3, 41. [Google Scholar] [CrossRef]
- Jain, V.; Pundir, S.; Sekhon, R.; Mishra, A.; Nayyar, N.; Vishwakarma, G.; Kamboj, M.; Rawal, S. Carcinosarcoma of the Ovary: A Single-Institute Experience with Surgical Cytoreduction and Platinum-Based Chemotherapy. J. Gynecol. Surg. 2019, 35, 224–231. [Google Scholar] [CrossRef]
- Lu, C.-H.; Chen, I.-H.; Chen, Y.-J.; Wang, K.-L.; Qiu, J.-T.T.; Lin, H.; Lin, W.-C.; Liou, W.-S.; Huang, Y.-F.; Lin, Y.-S.; et al. Primary Treatment and Prognostic Factors of Carcinosarcoma of the Ovary, Fallopian Tube, and Peritoneum: A Taiwanese Gynecologic Oncology Group Study. Int. J. Gynecol. Cancer 2014, 24, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Loizzi, V.; Cormio, G.; Camporeale, A.; Falagario, M.; De Mitri, P.; Scardigno, D.; Putignano, G.; Selvaggi, L.E. Carcinosarcoma of the Ovary: Analysis of 13 Cases and Review of the Literature. Oncology 2011, 80, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Kitajima, M.; Hachisuga, T.; Tanimoto, A.; Okura, N.; Kihara, I. Malignant mixed müllerian tumor with malignant neuroectodermal components (teratoid carcinosarcoma) of the ovary: Report of a case with clinicopathologic findings. J. Obstet. Gynaecol. Res. 2010, 36, 907–911. [Google Scholar] [CrossRef]
- Carnevali, I.W.; Cimetti, L.; Sahnane, N.; Libera, L.; Cavallero, A.; Formenti, G.; Riva, C.; Tibiletti, M.G. Two Cases of Carcinosarcomas of the Ovary Involved in Hereditary Cancer Syndromes. Int. J. Gynecol. Pathol. 2017, 36, 64–70. [Google Scholar] [CrossRef]
- D’Amati, A.; Pezzuto, F.; Serio, G.; Marzullo, A.; Fortarezza, F.; Lettini, T.; Cazzato, G.; Cormio, G.; Resta, L. Mesonephric-Like Carcinosarcoma of the Ovary Associated with Low-Grade Serous Carcinoma: A Case Report. Diagnostics 2021, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Belbaraka, R.; Taleb, A.; Errihani, H. A Rare Tumor of the Ovary: Carcinosarcoma. J. Med. Cases 2010, 1, 55–57. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, I.; Meydanli, M.M.; Turan, A.T.; Taşkın, S.; Sari, M.E.; Gungor, T.; Akbayir, O.; Ayhan, A. Carcinosarcoma of the ovary compared to ovarian high-grade serous carcinoma: Impact of optimal cytoreduction and standard adjuvant treatment. Int. J. Clin. Oncol. 2018, 23, 329–337. [Google Scholar] [CrossRef]
- Pushpagiri, N.; Dinakaran, S.; Chidambaram, A.; Pari, P. Carcinosarcoma of ovary—A rare case report and literature overview. Int. J. Adv. Med. 2016, 3, 440–442. [Google Scholar] [CrossRef]
- Powell, M.A.; Filiaci, V.L.; Hensley, M.L.; Huang, H.Q.; Moore, K.N.; Tewari, K.S.; Copeland, L.J.; Secord, A.A.; Mutch, D.G.; Santin, A.; et al. A randomized phase 3 trial of paclitaxel (P) plus carboplatin (C) versus paclitaxel plus ifosfamide (I) in chemotherapy-naive patients with stage I-IV, persistent or recurrent carcinosarcoma of the uterus or ovary: An NRG Oncology trial. J. Clin. Oncol. 2019, 37, 5500. [Google Scholar] [CrossRef]
- Goodall, E.J.; Madhuri, T.; Manuel, S.B. The Management Dilemma of Leiomyosarcoma of the Ovary. World J. Oncol. 2011, 2, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Nazneen, S.; Kumari, A.; Choudhary, V.; Kumari, S.; Pankaj, S. Prolonged Survival of a Young Female with High Grade Pleomorphic Leiomyosarcoma of Ovary Without Recurrence. J. Obstet. Gynecol. India 2016, 66, 639–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvi, K.; Toi, P.C.; Badhe, B.A. A Rare Case of Primary Leiomyosarcoma of the Ovary in Association with Cellular Leiomyoma of the Broad Ligament and Uterus. J. Gynecol. Surg. 2014, 30, 363–366. [Google Scholar] [CrossRef]
- Na Lee, B.; Ouh, Y.T.; Choi, H.J.; Yang, S.Y.; Lee, J.K.; Hong, J.H. Leiomyosarcoma of the Ovary Mimicking Gastrointestinal Stromal Tumor Originating from Small Bowel: A Case Report and Literature Review. Gynecol. Obstet. 2016, 6, 1000359. [Google Scholar] [CrossRef]
- Divya, N.S.; Srinivasamurthy, V. Myxoid Leiomyosarcoma of Ovary-A Rare Case Report. J. Clin. Diagn. Res. 2014, 8, FD05–FD06. [Google Scholar] [CrossRef]
- He, M.; Deng, Y.-J.; Zhao, D.-Y.; Zhang, Y.; Wu, T. Synchronous leiomyosarcoma and fibroma in a single ovary: A case report and review of the literature. Oncol. Lett. 2016, 11, 2510–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, J.V.; Khurana, A.; Kaur, P.; Chuahan, A.K.; Singh, S. A rare presentation of primary leiomyosarcoma of ovary in a young woman. Ecancermedicalscience 2015, 9, 524. [Google Scholar] [CrossRef] [Green Version]
- Vishwanath; Vyas, N.M.; Goripally, S.; Rai, S. A Rare Case of Primary Leiomyosarcoma of the Ovary. J. Clin. Diagn. Res. 2018. [Google Scholar] [CrossRef]
- Pongsuvareeyakul, T.; Sukpan, K.; Chaicharoen, S.; Khunamornpong, S. Leiomyosarcoma and Squamous Cell Carcinoma Arising in Mature Cystic Teratoma of the Ovary. Case Rep. Pathol. 2017, 2017, 7907359. [Google Scholar] [CrossRef] [Green Version]
- Rivas, G.; Bonilla, C.; Rubiano, J.; Arango, N. Primary Leiomyosarcoma of the Ovary: A Case Report. Case Rep. Clin. Med. 2014, 3, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Kudva, R.; Ayachit, G.S.; Ayachit, A. Malignant Melanoma Arising in an Ovarian Mature Cystic Teratoma—A Rare Entity. J. Clin. Diagn. Res. 2015, 9, ED14–ED16. [Google Scholar] [CrossRef]
- Paola, A.; Maria, R.S.; Laura, C.; Elena, N.; Orlando, C.; Teresio, M. A rare melanoma feature with primary ovarian origin: A case report and the literature review. Obstet. Gynecol. Sci. 2018, 61. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-B.; Lee, S.-H.; Shin, J.-W.; Chung, D.-H.; Park, C.-Y. Ovarian malignant melanoma without evidence of teratoma. J. Obstet. Gynaecol. Res. 2010, 36, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.K.; Lee, D.H.; Cho, D.H.; Jang, K.Y.; Kim, K.M. Primary malignant melanoma arising from ruptured ovarian mature cystic teratoma with elevated serum CA 19–9: A case report and review of literature. BMC Womens Health 2019, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Genc, M.; Sivrikoz, O.; Genç, B.; Kurt, S.; Celik, E. Synchronous primary endometrial carcinoma and metastatic malignant melanoma in an ovarian cystic teratoma. Turk. J. Pathol. 2015, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Liu, N.-F.; Sheng, X.-G. Malignant ovarian melanoma with extensive pelvic and peritoneal metastasis: A case report and literature review. Chin. J. Cancer 2010, 29, 460–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajbhanari, A.; Manandhar, U. Malignant melanoma arising within ovarian mature cystic teratoma. J. Pathol. Nepal 2019, 9, 1508–1510. [Google Scholar] [CrossRef]
- Sioulas, V.D.; Panayiotides, I.G.; Chrelias, C.; Grammatikakis, I.; Vaggopoulos, V.; Kefala, M.; Kassanos, D. Ovarian melanoma complicating pregnancy achieved byin-vitrofertilisation. J. Obstet. Gynaecol. 2013, 33, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, K.; Young, F.; Kucukmetin, A.; Cresti, N.; Plummer, R.; Ralte, A.; O’Donnell, R.L. BRAF Wild-type, PTEN Mutant Malignant Uveal Melanoma Arising Within a Mature Ovarian Teratoma: A Case Report and Review of the Literature. Int. J. Gynecol. Pathol. 2020, 39, 321–326. [Google Scholar] [CrossRef]
- Shah, H.A.; Chandra, K.; Shah, A.A.; Verma, P.K. Bilateral Primary Ovarian Amelanotic Malignant Melano-ma Arising in a Mature Teratoma in Pediatric Age Group. Indian J. Clin. Pract. 2016, 27, 367–369. Available online: https://www.researchgate.net/profile/Jay-Prakash-5/publication/325206152_Minimal_Intubating_Dose_of_Succinylcholine_A_Comparative_Study_of_04_05_and_06_mgkg_Dose_Indian_Journal_of_Clinical_Practice_2742016322-5/links/5b13793ea6fdcc4611df9a73/Minimal-Intub (accessed on 15 September 2016).
- Ueng, S.-H.; Pinto, M.M.; Alvarado-Cabrero, I.; Lee, L.-Y.; Tavassoli, F.A. Ovarian Malignant Melanoma: A Clinicopathologic Study of 5 Cases. Int. J. Surg. Pathol. 2010, 18, 184–192. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.H.; Chon, G.R.; Kim, A.; Kim, B.-H. Primary Malignant Melanoma Arising in an Ovarian Mature Cystic Teratoma—A Case Report and Literature Review. Korean J. Pathol. 2011, 45, 659. [Google Scholar] [CrossRef]
- Kaplan, M.A.; Küçüköner, M.; Inal, A.; Işıkdoğan, A.; Urakçı, Z. Ovarian malignant melanoma presenting with hypercalcemia and bone marrow infiltration: A case report and review of the literature. J. Clin. Exp. Investig. 2012, 3. [Google Scholar] [CrossRef]
- Samiee-Rad, F.; Zangivand, A.A.; Soleimanitadi, K. Primary form of malignant melanoma in an ovarian mature cystic teratoma: Case report and literature review. Comp. Haematol. Int. 2017, 26, 989–992. [Google Scholar] [CrossRef]
- Mahapatra, L.; Andruska, N.; Mao, C.; Le, J.; Shapiro, D.J. A Novel IMP1 Inhibitor, BTYNB, Targets c-Myc and Inhibits Melanoma and Ovarian Cancer Cell Proliferation. Transl. Oncol. 2017, 10, 818–827. [Google Scholar] [CrossRef]
- Chai, W.; Zhang, W.; Zhou, L.; Sun, X.; Jia, G. Strumal carcinoid tumor of the ovary. Medicine 2019, 98, e18009. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kumarapeli, A.; Chen, F.; Paczos, T. Primary Mucinous Carcinoid of the Ovary Arising in a Mature Cystic Teratoma: A Case Report with Review of the Literature. Am. Chin. J. Med. Sci. 2012, 5, 239. [Google Scholar] [CrossRef]
- Lenicek, T.; Tomas, D.; Soljacić-Vranes, H.; Kraljević, Z.; Klarić, P.; Kos, M.; Kos, M. Strumal carcinoid of the ovary: Report of two cases. Acta Clin. Croat. 2012, 51, 649–653. [Google Scholar]
- Yamada, S.; Hisaoka, M.; Tanimoto, A.; Urabe, R.; Sasaguri, Y. Two cases of strumal carcinoid of the ovary: The enigma of its histogenesis. Rev. Esp. Patol. 2011, 44, 49–54. [Google Scholar] [CrossRef]
- Rao, K.N.; Vijayasree, M.; Devi, C.P.; Sailabala, G.; Katta, R. Primary Strumal Carcinoid of the Ovary—A Rare Entity. Int. J. Sci. Res. 2015, 4, 2310–2311. Available online: https://www.ijsr.net/archive/v4i1/SUB15829.pdf (accessed on 15 January 2015).
- Gokhan, B. A Primary Insular Type Carcinoid Tumor Arising in a Mature Cystic Teratoma of the Ovary: A Case Report. J. Clin. Case Rep. 2012, 2, 2. [Google Scholar] [CrossRef]
- Ghanbarzadeh, N.; Nadjafi-Semnani, M.; Azarkar, Z.; Haghighi, F.; Nadjafi-Semnani, A. Primary strumal carcinoid tu-mor of the ovary: A case report. Iran. J. Pathol. 2014, 9, 285–290. [Google Scholar]
- Macháleková, K.; Kolníková, G.; Redecha, M.; Žúbor, P.; Kajo, K. Strumal carcinoid of the ovary—Report of two cases and review of literature. Ceska Gynekol. 2018, 83, 452–457. [Google Scholar] [PubMed]
- Fiore, M.G.; Rossi, R.; Covelli, C.; Loizzi, V.; Piscitelli, D.; Cormio, G. Goblet-cell carcinoid of the ovary: A case report with ultrastructural analysis. J. Obstet. Gynaecol. 2017, 37, 266–267. [Google Scholar] [CrossRef]
- Sulaiman, S.; Chia, Y.N.; Namuduri, R.V.D. Strumal carcinoid tumour of the ovary presenting with severe constipation. Singap. Med. J. 2013, 54, e21–e23. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Rajwanshi, A.; Dey, P. Carcinoid of the Ovary. Diagn. Cytopathol. 2013, 42, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Arora, R.; Bhasin, S.; Khurana, N. An Unusual Case of Synchronous Carcinoid of Ovary and Gall Bladder. Case Rep. Obstet. Gynecol. 2013, 2013, 737016. [Google Scholar] [CrossRef]
- Van Rompuy, A.-S.; Vanderstichele, A.; Vergote, I.; Moerman, P. Diffusely Metastasized Adenocarcinoma Arising in a Mucinous Carcinoid of the Ovary. Int. J. Gynecol. Pathol. 2018, 37, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, B.; Grana, C.; Fazio, N.; Cavaliere, A.F.; Ferrari, M.; Bodei, L.; Baio, S.; Scambia, G.; Paganelli, G.; Peccatori, F. Pregnant with metastatic neuroendocrine tumour of the ovary: What now? Ecancermedicalscience 2012, 6, 240. [Google Scholar] [CrossRef] [PubMed]
- Kaiho-Sakuma, M.; Toyoshima, M.; Watanabe, M.; Toki, A.; Kameda, S.; Minato, T.; Niikura, H.; Yaegashi, N. Aggressive neuroendocrine tumor of the ovary with multiple metastases treated with everolimus: A case report. Gynecol. Oncol. Rep. 2018, 23, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Delić, R. Mucinous cystadenoma of the ovary with carcinoid tumour in a 23-year-old nulliparous woman. J. Obstet. Gynaecol. 2017, 37, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Buis, C.C.; van Doorn, H.C.; Dinjens, W.N.; Ewing, P.C. Mucinous carcinoid of the ovary: Report of a case with metastasis in the contralateral ovary after ten years. Rare Tumors 2010, 2, 106–109. [Google Scholar] [CrossRef] [Green Version]
- Ayyanar, P.; Begum, J.; Rout, S.; Mishra, P. Synchronous colonic adenocarcinoma and well-differentiated neuroendocrine tumor arising in a mature cystic teratoma of ovary—Rare presentation in a postmenopausal woman with literature review. Indian J. Pathol. Microbiol. 2021, 64, 385–389. [Google Scholar] [PubMed]
- Noh, H.K.; Kwon, B.S.; Kim, Y.H.; Lee, N.K.; Choi, K.U.; Suh, D.S.; Lee, D.H.; Kim, K.H. Peptide YY producing strumal carcinoid tumor of the ovary in a postmenopausal woman: A rare cause of chronic constipation. Obstet. Gynecol. Sci. 2017, 60, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Buda, A.; Giuliani, D.; Montano, N.; Perego, P.; Milani, R. Primary insular carcinoid of the ovary with carcinoid heart disease: Unfavourable outcome of a case. Int. J. Surg. Case Rep. 2012, 3, 59–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vora, M.; Lacour, R.A.; Black, D.R.; Turbat-Herrera, E.A.; Gu, X. Neuroendocrine tumors in the ovary: Histogenesis, pathologic differentiation, and clinical presentation. Arch. Gynecol. Obstet. 2016, 293, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Doraiswamy, J.; Reddy, K.; Joshi, P.A.; Gornall, R. A virilising primary mucinous carcinoid tumour of the ovary in a postmenopausal woman: A diagnostic challenge! J. Obstet. Gynaecol. 2017, 37, 123–124. [Google Scholar] [CrossRef]
- Gupta, B.; Suneja, A.; Vaid, N.; Bhatia, A. Primary ovarian carcinoid tumor simulating virilizing tumor of the ovary: A rare entity. Indian J. Cancer 2014, 51, 529. [Google Scholar] [CrossRef]
- Kurabayashi, T.; Minamikawa, T.; Nishijima, S.; Tsuneki, I.; Tamura, M.; Yanase, T.; Hashidate, H.; Shibuya, H.; Motoyama, T. Primary strumal carcinoid tumor of the ovary with multiple bone and breast metastases. J. Obstet. Gynaecol. Res. 2010, 36, 567–571. [Google Scholar] [CrossRef]
- Niu, D.; Li, Z.; Sun, L.; Cao, D. Carcinoid Arising From the Teratomatous Bronchial Mucosa in a Mature Cystic Teratoma of the Ovary. Int. J. Gynecol. Pathol. 2018, 37, 123–127. [Google Scholar] [CrossRef]
- Ulker, V.; Numanoglu, C.; Akbayir, O.; Akyol, A.; Tuncel, A.; Akca, A.; Aydin, O. Malignant transformation arising from mature cystic teratoma of the ovary: A report of six cases. J. Obstet. Gynaecol. Res. 2012, 38, 849–853. [Google Scholar] [CrossRef]
- Bohara, S.; Agarwal, S.; Khuraijam, B.; Khurana, N.; Arora, R. Strumal carcinoid ovary with mucinous cystadenoma presenting as a large abdominal mass and increased tumour marker level. J. Obstet. Gynaecol. 2013, 33, 637–638. [Google Scholar] [CrossRef]
- Liu, K.-T.; Chang, Y.-C.; Lin, Y.-C.; Chang, J.-L. Primary strumal carcinoid tumor of the ovary arising from a heter-ochronous struma ovarii in young female patient. J. Clin. Images Med. Case Rep. 2021, 2. Available online: http://jcimcr.org/pdfs/JCIMCR-v2-1057.pdf (accessed on 21 April 2021).
- Takatori, E.; Shoji, T.; Miura, J.; Takeuchi, S.; Yoshizaki, A.; Sugiyama, T. Case of peptide-YY-producing strumal carcinoid of the ovary: A case report and review. J. Obstet. Gynaecol. Res. 2012, 38, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Arimoto, T.; Sandoh, K.; Okano, K.; Ebisu, Y.; Ito, H.; Matsumoto, M.; Mizokami, T.; Kita, M.; Okada, H.; et al. Imprint cytology of strumal carcinoid of the ovary: A case report with immunocytochemical analysis. Diagn. Cytopathol. 2019, 47, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Tashiro, H.; Motohara, K.-I.; Ohba, T.; Katabuchi, H. Primary strumal carcinoid tumor of the ovary: A pregnant patient exhibiting severe constipation and CEA elevation. Gynecol. Oncol. Case Rep. 2013, 4, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Bhardwaj, M.; Ahuja, A. Rare case of primary trabecular carcinoid tumor of the ovary with unusual presentation. Taiwan. J. Obstet. Gynecol. 2016, 55, 748–750. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Katsuki, N.; Miyai, Y.; Shibuya, S.; Sasaki, M.; Bando, K.; Matsunaga, T.; Haba, R.; Kushida, Y.; Kadota, K.; et al. Cytopathologic characteristics of the primary strumal carcinoid tumor of the ovary: A case report with emphasis on differential diagnostic considerations. Diagn. Cytopathol. 2013, 41, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.M.; Goheen, M.P.; Broshears, J.R. Malignant Brenner Tumor of the Ovary with Transformation to Trabecular Carcinoid. Int. J. Gynecol. Pathol. 2012, 31, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sehouli, J.; Woopen, H.; Pavel, M.; Richter, R.; Lauterbach, L.K.; Taube, E.; Darb-Esfahani, S.; Fotopoulou, C.; Pietzner, K. Neuroendocrine neoplasms of the ovary: A retrospective study of the north eastern German Society of Gynecologic Oncology (NOGGO). Anticancer Res. 2016, 36, 1003–1009. [Google Scholar]
| Characteristics | Surgical Treatment Only | Surgical Treatment and Adjuvant Chemotherapy | |
|---|---|---|---|
| Cases Number (n = 14) | 4 | 10 | |
| Mean of patient age at diagnosis (min.-max.) | 51 years (22–72) | 58.8 years (40–80) | |
| Neoadjuvant chemotherapy | 1 | 1 | |
| Recurrences number | 1 | 1 | |
| Follow up | Death (n/min. OS/max. OS) | 1/25 mon. | 1/46 mon. |
| Survival (n/min. OS/max. OS) | 1/72 mon. | 8/6 mon./76 mon. | |
| Not specified/undergoing treatment (n) | 2 | 1 | |
| Characteristics | Surgical Treatment Only | Surgical Treatment and Adjuvant Chemotherapy | |
|---|---|---|---|
| Cases Number (n = 10) | 6 | 4 | |
| Mean of patient age at diagnosis (min.-max.) | 53.2 years (26–67) | 44.3 years (27–65) | |
| Recurrences number | 1 | 1 | |
| Follow up | Death (n/min. OS/max. OS) | 2/1 mon./51 mon. | 1/36 mon. |
| Survival (n/min. OS/max. OS) | 1/22 mon. | 3/2 mon./47 mon. | |
| Not specified/undergoing treatment (n) | 3 | 0 | |
| Characteristics | Surgical Treatment Only | Surgical and Adjuvant Treatment | |
|---|---|---|---|
| Cases Number (n = 18) | 9 | 9 | |
| Mean of patient age at diagnosis (years) | 47.6 | 55.2 | |
| Isolated ovarian melanoma | 2 | 2 | |
| Associated to ovarian teratoma | 7 | 7 | |
| Recurrences number | 6 | 2 | |
| Follow up | Death (n/min. OS/max. OS) | 6/3 mon./17 mon. | 5/2 mon./28 mon. |
| Survival (n/min. OS/max. OS) | 1/48 mon. | 2/6 mon./12 mon. | |
| Not specified/undergoing treatment (n) | 2 | 2 | |
| Characteristics | Surgical Treatment Only | Surgical and Adjuvant Treatment | |
|---|---|---|---|
| Cases Number (n = 40) | 35 | 5 | |
| Mean of patient age at diagnosis (min.–max.) (years) | 44.3 (14–78) | 58.8 (45–85) | |
| Recurrences number | 3 | 1 | |
| Follow up | Death (n/min. OS/max. OS) | 2/20 days/10 mon. | 2/24 mon./34 mon. |
| Survival (n/min. OS/max. OS) | 27/2 mon./213 mon. | 3/9 weeks/7 mon. | |
| Not specified/undergoing treatment (n) | 6 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowski, M.; Nowak, K.; Kordek, A.; Cymbaluk-Płoska, A. Therapeutic Management of Rare Primary Ovarian Neoplasms: Carcinosarcoma, Leiomyosarcoma, Melanoma and Carcinoid. Int. J. Environ. Res. Public Health 2021, 18, 7819. https://doi.org/10.3390/ijerph18157819
Kozłowski M, Nowak K, Kordek A, Cymbaluk-Płoska A. Therapeutic Management of Rare Primary Ovarian Neoplasms: Carcinosarcoma, Leiomyosarcoma, Melanoma and Carcinoid. International Journal of Environmental Research and Public Health. 2021; 18(15):7819. https://doi.org/10.3390/ijerph18157819
Chicago/Turabian StyleKozłowski, Mateusz, Katarzyna Nowak, Agnieszka Kordek, and Aneta Cymbaluk-Płoska. 2021. "Therapeutic Management of Rare Primary Ovarian Neoplasms: Carcinosarcoma, Leiomyosarcoma, Melanoma and Carcinoid" International Journal of Environmental Research and Public Health 18, no. 15: 7819. https://doi.org/10.3390/ijerph18157819
APA StyleKozłowski, M., Nowak, K., Kordek, A., & Cymbaluk-Płoska, A. (2021). Therapeutic Management of Rare Primary Ovarian Neoplasms: Carcinosarcoma, Leiomyosarcoma, Melanoma and Carcinoid. International Journal of Environmental Research and Public Health, 18(15), 7819. https://doi.org/10.3390/ijerph18157819






